Drug development Efficacy tests Safety toxicty test Preclinical









- Slides: 13







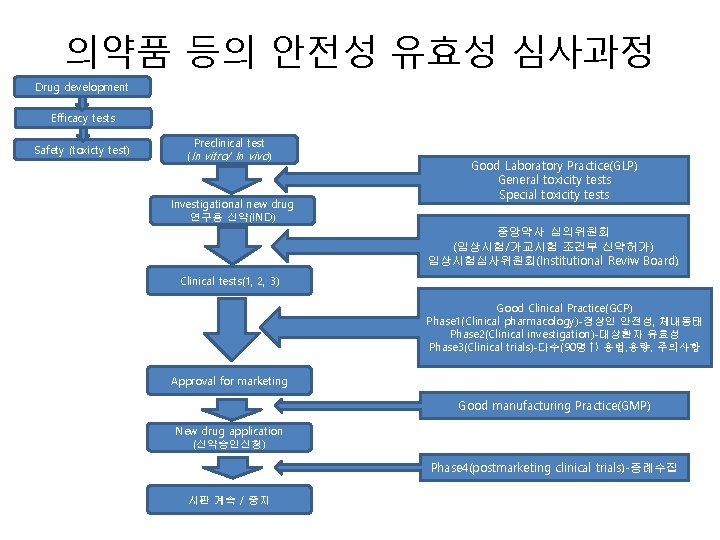
의약품 등의 안전성 유효성 심사과정 Drug development Efficacy tests Safety (toxicty test) Preclinical test (In vitro/ In vivo ) Investigational new drug 연구용 신약(IND) Good Laboratory Practice(GLP) General toxicity tests Special toxicity tests 중앙약사 심의위원회 (임상시험/가교시험 조건부 신약허가) 임상시험심사위원회(Institutional Reviw Board) Clinical tests(1, 2, 3) Good Clinical Practice(GCP) Phase 1(Clinical pharmacology)-정상인 안전성, 체내동태 Phase 2(Clinical investigation)-대상환자 유효성 Phase 3(Clinical trials)-다수(90명↑) 용법, 용량, 주의사항 Approval for marketing Good manufacturing Practice(GMP) New drug application (신약승인신청) Phase 4(postmarketing clinical trials)-증례수집 시판 계속 / 중지




Product of Silymarin Milk thistle has not been evaluated by the FDA for safety, effectiveness, or purity

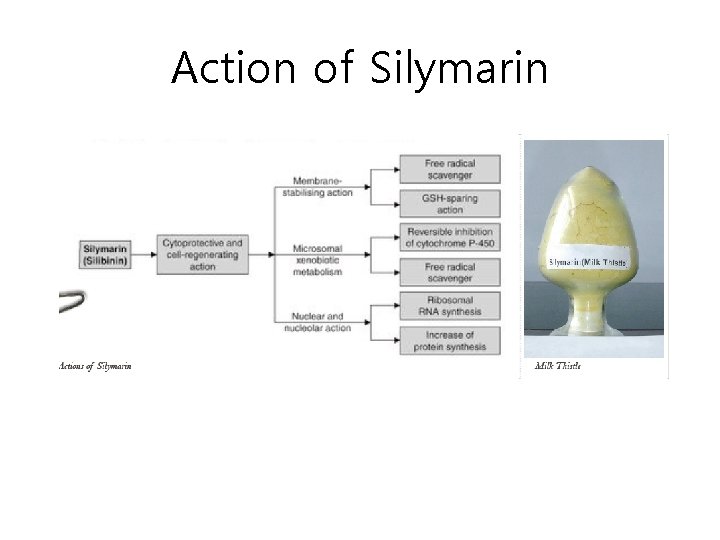
Action of Silymarin

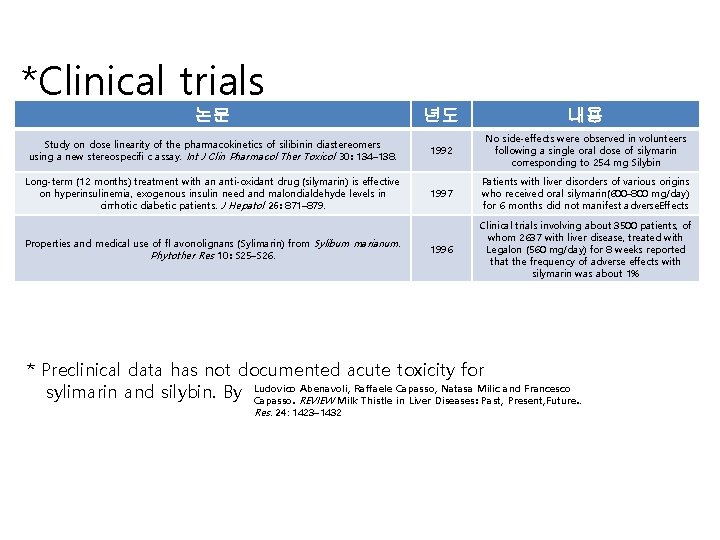
*Clinical trials 논문 년도 내용 Study on dose linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecifi c assay. Int J Clin Pharmacol Ther Toxicol 30: 134– 138. 1992 No side-effects were observed in volunteers following a single oral dose of silymarin corresponding to 254 mg Silybin Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol 26: 871– 879. 1997 Patients with liver disorders of various origins who received oral silymarin(600– 800 mg/day) for 6 months did not manifest adverse. Effects 1996 Clinical trials involving about 3500 patients, of whom 2637 with liver disease, treated with Legalon (560 mg/day) for 8 weeks reported that the frequency of adverse effects with silymarin was about 1% Properties and medical use of fl avonolignans (Sylimarin) from Sylibum marianum. Phytother Res 10: S 25–S 26. * Preclinical data has not documented acute toxicity for Abenavoli, Raffaele Capasso, Natasa Milic and Francesco sylimarin and silybin. By Ludovico Capasso. REVIEW Milk Thistle in Liver Diseases: Past, Present, Future. . Res. 24: 1423– 1432
 Preclinical drug development process
Preclinical drug development process Drug efficacy
Drug efficacy Drug efficacy
Drug efficacy Ace different tests iq tests but
Ace different tests iq tests but Explain tims in computer
Explain tims in computer Exhausted drug meaning
Exhausted drug meaning Self monitoring in organisational behaviour
Self monitoring in organisational behaviour Role efficacy
Role efficacy Potency vs efficacy
Potency vs efficacy Potency vs efficacy
Potency vs efficacy Nebido efficacy
Nebido efficacy Self confidence model
Self confidence model Christina mellon
Christina mellon Collective teacher efficacy
Collective teacher efficacy