Quantum dynamics of excited states of optically active










- Slides: 39

Quantum dynamics of excited states of optically active biomolecules Ross Mc. Kenzie condensedconcepts. blogspot. com

Outline • Optically active biomolecules are complex quantum many-body systems at the quantum-classical boundary • An effective Hamiltonian for quantum decoherence of optically excited states • Spectral density for chromophore-environment interaction is well characterised and can be described by dielectric continuum models. • Observing the ``collapse’’ of the wavefunction! • Ref: J. Gilmore and RHM, J. Phys. Chem. A 112, 2162 (2008) [Review article]

Some key questions concerning biomolecular functionality • Which details matter? • What role does water play? • Do biomolecules have the optimum structure to exploit dynamics for their functionality? • When is quantum dynamics (e. g. , tunneling, coherence, entanglement) necessary for functionality?

Specificity vs. universality For complex molecular materials when do the details matter? • Physicists say the details don’t matter. They think cows are spherical! • Chemists say details do matter. • Biologists say the details are a matter of life and death!

Quantum biology at amazon. com?

Engaging with Hameroff & Penrose

Kauzmann’s maxim • Walter Kauzmann (1916 -2009) was first to understand the hydrophobic interaction • “people will tend to believe what they want to believe rather than what the evidence before them suggests they should believe” Reminiscences of a life in protein physical chemistry, Protein Science 2, 671 (1992) condensedconcepts. blogspot. com


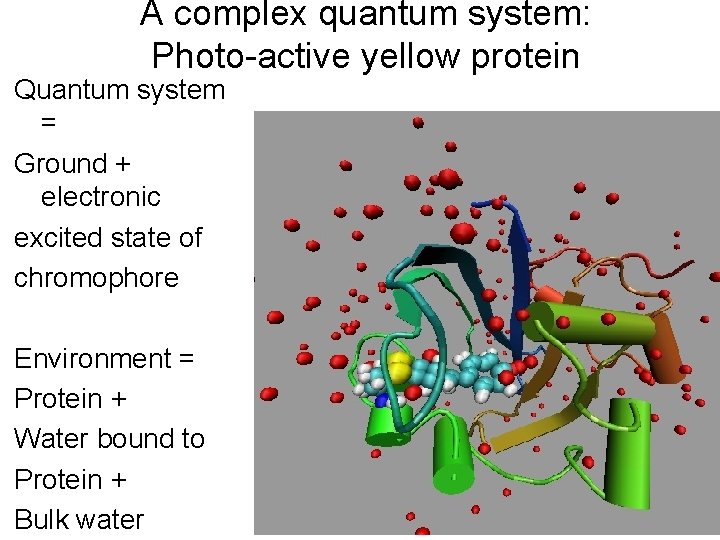
A complex quantum system: Photo-active yellow protein Quantum system = Ground + electronic excited state of chromophore Environment = Protein + Water bound to Protein + Bulk water

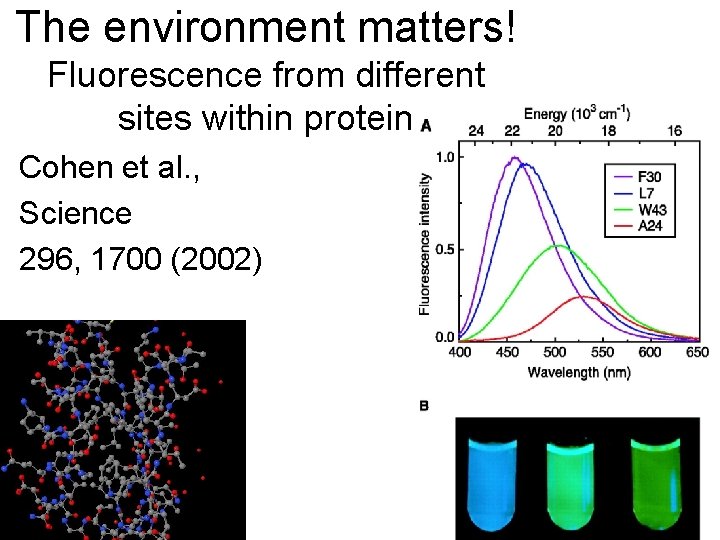
The environment matters! Fluorescence from different sites within protein Cohen et al. , Science 296, 1700 (2002)

Seeking a minimal model for this quantum system and its environment • Must capture and give insights into essential physics. • Tells us which physical parameters lead to qualitative changes in quantum dynamics.

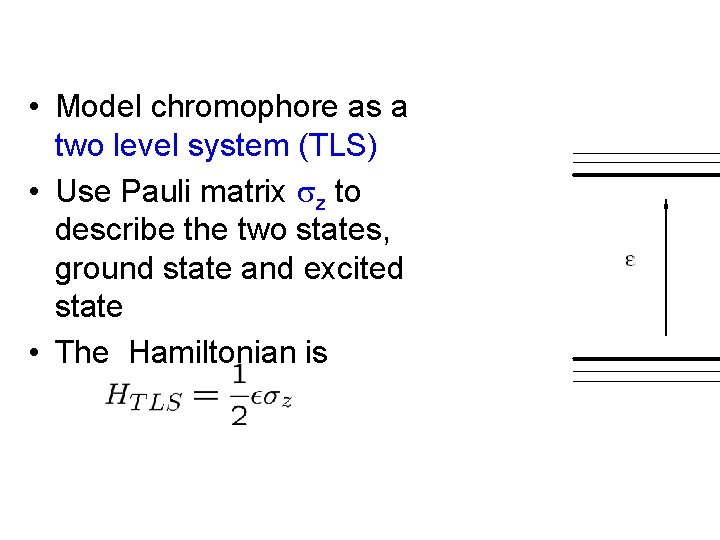
• Model chromophore as a two level system (TLS) • Use Pauli matrix z to describe the two states, ground state and excited state • The Hamiltonian is

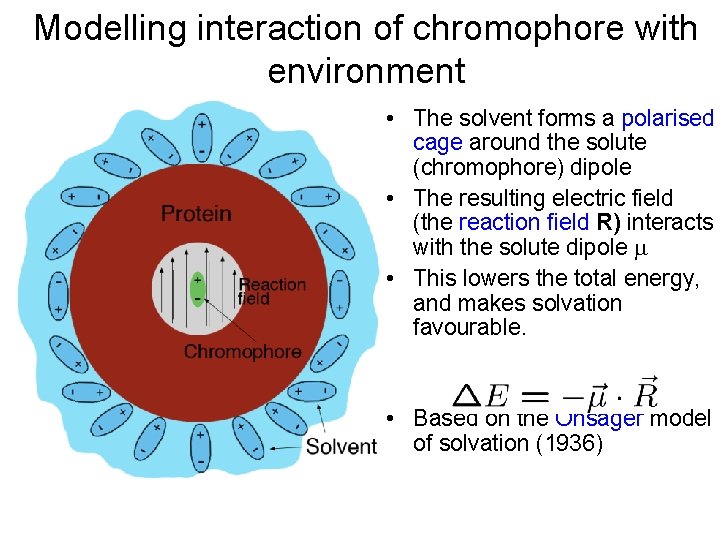
Modelling interaction of chromophore with environment • The solvent forms a polarised cage around the solute (chromophore) dipole • The resulting electric field (the reaction field R) interacts with the solute dipole • This lowers the total energy, and makes solvation favourable. • Based on the Onsager model of solvation (1936)

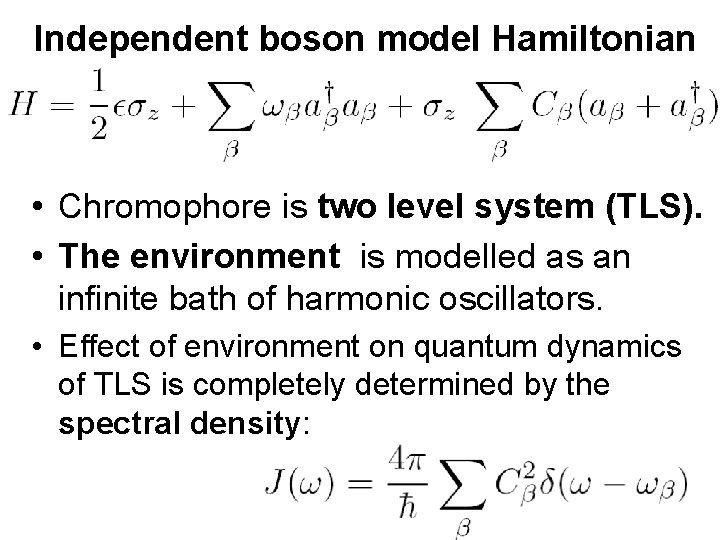
Independent boson model Hamiltonian • Chromophore is two level system (TLS). • The environment is modelled as an infinite bath of harmonic oscillators. • Effect of environment on quantum dynamics of TLS is completely determined by the spectral density:

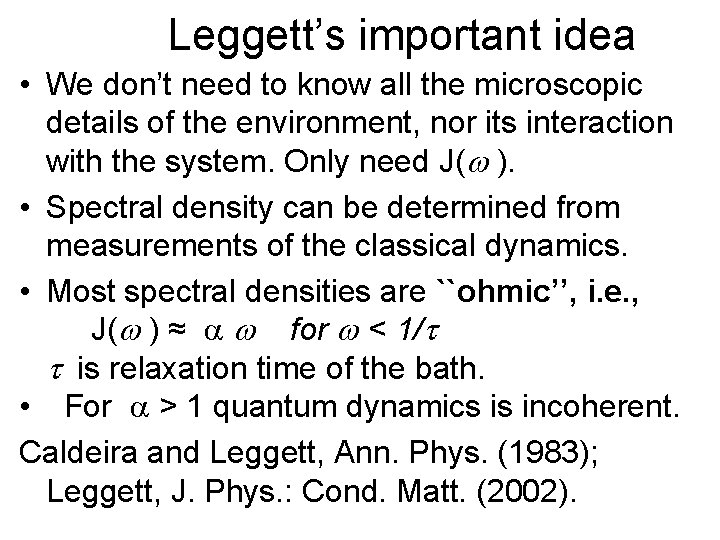
Leggett’s important idea • We don’t need to know all the microscopic details of the environment, nor its interaction with the system. Only need J( ). • Spectral density can be determined from measurements of the classical dynamics. • Most spectral densities are ``ohmic’’, i. e. , J( ) ≈ for < 1/t t is relaxation time of the bath. • For > 1 quantum dynamics is incoherent. Caldeira and Leggett, Ann. Phys. (1983); Leggett, J. Phys. : Cond. Matt. (2002).

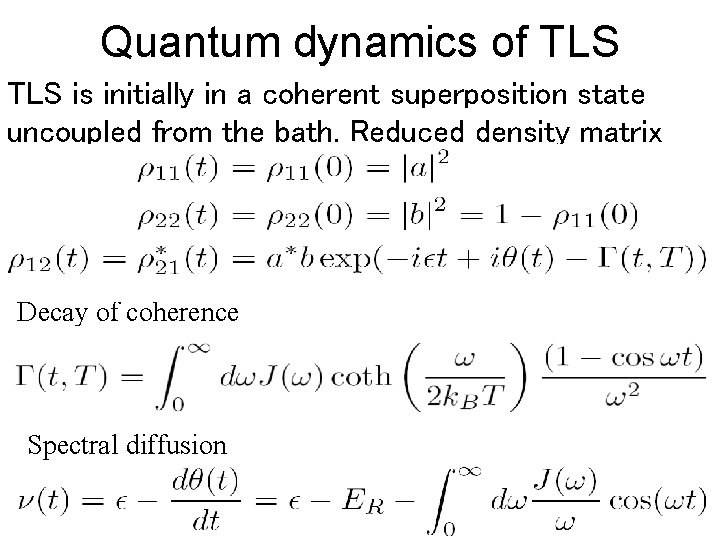
Quantum dynamics of TLS is initially in a coherent superposition state uncoupled from the bath. Reduced density matrix of TLS is Decay of coherence Spectral diffusion

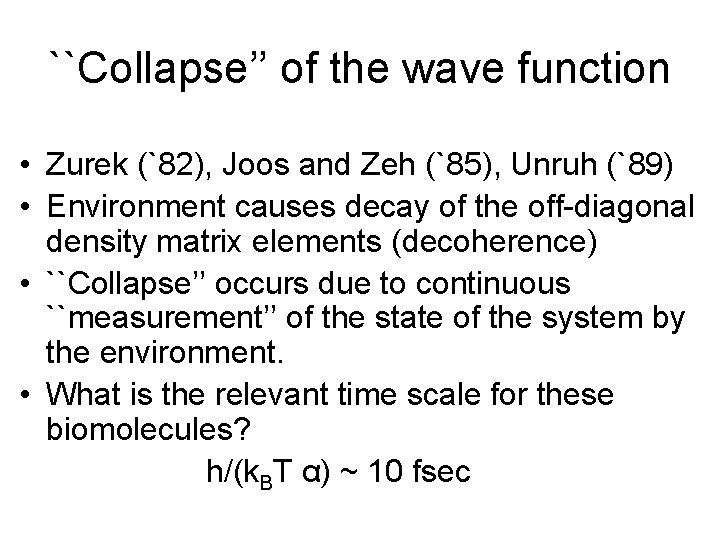
``Collapse’’ of the wave function • Zurek (`82), Joos and Zeh (`85), Unruh (`89) • Environment causes decay of the off-diagonal density matrix elements (decoherence) • ``Collapse’’ occurs due to continuous ``measurement’’ of the state of the system by the environment. • What is the relevant time scale for these biomolecules? h/(k. BT α) ~ 10 fsec

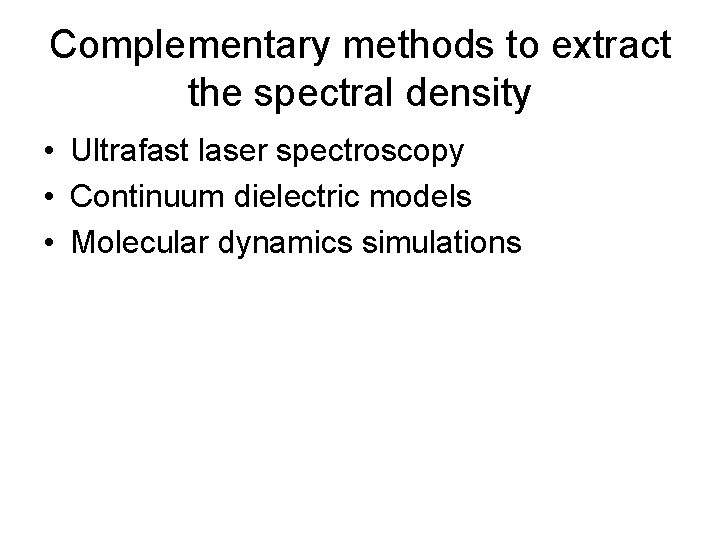
Complementary methods to extract the spectral density • Ultrafast laser spectroscopy • Continuum dielectric models • Molecular dynamics simulations

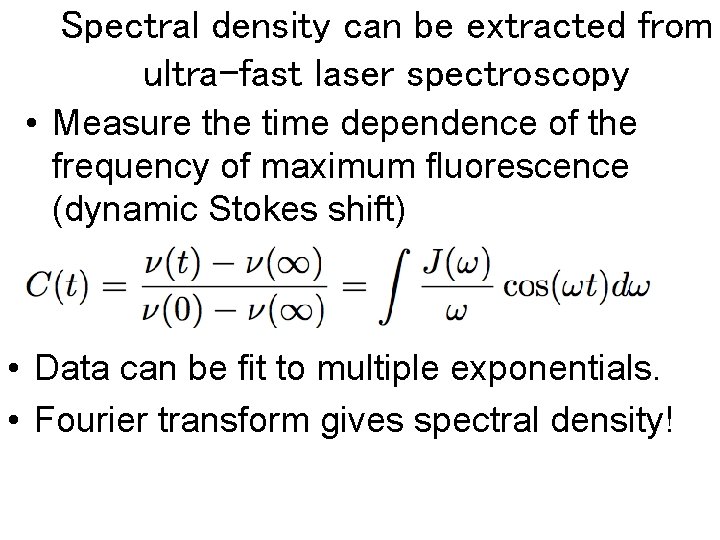
Spectral density can be extracted from ultra-fast laser spectroscopy • Measure the time dependence of the frequency of maximum fluorescence (dynamic Stokes shift) • Data can be fit to multiple exponentials. • Fourier transform gives spectral density!

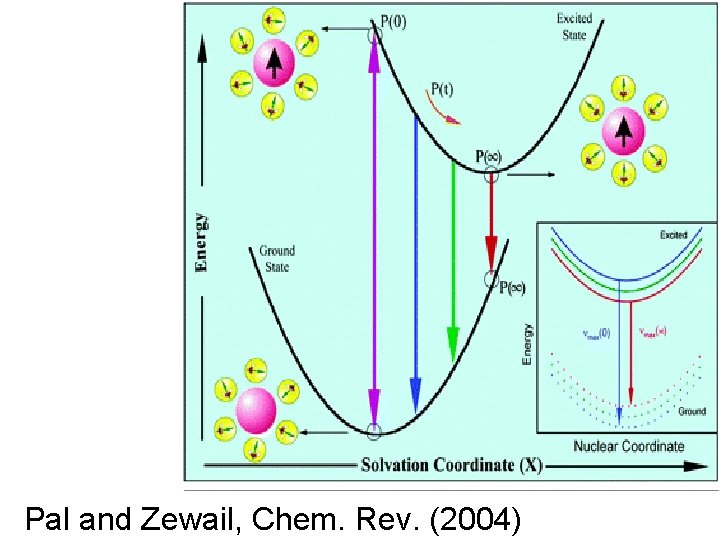
Pal and Zewail, Chem. Rev. (2004)

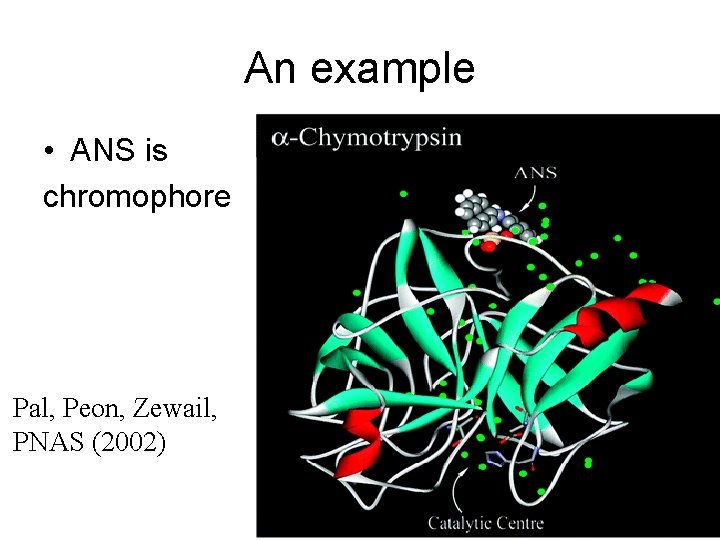
An example • ANS is chromophore Pal, Peon, Zewail, PNAS (2002)

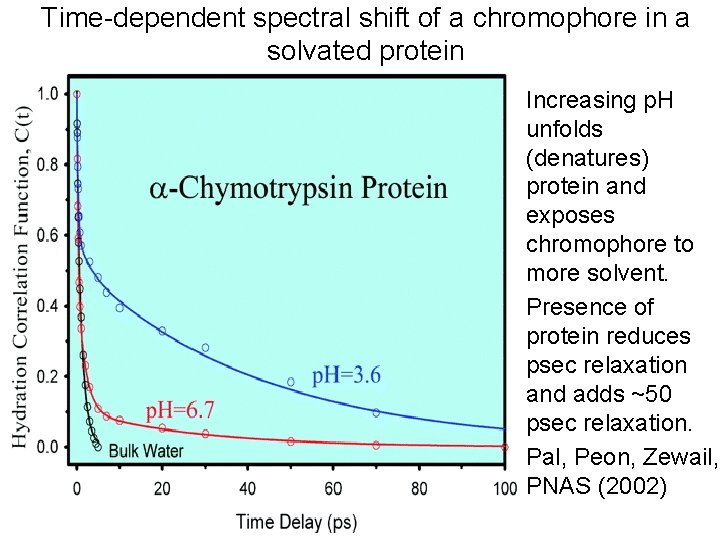
Time-dependent spectral shift of a chromophore in a solvated protein • Increasing p. H unfolds (denatures) protein and exposes chromophore to more solvent. • Presence of protein reduces psec relaxation and adds ~50 psec relaxation. • Pal, Peon, Zewail, PNAS (2002)

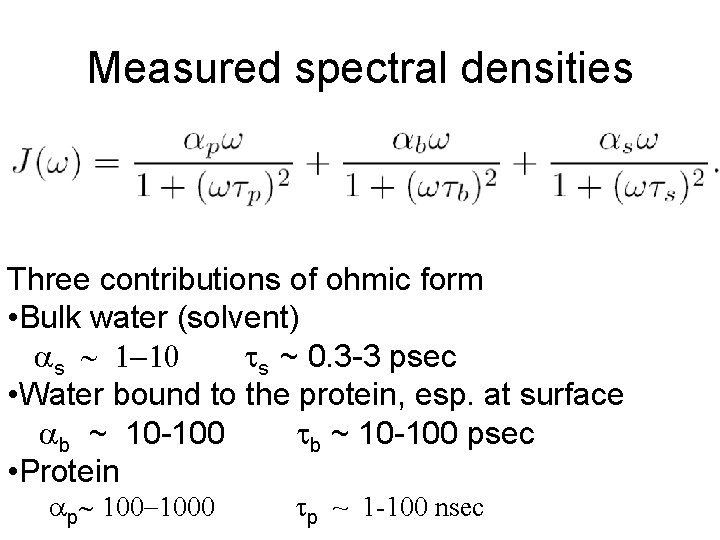
Measured spectral densities Three contributions of ohmic form • Bulk water (solvent) s ~ 1 -10 ts ~ 0. 3 -3 psec • Water bound to the protein, esp. at surface b ~ 10 -100 tb ~ 10 -100 psec • Protein p~ 100 -1000 tp ~ 1 -100 nsec

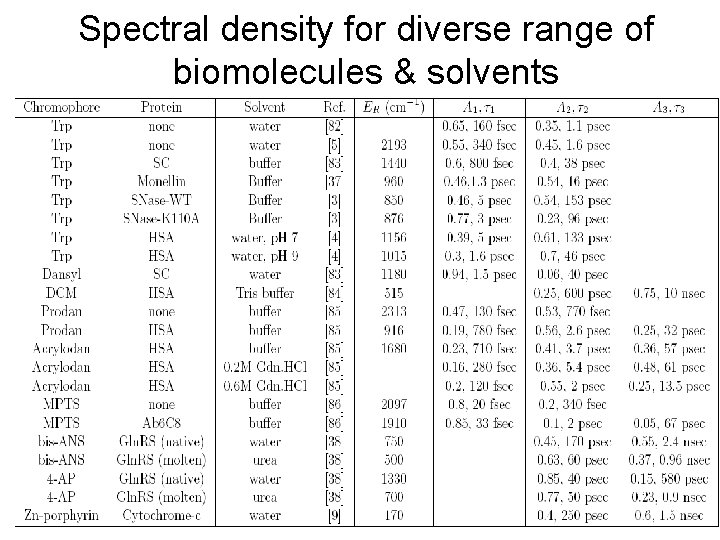
Spectral density for diverse range of biomolecules & solvents

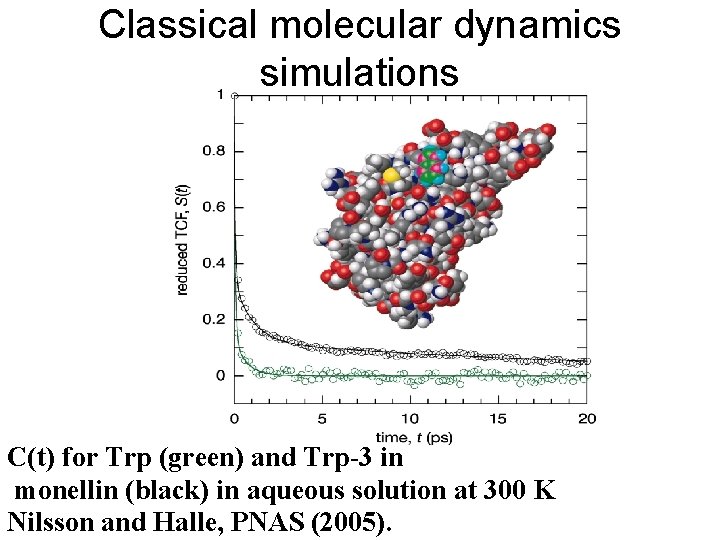
Classical molecular dynamics simulations C(t) for Trp (green) and Trp-3 in monellin (black) in aqueous solution at 300 K Nilsson and Halle, PNAS (2005).

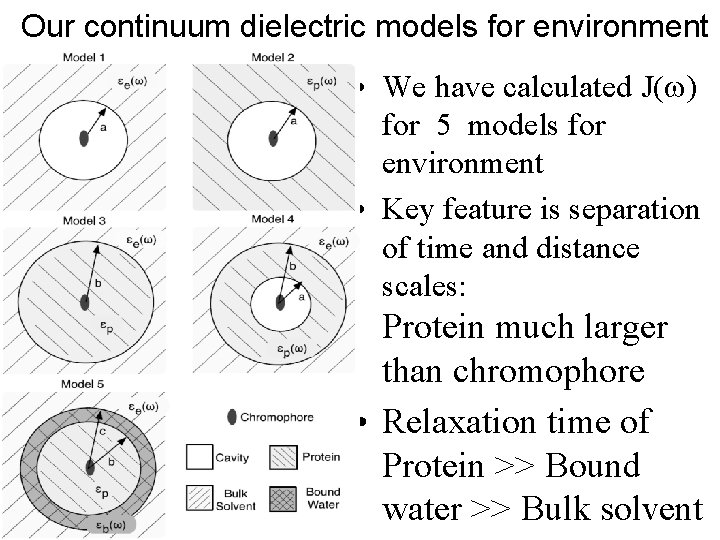
Our continuum dielectric models for environment • We have calculated J(w) for 5 models for environment • Key feature is separation of time and distance scales: Protein much larger than chromophore • Relaxation time of Protein >> Bound water >> Bulk solvent

Key physics behind decoherence • Most chromophores have a large difference between electric dipole moment of ground and excited states. • Water is a very polar solvent (static dielectric constant s = 80) – Water molecules have a net electric dipole moment – Dipole direction fluctuates due to thermal fluctuations (typical relaxation time at 300 K is ~1 psec) • Chromophore experiences fluctuating electric fiel • Surrounding protein does not completely shield chromophore from solvent.

Conclusions • Biochemistry happens in a hot wet environment • Spectral density characterises quantum system-environment (protein+ water) interaction for biomolecular chromophores. • These spectral densities can be used to assess claims about the role of quantum effects in biomolecular processes. J. Gilmore and RHM, J. Phys. Chem. A 112, 2162 (2008) condensedconcepts. blogspot. com


What have we learned? • Complete characterisation of system-environment interaction for biomolecular chromophores. • These spectral densities can be used to make definitive statements about the importance of quantum effects in biomolecular processes. • Due to their tuneable coupling to their environment biomolecular systems may be model systems to use to test ideas in quantum measurement theory. • For chromophores the timescale of the ``collapse’’ is less than 100 fsec.

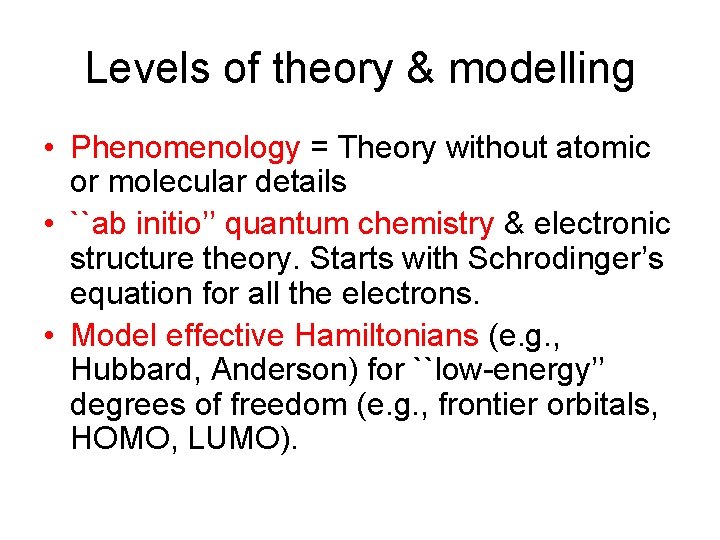
Levels of theory & modelling • Phenomenology = Theory without atomic or molecular details • ``ab initio’’ quantum chemistry & electronic structure theory. Starts with Schrodinger’s equation for all the electrons. • Model effective Hamiltonians (e. g. , Hubbard, Anderson) for ``low-energy’’ degrees of freedom (e. g. , frontier orbitals, HOMO, LUMO).



Why should quantum physicists be interested in biomolecules? Photo-active • biomolecules are tuneable systems at Retinal, responsible for vision – Single photon detector the quantum-classical – Quantum dynamics when the boundary Born-Oppenheimer approx. breaks down - Entanglement of electrons & nuclei - Effect of decoherence on Berry’s phase Photosynthetic Light harvesting complexes Quantum coherence over large


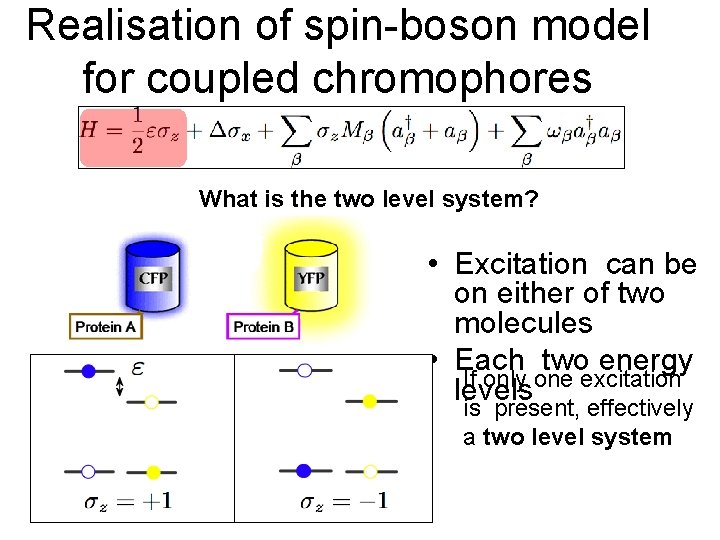
Realisation of spin-boson model for coupled chromophores What is the two level system? • Excitation can be on either of two molecules • Each two energy If only one excitation levels is present, effectively a two level system

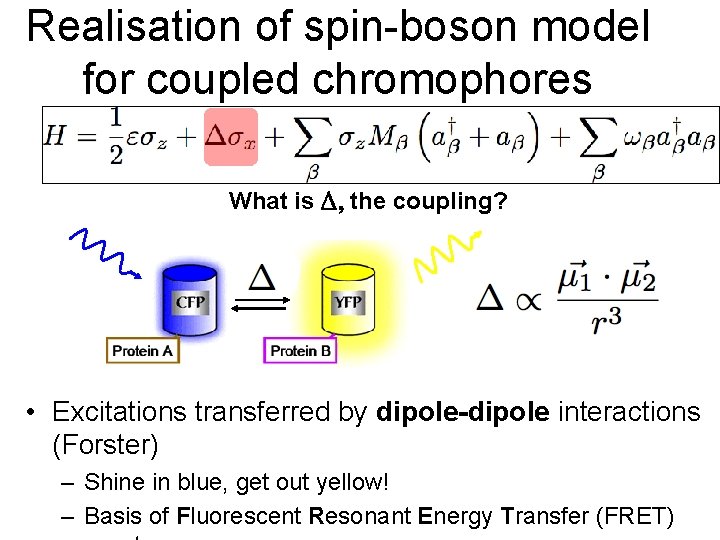
Realisation of spin-boson model for coupled chromophores What is the coupling? • Excitations transferred by dipole-dipole interactions (Forster) – Shine in blue, get out yellow! – Basis of Fluorescent Resonant Energy Transfer (FRET)

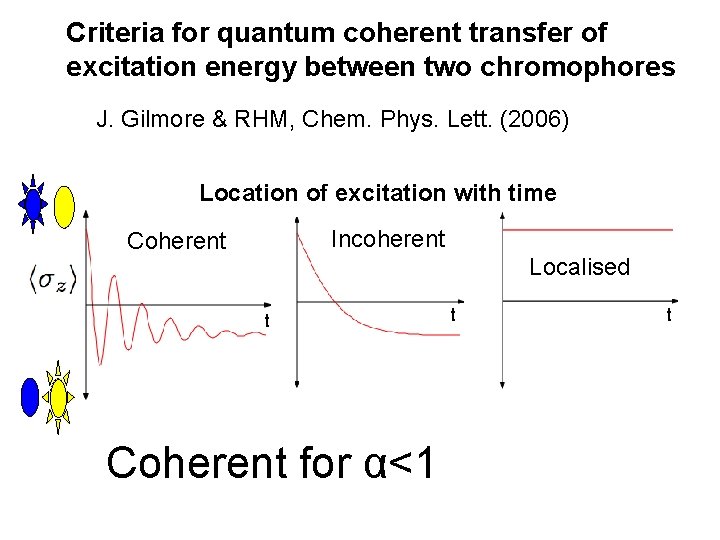
Criteria for quantum coherent transfer of excitation energy between two chromophores J. Gilmore & RHM, Chem. Phys. Lett. (2006) Location of excitation with time Incoherent Coherent Localised t Coherent for α<1 t t

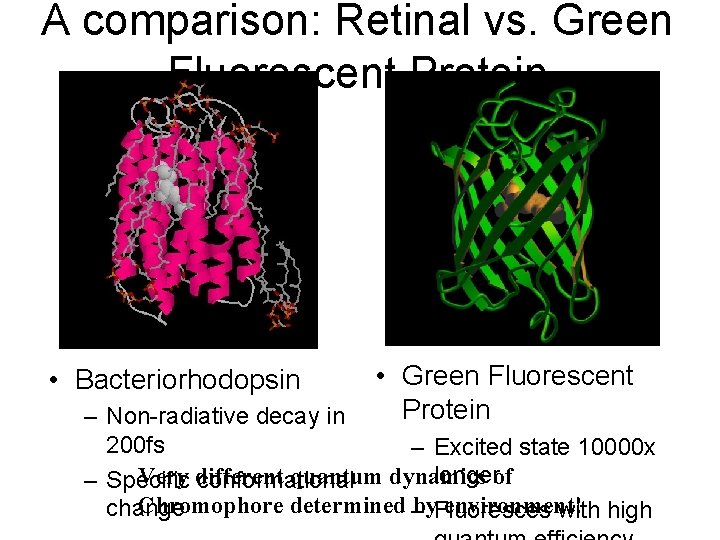
A comparison: Retinal vs. Green Fluorescent Protein • Bacteriorhodopsin • Green Fluorescent Protein – Non-radiative decay in 200 fs – Excited state 10000 x longerof Very different quantum dynamics – Specific conformational Chromophore determined –by. Fluoresces environment! change with high
 Optical purity formula
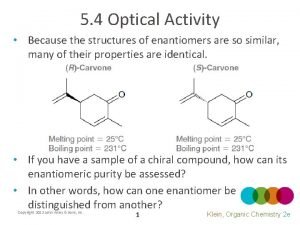
Optical purity formula Are cis and trans diastereomers
Are cis and trans diastereomers Ochem optically active
Ochem optically active A ray of light travels from an optical denser
A ray of light travels from an optical denser Origin of quantum mechanics
Origin of quantum mechanics Quantum physics vs mechanics
Quantum physics vs mechanics Shadow tomography of quantum states
Shadow tomography of quantum states Ashwin nayak
Ashwin nayak There were 11
There were 11 Southern states
Southern states Tyranny
Tyranny And or boolean
And or boolean Primary active transport and secondary active transport
Primary active transport and secondary active transport Primary active transport vs secondary active transport
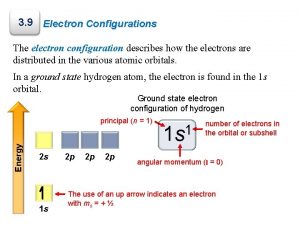
Primary active transport vs secondary active transport Excited state electron configuration
Excited state electron configuration Excited
Excited Excited state electron configuration
Excited state electron configuration Et 332
Et 332 What title does napoleon eventually assume for himself? *
What title does napoleon eventually assume for himself? * You excited
You excited Excited
Excited Shell and subshell
Shell and subshell My very excited mother just
My very excited mother just Self excited mixer
Self excited mixer I am awfully excited noun form
I am awfully excited noun form Boring comparative and superlative
Boring comparative and superlative Electron configuration special cases
Electron configuration special cases Sinusoidally excited linear circuit
Sinusoidally excited linear circuit Bohr model excited state
Bohr model excited state Excited comparative and superlative
Excited comparative and superlative The most helpful classmates are the ones who
The most helpful classmates are the ones who Superlativo de estudioso
Superlativo de estudioso Tictle
Tictle Excited to get started
Excited to get started Meaning
Meaning Show don't tell excitement
Show don't tell excitement Thematic statement for betrayal
Thematic statement for betrayal Superlative examples
Superlative examples Excited part of speech
Excited part of speech Word for excited
Word for excited