Protocol Version 19 0 Site Training STAMPEDE Systemic










































- Slides: 73

Protocol Version 19. 0 Site Training STAMPEDE Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy November 2018

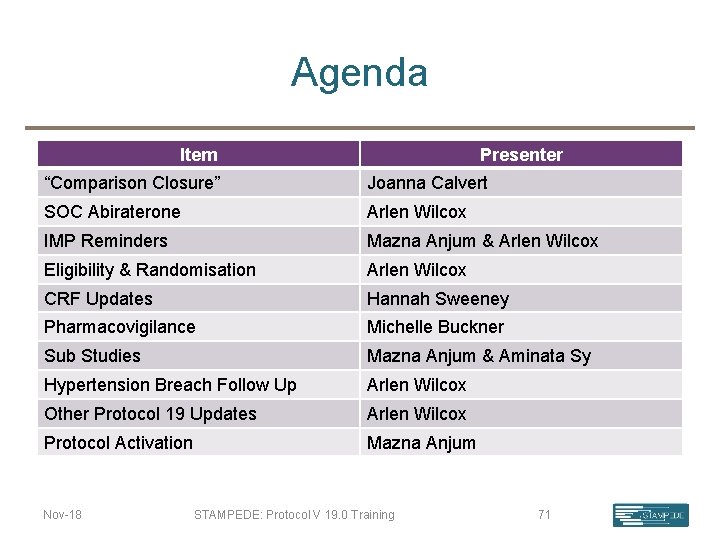
Agenda Item Presenter “Comparison Closure” Joanna Calvert SOC Abiraterone Arlen Wilcox IMP Reminders Mazna Anjum & Arlen Wilcox Eligibility & Randomisation Arlen Wilcox & Hannah Sweeney CRF Updates Hannah Sweeney Pharmacovigilance Michelle Buckner Sub Studies Mazna Anjum & Aminata Sy Hypertension Breach Follow Up Arlen Wilcox Other Protocol 19 Updates Arlen Wilcox Protocol Activation Mazna Anjum Nov-18 STAMPEDE: Protocol V 19. 0 Training 2

Joanna Calvert “COMPARISON CLOSURE” Nov-18 STAMPEDE: Protocol V 19. 0 Training 3

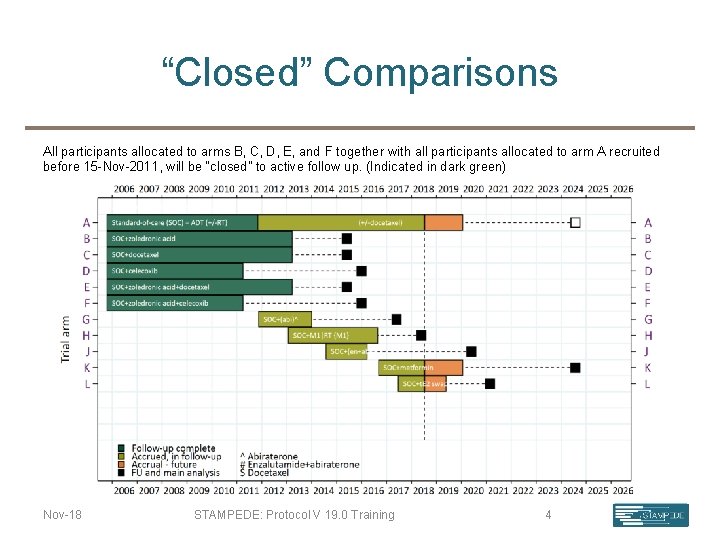
“Closed” Comparisons All participants allocated to arms B, C, D, E, and F together with all participants allocated to arm A recruited before 15 -Nov-2011, will be “closed” to active follow up. (Indicated in dark green) Nov-18 STAMPEDE: Protocol V 19. 0 Training 4

“Comparison Closure” Active Follow Up *Ongoing Data Collection “Comparison closure” is the stopping of active follow up of patients within a particular comparison/s. There may still be some data collection requested from sites to support ongoing sub-studies on closed comparisons. These requests may be for confirmation of health status or data already collected at sites e. g. baseline imaging data and FFPE tumour blocks. Active follow up is defined as hospital-based or telephone assessments, required to be able to complete the scheduled follow-up assessments. Stopping active follow up includes the ceasing of: Ø All future data collection* Ø Historic outstanding queries Ø Historic outstanding missing CRFs Ø SAE reporting There is a small cohort of interest for which a particular data point will continue to be collected. For M 1 participants on arm C and contemporaneous arm A, the PSA at baseline will be collected retrospectively where available. (A list of these patients will be provided) Any longer term analyses of data beyond comparison closure will be preferentially performed using observational data collected through national registers and NHS Digital or other datasets, providing such data are accessible. Nov-18 STAMPEDE: Protocol V 19. 0 Training 5

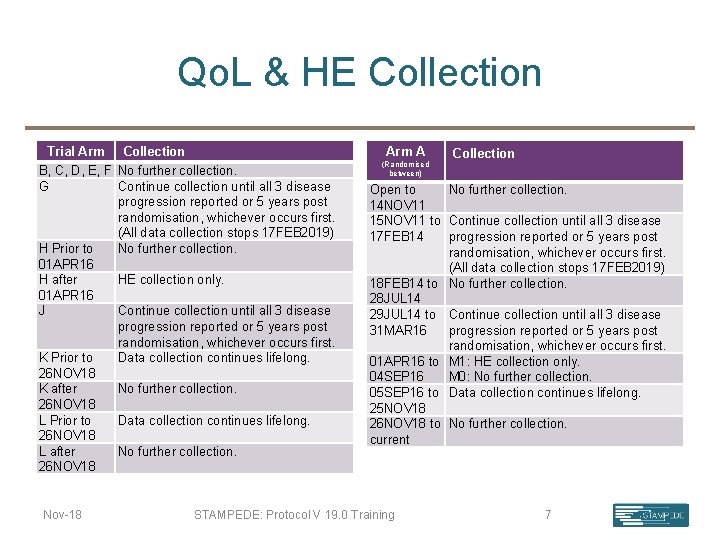
Participant Reported Outcome Collection (Qo. L & HE) Qo. L & HE collection stops for most participants. Collection will continue as planned in participants in the: • “abiraterone comparison” • “M 1 RT comparison” randomised after 01 APR 16 • “abiraterone & enzalutamide comparison” • “metformin comparison” randomised before 26 NOV 18 • “transdermol oestradiol comparison” randomised before 26 NOV 18 Nov-18 STAMPEDE: Protocol V 19. 0 Training 6

Qo. L & HE Collection Trial Arm Collection B, C, D, E, F No further collection. G Continue collection until all 3 disease progression reported or 5 years post randomisation, whichever occurs first. (All data collection stops 17 FEB 2019) H Prior to No further collection. 01 APR 16 H after HE collection only. 01 APR 16 J Continue collection until all 3 disease progression reported or 5 years post randomisation, whichever occurs first. K Prior to Data collection continues lifelong. 26 NOV 18 K after No further collection. 26 NOV 18 L Prior to Data collection continues lifelong. 26 NOV 18 L after No further collection. 26 NOV 18 Nov-18 Arm A (Randomised between) Collection Open to No further collection. 14 NOV 11 15 NOV 11 to Continue collection until all 3 disease 17 FEB 14 progression reported or 5 years post randomisation, whichever occurs first. (All data collection stops 17 FEB 2019) 18 FEB 14 to No further collection. 28 JUL 14 29 JUL 14 to Continue collection until all 3 disease 31 MAR 16 progression reported or 5 years post randomisation, whichever occurs first. 01 APR 16 to M 1: HE collection only. 04 SEP 16 M 0: No further collection. 05 SEP 16 to Data collection continues lifelong. 25 NOV 18 26 NOV 18 to No further collection. current STAMPEDE: Protocol V 19. 0 Training 7

Archiving CRFs, clinical notes and administrative documentation should continue to be kept in a secure location. It is permissible to archive this information, for these comparisons only, providing that its location is referenced in the investigator site file, it can be made accessible and available to the competent or equivalent authorities, the Sponsor, and other delegated authorities with suitable notice. STAMPEDE as a trial is still open, and as such, the data may be subject to audit or inspection from any of the above. Consent forms for all participants should remain in the ISF (please make sure an anonymised copy has been sent through to the MRCCTU at UCL, we will provide a list of those outstanding) Information must be held for 15 years after the end of the trial NB 1: 15 years after ‘end of trial’, not 15 years after “comparison closure” Nov-18 STAMPEDE: Protocol V 19. 0 Training 8

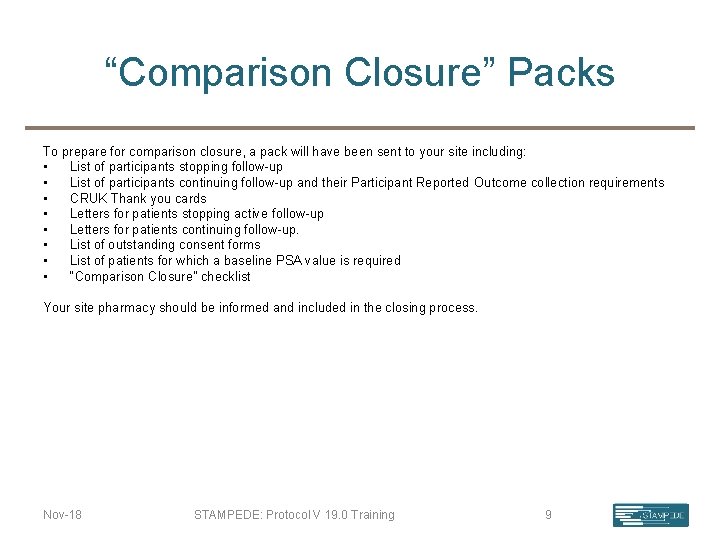
“Comparison Closure” Packs To prepare for comparison closure, a pack will have been sent to your site including: • List of participants stopping follow-up • List of participants continuing follow-up and their Participant Reported Outcome collection requirements • CRUK Thank you cards • Letters for patients stopping active follow-up • Letters for patients continuing follow-up. • List of outstanding consent forms • List of patients for which a baseline PSA value is required • “Comparison Closure” checklist Your site pharmacy should be informed and included in the closing process. Nov-18 STAMPEDE: Protocol V 19. 0 Training 9

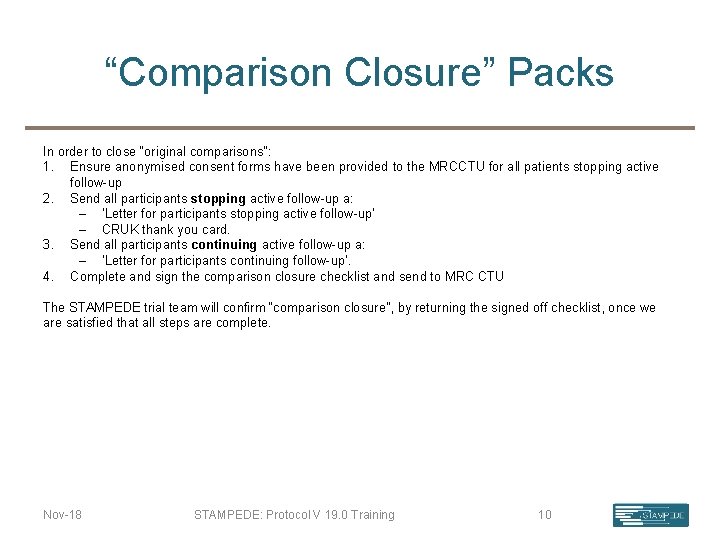
“Comparison Closure” Packs In order to close “original comparisons”: 1. Ensure anonymised consent forms have been provided to the MRCCTU for all patients stopping active follow-up 2. Send all participants stopping active follow-up a: – ‘Letter for participants stopping active follow-up’ – CRUK thank you card. 3. Send all participants continuing active follow-up a: – ‘Letter for participants continuing follow-up’. 4. Complete and sign the comparison closure checklist and send to MRC CTU The STAMPEDE trial team will confirm “comparison closure”, by returning the signed off checklist, once we are satisfied that all steps are complete. Nov-18 STAMPEDE: Protocol V 19. 0 Training 10

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 11

Arlen Wilcox SOC ABIRATERONE Nov-18 STAMPEDE: Protocol V 19. 0 Training 12

SOC Abiraterone The primary analysis of the “abiraterone comparison” of STAMPEDE and the co-published LATITUDE trial are consistent in showing abiraterone to improve survival in this patient setting i. e. newly-diagnosed high-risk metastatic patients From the date that protocol v 19. 0 is activated at site, the treating clinician and participant may consider the use of abiraterone in the castrate‑sensitive setting, termed SOC abiraterone, where this is available. SOC abiraterone is currently only permitted for participants within the metformin comparison. (Arms A and K) Nov-18 STAMPEDE: Protocol V 19. 0 Training 13

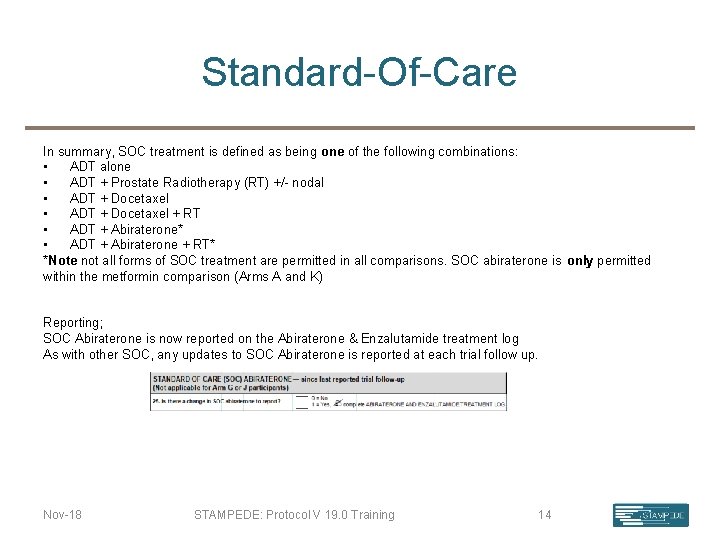
Standard-Of-Care In summary, SOC treatment is defined as being one of the following combinations: • ADT alone • ADT + Prostate Radiotherapy (RT) +/- nodal • ADT + Docetaxel + RT • ADT + Abiraterone* • ADT + Abiraterone + RT* *Note not all forms of SOC treatment are permitted in all comparisons. SOC abiraterone is only permitted within the metformin comparison (Arms A and K) Reporting; SOC Abiraterone is now reported on the Abiraterone & Enzalutamide treatment log As with other SOC, any updates to SOC Abiraterone is reported at each trial follow up. Nov-18 STAMPEDE: Protocol V 19. 0 Training 14

Abiraterone with t. E 2 Patches Castrate Sensitive PCa Castrate Resistant PCa (CRPC) Transdermal oestradiol cannot be given in addition to abiraterone in the castrate sensitive setting within the STAMPEDE protocol due to lack of safety data available so far. Therefore the use of SOC abiraterone is only permitted in participants randomised to the “metformin comparison” (arm K and comparable arm A patients). Following progression, where the patient is in the castrate resistant setting, transdermal oestradiol may continue at the discretion of the patient and clinician. Second line / castrate resistant setting treatments, including abiraterone, may be introduced at the treating clinician’s discretion. Nov-18 STAMPEDE: Protocol V 19. 0 Training 15

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 16

Mazna Anjum & Arlen Wilcox IMP REMINDERS Nov-18 STAMPEDE: Protocol V 19. 0 Training 17

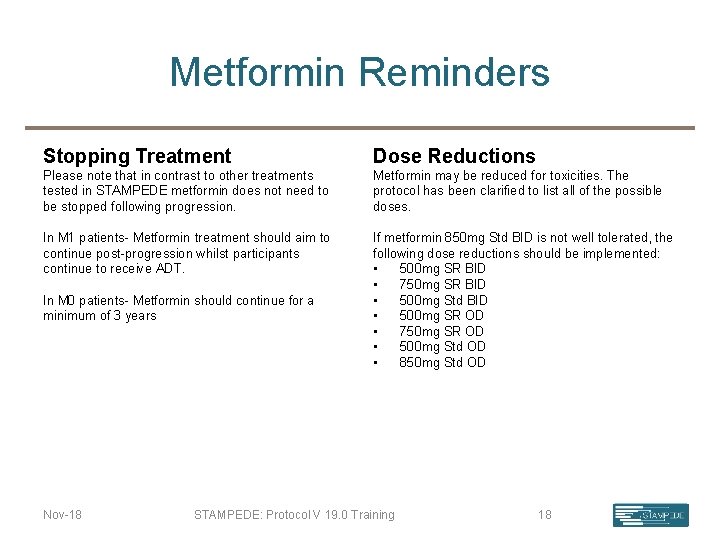
Metformin Reminders Stopping Treatment Dose Reductions Please note that in contrast to other treatments tested in STAMPEDE metformin does not need to be stopped following progression. Metformin may be reduced for toxicities. The protocol has been clarified to list all of the possible doses. In M 1 patients- Metformin treatment should aim to continue post-progression whilst participants continue to receive ADT. If metformin 850 mg Std BID is not well tolerated, the following dose reductions should be implemented: • 500 mg SR BID • 750 mg SR BID • 500 mg Std BID • 500 mg SR OD • 750 mg SR OD • 500 mg Std OD • 850 mg Std OD In M 0 patients- Metformin should continue for a minimum of 3 years Nov-18 STAMPEDE: Protocol V 19. 0 Training 18

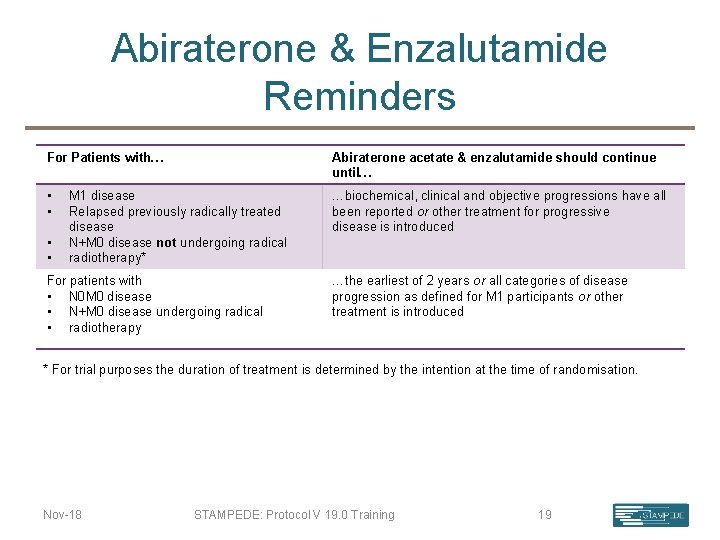
Abiraterone & Enzalutamide Reminders For Patients with… Abiraterone acetate & enzalutamide should continue until… • • …biochemical, clinical and objective progressions have all been reported or other treatment for progressive disease is introduced • • M 1 disease Relapsed previously radically treated disease N+M 0 disease not undergoing radical radiotherapy* For patients with • N 0 M 0 disease • N+M 0 disease undergoing radical • radiotherapy …the earliest of 2 years or all categories of disease progression as defined for M 1 participants or other treatment is introduced * For trial purposes the duration of treatment is determined by the intention at the time of randomisation. Nov-18 STAMPEDE: Protocol V 19. 0 Training 19

Abiraterone & Enzalutamide Reminders Stopping Treatment Restarting Treatment Trial abiraterone must stop if other systemic treatments are initiated at any time for disease progression control (including chemotherapy, radium -223 etc). Anti-androgens (e. g. bicalutamide) should not be given in combination with abiraterone or enzalutamide due to the risk of toxicity. For patients for whom treatment was discontinued but who have not met the above criteria for stopping, treatment can be re-introduced at any time if this is considered safe and in the patients best interest. However, participants may continue on abiraterone or abiraterone and enzalutamide if they receive radiotherapy on a single occasion for a skeletalrelated event. Sites must contact the STAMPEDE trial team for further guidance as appropriate. Nov-18 If it is only possible to restart one of the treatments, STAMPEDE investigators may consider if this should be abiraterone, given that we now know of its positive effects and that we do not yet have data on enzalutamide alone in this setting STAMPEDE: Protocol V 19. 0 Training 20

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 21

Arlen Wilcox & Hannah Sweeney ELIGIBILITY & RANDOMISATION Nov-18 STAMPEDE: Protocol V 19. 0 Training 22

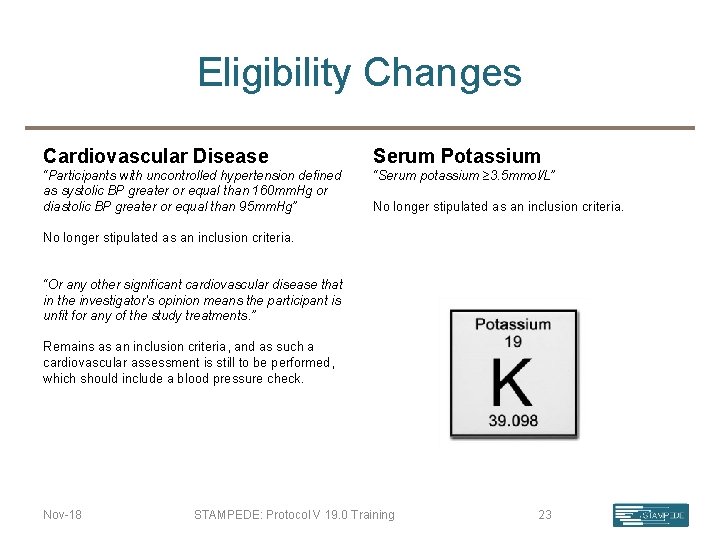
Eligibility Changes Cardiovascular Disease Serum Potassium “Participants with uncontrolled hypertension defined as systolic BP greater or equal than 160 mm. Hg or diastolic BP greater or equal than 95 mm. Hg” “Serum potassium ≥ 3. 5 mmol/L” No longer stipulated as an inclusion criteria. “Or any other significant cardiovascular disease that in the investigator's opinion means the participant is unfit for any of the study treatments. ” Remains as an inclusion criteria, and as such a cardiovascular assessment is still to be performed, which should include a blood pressure check. Nov-18 STAMPEDE: Protocol V 19. 0 Training 23

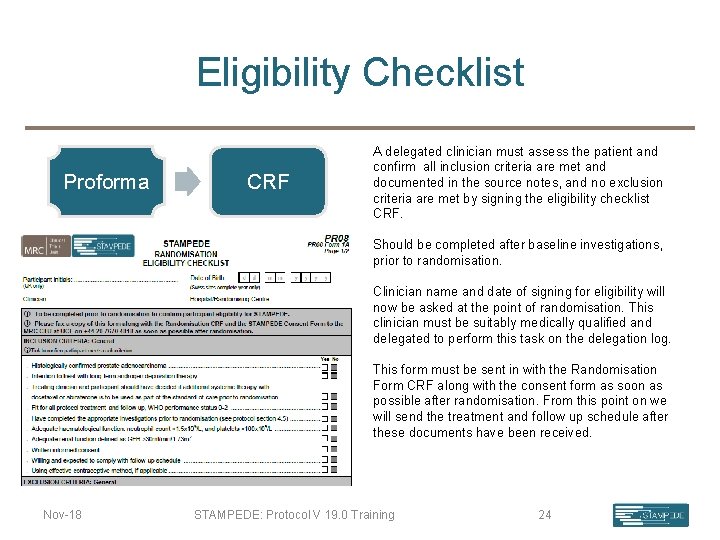
Eligibility Checklist Proforma CRF A delegated clinician must assess the patient and confirm all inclusion criteria are met and documented in the source notes, and no exclusion criteria are met by signing the eligibility checklist CRF. Should be completed after baseline investigations, prior to randomisation. Clinician name and date of signing for eligibility will now be asked at the point of randomisation. This clinician must be suitably medically qualified and delegated to perform this task on the delegation log. This form must be sent in with the Randomisation Form CRF along with the consent form as soon as possible after randomisation. From this point on we will send the treatment and follow up schedule after these documents have been received. Nov-18 STAMPEDE: Protocol V 19. 0 Training 24

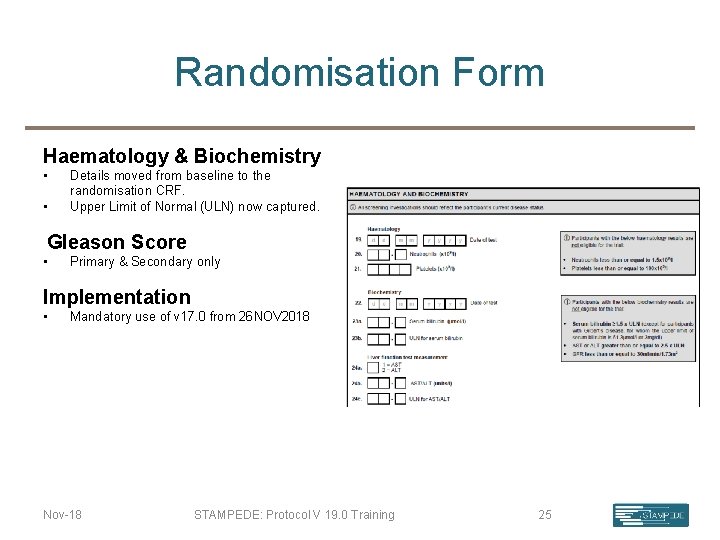
Randomisation Form Haematology & Biochemistry • • Details moved from baseline to the randomisation CRF. Upper Limit of Normal (ULN) now captured. Gleason Score • Primary & Secondary only Implementation • Mandatory use of v 17. 0 from 26 NOV 2018 Nov-18 STAMPEDE: Protocol V 19. 0 Training 25

Randomisation Form PSA Doubling Time • • New addition to the Randomisation form. Patients falling in the following eligibility category are required to provide the doubling time: Use the PSA calculator to report doubling time: • Enter the patient’s PSAs and dates obtained into the space provided. • Click the add button each time a PSA is entered to compile the list of data. • Click calculate and enter the Doubling time result onto the Randomisation form. Nov-18 STAMPEDE: Protocol V 19. 0 Training 26

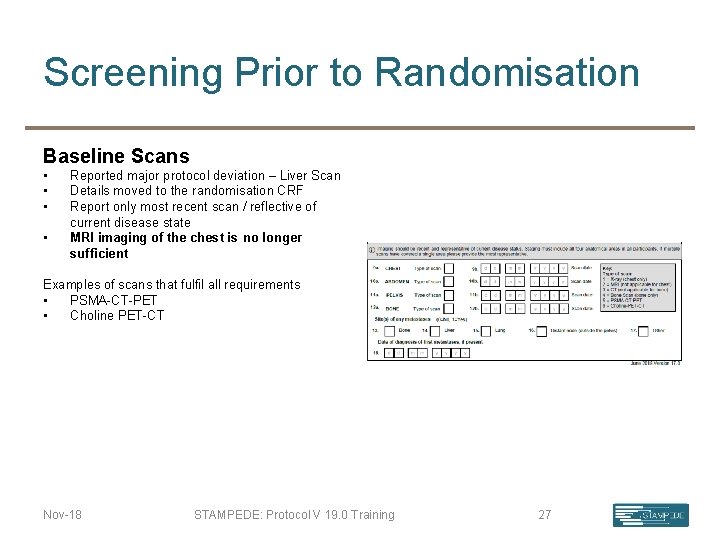
Screening Prior to Randomisation Baseline Scans • • Reported major protocol deviation – Liver Scan Details moved to the randomisation CRF Report only most recent scan / reflective of current disease state MRI imaging of the chest is no longer sufficient Examples of scans that fulfil all requirements • PSMA-CT-PET • Choline PET-CT Nov-18 STAMPEDE: Protocol V 19. 0 Training 27

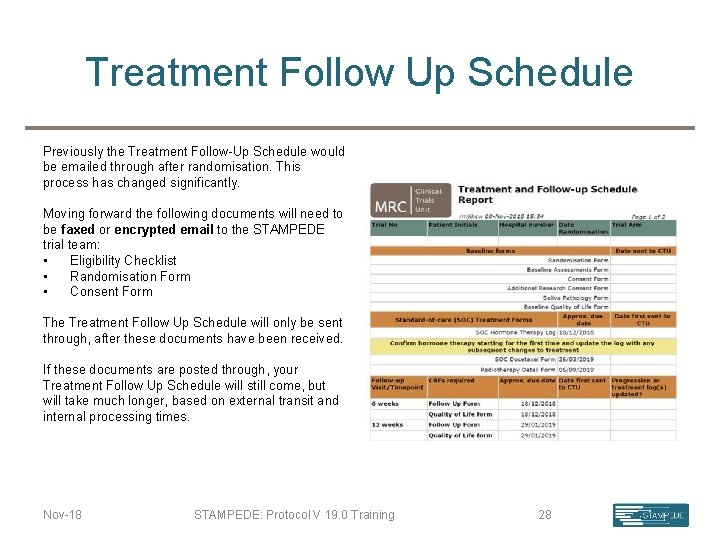
Treatment Follow Up Schedule Previously the Treatment Follow-Up Schedule would be emailed through after randomisation. This process has changed significantly. Moving forward the following documents will need to be faxed or encrypted email to the STAMPEDE trial team: • Eligibility Checklist • Randomisation Form • Consent Form The Treatment Follow Up Schedule will only be sent through, after these documents have been received. If these documents are posted through, your Treatment Follow Up Schedule will still come, but will take much longer, based on external transit and internal processing times. Nov-18 STAMPEDE: Protocol V 19. 0 Training 28

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 29

Hannah Sweeney CRF UPDATES Nov-18 STAMPEDE: Protocol V 19. 0 Training 30

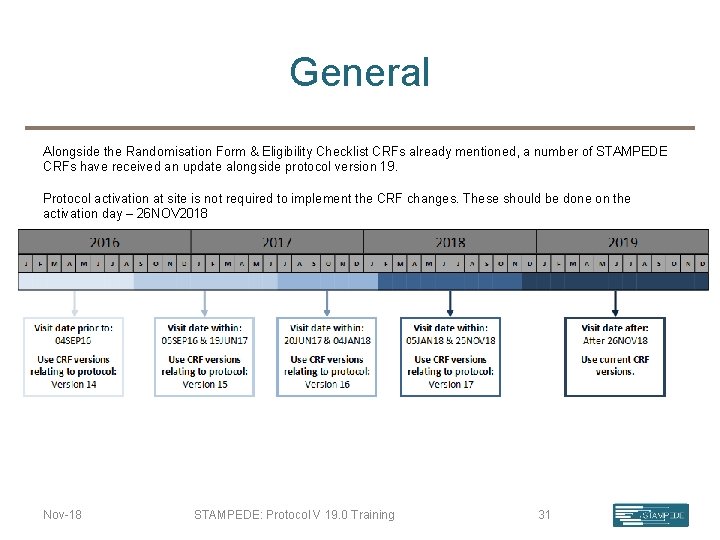
General Alongside the Randomisation Form & Eligibility Checklist CRFs already mentioned, a number of STAMPEDE CRFs have received an update alongside protocol version 19. Protocol activation at site is not required to implement the CRF changes. These should be done on the activation day – 26 NOV 2018 Nov-18 STAMPEDE: Protocol V 19. 0 Training 31

CRF Updates Baseline Death Lots of items removed from this version of the form, notably: • Blood markers • Bone density • History of cardiovascular events Cause of death codes now correspond to CTCAE v. 4. 03 common terminology. Question 6 added to provide further detail regarding causality. Guidance added to the back page of the form. Clinician signature added to the bottom of the form. Forms will be queried if this is not completed by a delegated clinician. An addition has been made in this form; whereby the most recent PSA prior to randomisation is now required: (ideally 2 weeks prior to randomisation) Nov-18 STAMPEDE: Protocol V 19. 0 Training 32

Death Form - Continued The death form allows us to report the number of deaths attributable to prostate cancer versus deaths not related to prostate cancer in our outcome analysis. If a form clearly indicates prostate cancer is a significant factor in the death this will be automatically coded as a prostate cancer death; Eg: Primary cause of death = pneumonia, secondary cause of death = prostate cancer, AND no other causes listed AND the clinical reviewer ticks Q 6 as prostate cancer death > automatically coded as prostate cancer death. If there is any ambiguity about the cause of death it will be reviewed by a clinician at the CTU to decide if the death is likely to be related to prostate cancer or not. Therefore it is particularly helpful if we could have as much detail as possible about the cause of death Nov-18 STAMPEDE: Protocol V 19. 0 Training 33

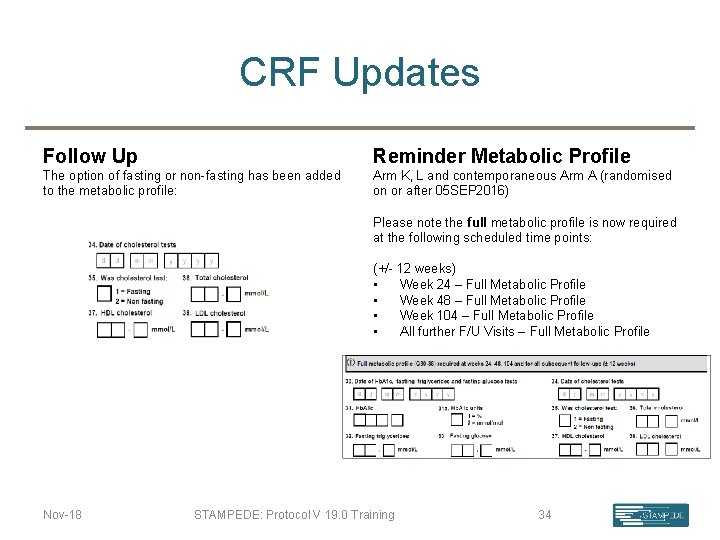
CRF Updates Follow Up Reminder Metabolic Profile The option of fasting or non-fasting has been added to the metabolic profile: Arm K, L and contemporaneous Arm A (randomised on or after 05 SEP 2016) Please note the full metabolic profile is now required at the following scheduled time points: (+/- 12 weeks) • Week 24 – Full Metabolic Profile • Week 48 – Full Metabolic Profile • Week 104 – Full Metabolic Profile • All further F/U Visits – Full Metabolic Profile Nov-18 STAMPEDE: Protocol V 19. 0 Training 34

CRF Updates Toxicity Form Now corresponds to CTCAE 4. 03 common terminology, which can be found here: http: //www. stampedetrial. org/centres/toolstraining/training-materials-resouces/ Guidance has been added to a number of toxicity categories with directions to required treatment actions. If a toxicity is reported but not listed on the CRF: 1. Check CTCAE v. 4. 03 for suitable category and grading, use the search bar to identify the related category. Please record the toxicity name as it appears in the CTCAE, we can only record events that match this guideline. 2. Turn to page 2 of the toxicity CRF and enter the body system code using the table provided, enter the toxicity and provide the suitable grade. Nov-18 STAMPEDE: Protocol V 19. 0 Training 35

CRF Updates Abiraterone & Enzalutamide Treatment Log To be used for both SOC Abiraterone and protocol treatment. • Report permanent stopping for Standard-of-care treatment but not for protocol treatment, where End Of Research Treatment CRF still required. • Steroid dose has been added to the form. Enter dose used at time of treatment change. (For Arms G & J) Nov-18 STAMPEDE: Protocol V 19. 0 Training 36

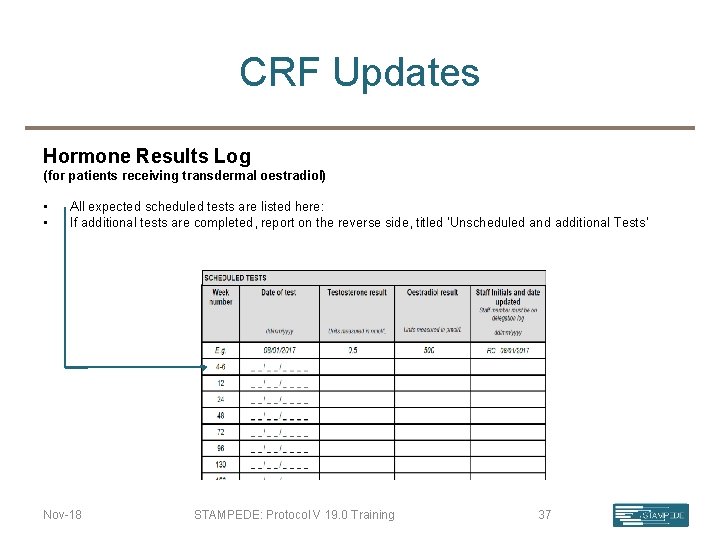
CRF Updates Hormone Results Log (for patients receiving transdermal oestradiol) • • All expected scheduled tests are listed here: If additional tests are completed, report on the reverse side, titled ‘Unscheduled and additional Tests’ Nov-18 STAMPEDE: Protocol V 19. 0 Training 37

CRF & Data Management Reminder; The STAMPEDE team have revamped our regular training sessions and will now periodically hold training sessions designed for new staff into STAMPEDE and those wanting a refresher. The two sessions available are; 1. STAMPEDE Basic Data Management Practices Training Session 2. STAMPEDE CRF Completion Training Session Each will run for 30 minutes and give attendees an overview of tasks, our timesaving tips, and guidance to minimise queries. Please contact your site’s STAMPEDE Data Manager or keep an eye out for the registration communication to be included. Nov-18 STAMPEDE: Protocol V 19. 0 Training 38

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 39

Michelle Buckner PHARMACOVIGILANCE Nov-18 STAMPEDE: Protocol V 19. 0 Training 40

Event Reporting Changes As described previously, with “Comparison Closure”; • No further reporting SAEs or Notable AEs for patients with the “original comparisons” after the “comparison closure” has been implemented at your site. Reporting Notification Periods All Serious Adverse Events (SAEs) are reportable from the time of randomisation until 30 days after discontinuation of protocol treatment (refer to Section 11. 1. 2), • LHRH analogues & other depot administered treatments; are reportable until 30 days after the depot expiration date e. g. up to 8 weeks after administration of a 4 -week depot or 16 weeks after administration of a 12 -week depot. • Exception; reactions (SARs and SUSARs) which continue to be reportable until comparison closure. All Notable Adverse Events are reportable from randomisation until comparison closure. NB: Reminder that Metformin is a protocol treatment that is taken lifelong. SAEs are reported when on protocol treatment – in this case lifelong. Nov-18 STAMPEDE: Protocol V 19. 0 Training 41

Trial Specific Exemptions Serious adverse events unrelated to protocol treatment i. e. unrelated SAEs (refer to Section 11. 1. 2) occurring more than 30 days after stopping protocol treatment. Serious adverse events occurring after disease progression that are unrelated (i. e. not SARs or SUSARs) to protocol treatment are exempt, providing protocol treatment stopped at least 30 days ago. • N. B non-protocol treatment includes ADT in CRPC setting, therefore the 30 day rule does not apply for patients continuing on ADT alone. (Refer to Section 11. 1. 2) Non-fatal progression events: events that fulfil the definition of serious e. g. result in hospital admission, but are due to disease progression are exempt from reporting as an SAE , instead details should be provided on the Progression Log. Death as a result of disease progression or disease-related deaths : Do not complete an SAE CRF, instead details should be reported on the Death Form. Elective hospitalisation and surgery for treatment of locally-advanced or metastatic prostate cancer or its complications. These should be recorded as a non-trial inpatient admission on the follow-up form under Non. Trial visits. Elective hospitalisation to simplify treatment or procedures. If related to prostate cancer , record as non-trial inpatient admission on the follow-up form. If unrelated e. g. pre-existing conditions that have not been exacerbated by protocol treatment, do not report. Nov-18 STAMPEDE: Protocol V 19. 0 Training 42

Reference Safety Information Updated Investigators Brochures Enzalutamide Version 10. 0 Abiraterone Version 13. 0 Addendum 2 Ensure the previous versions of the IB are marked as superseded- both ISF and PSF. Sites should now use the new versions to assess the expectedness for their safety events. Nov-18 STAMPEDE: Protocol V 19. 0 Training 43

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 44

Mazna Anjum & Aminata Sy SUB STUDIES UPDATE Nov-18 STAMPEDE: Protocol V 19. 0 Training 45

Additional Research Consent for additional research has been recorded in two places: 1. STAMPEDE Consent form version 13. 0 and earlier 2. From Mar-2016 onwards consent for participation in additional sub-studies is collected on a separate additional research consent form (part C) Nov-18 STAMPEDE: Protocol V 19. 0 Training 46

Combined Consent to the main study and to optional additional research will now be captured on the one consent form. Following this amendment: Section B: Q 15 15. I have read the Additional Research participant information sheet and I give my consent for my remaining prostate cancer tumour sample (including prostate biopsy or surgical samples) to be used for additional prostate cancer research. I understand that this may require the anonymised samples to be transferred outside of Europe and analysed by researchers (including both academic and commercial collaborators). This may include genetic analysis or use in animal models. I will not benefit financially from any resulting arising from this research. Nov-18 STAMPEDE: Protocol V 19. 0 Training 47

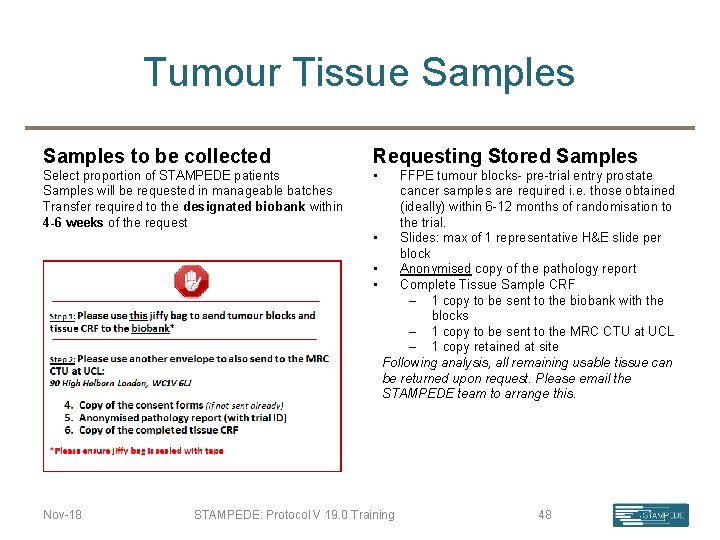
Tumour Tissue Samples to be collected Requesting Stored Samples Select proportion of STAMPEDE patients Samples will be requested in manageable batches Transfer required to the designated biobank within 4 -6 weeks of the request • Nov-18 FFPE tumour blocks- pre-trial entry prostate cancer samples are required i. e. those obtained (ideally) within 6 -12 months of randomisation to the trial. • Slides: max of 1 representative H&E slide per block • Anonymised copy of the pathology report • Complete Tissue Sample CRF – 1 copy to be sent to the biobank with the blocks – 1 copy to be sent to the MRC CTU at UCL – 1 copy retained at site Following analysis, all remaining usable tissue can be returned upon request. Please email the STAMPEDE team to arrange this. STAMPEDE: Protocol V 19. 0 Training 48

Tumour Tissue Samples Reminders What if blocks are not available: Complete the tissue sample CRF and send only to MRC CTU at UCL stating the reason of unavailability in question 2. What if more than 1 block is available: Please send all blocks, or the 3 containing the most tissue What if slides are available: Send a max of 1 representative H&E slide per block Invoices: Confirmed receipt by the biobank Email invoice: mrcctu. stampede@ucl. ac. uk Please include: FAO: Blocks Project Reimbursement amount per participant List of trial IDs Nov-18 STAMPEDE: Protocol V 19. 0 Training 49

Blood Collection Eligibility Logistics As per protocol v 19. 0 activation ONLY patients allocated to the “Abiraterone and Enzalutamide comparison” are eligible to participant in this substudy. ü Arm A: SOC (contemporaneous to Arm J) ü Arm J: SOC+ Enz + Abi • Randomised between 29 -Jul-2014 and 31 -Mar-2016 • • • Please DO NOT collect samples for patients in other arms (i. e. K and L) or Arm A’s that are not contemporaneous to Arm J, even if they are due a sampling time point. • • Blood kits are supplied to sites, please contact STAMPEDE team mrcctu. stampede@ucl. ac. uk if additional supplies are required Confirm consent prior to blood collection Collect 2 x 10 ml of blood in streck. TM tubes provided and invert 8 -10 times Please do not under fill tubes Ensure blood tubes are labelled and blood form is completed Store blood samples at room temp – do not refrigerate Post samples together with blood form within a maximum of 2 days Email ci. stampedeblood@ucl. ac. uk when samples are posted No Baseline sample collection Nov-18 STAMPEDE: Protocol V 19. 0 Training 50

Blood Collection Reminders • • • Progression samples are the most important If biochemical, clinical and radiological progression occurs at different times please provide a sample at each time point Please ensure consent forms are sent to MRC CTU, very important to ensure GCP compliance and HTA regulations Blood kits are not sent automatically at randomisation, please email trial team mrcctu. stampede@ucl. ac. uk should you require more kits Please do not sent blood samples without a trial ID on the samples and blood CRF Ensure blood samples are posted to: STAMPEDE BLOOD UCL Cancer Institute UCL ECMC GCLP Facility Paul O’Gorman Building 72 Huntley Street London WC 1 E 6 DD Please send an email to ci. stampede@ucl. ac. uk to inform the lab that a samples have been sent. Send a copy of the Blood CRF to MRC CTU at UCL. Nov-18 STAMPEDE: Protocol V 19. 0 Training 51

Saliva Samples Eligibility Collection Logistics • • • All patients enrolled in STAMPEDE are eligible to participate Patients must be provided with the additional research PIS Informed consent must be obtained using additional research consent form prior to Saliva collection, using consent form version 18. 0 Saliva sample can be collected at any time point during the study • • • A single saliva sample will be collected from patients who have consented Please ensure that patient does not eat/drink/chew gum/brush their teeth for 30 minutes before sample collection For full details refer to STAMPEDE Sample Collection and Handling manual v 7 (Section 1) Saliva collection Oragene kits are supplied to all sites, please email trial team mrcctu. stampede@ucl. ac. uk should you require more kits 1 x Saliva Collection Kit (Oragene DNA) 1 x White Postal Tube 2 x Sample ID Labels 1 x Plastic envelope 1 x Pre-Paid padded envelope Nov-18 STAMPEDE: Protocol V 19. 0 Training 52

Saliva Samples Reminders • • • Please do not send saliva samples without a trial ID on the samples and saliva CRF Saliva kits are not sent automatically at randomisation, please email trial team mrcctu. stampede@ucl. ac. uk should you require more kits Please ensure to use older kits before expiry date Do not sent consent form with the samples Ensure to send in consent forms to MRC CTU Shipping Checklist 1. Place labelled Oragene tube inside the postal tube and secure the screw cap. Complete Saliva CRF 2. Place Postal tube into the plastic polyseal bad and seal 3. Place both postal tubes into the transport box 4. Place the plastic polyseal bag and the saliva pathology form into the prepaid padded envelope Ensure saliva samples are posted to: Professor Ros Eeles STAMPEDE SALIVA Institute of Cancer Research 15 Cotswold Place Sutton Surrey SM 2 5 NG Please send a copy of the saliva form to MRC CTU at UCL Nov-18 STAMPEDE: Protocol V 19. 0 Training 53

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 54

Arlen Wilcox HYPERTENSION SERIOUS BREACH FOLLOW UP Nov-18 STAMPEDE: Protocol V 19. 0 Training 55

Monitoring Abiraterone and enzalutamide are associated with hypertension Regular blood pressure monitoring is required • Monitored; – Two-weekly for 12 weeks – Monthly thereafter • Monitoring may be done two-monthly, following a 12 month period without any abnormalities. • Self-monitoring (or GP monitoring) encouraged • Blood pressure control should be reviewed at each follow-up • Representative values documented in medical notes (source documentation) Please refer to protocol section 6. 2. 3 b.

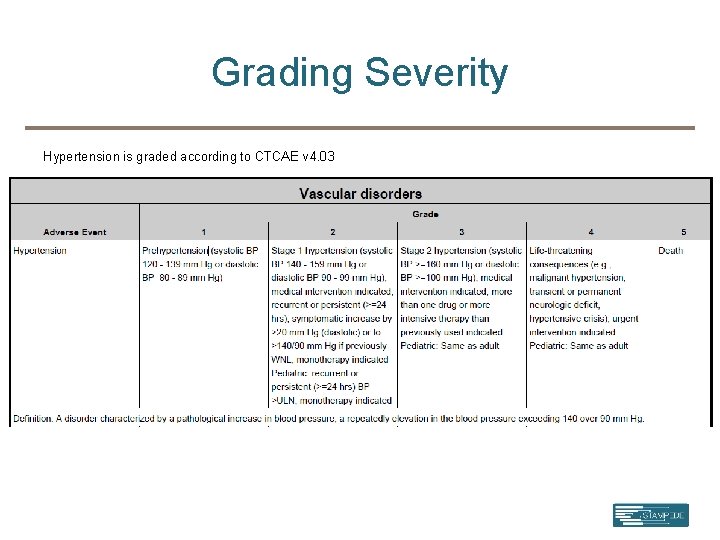
Grading Severity Hypertension is graded according to CTCAE v 4. 03

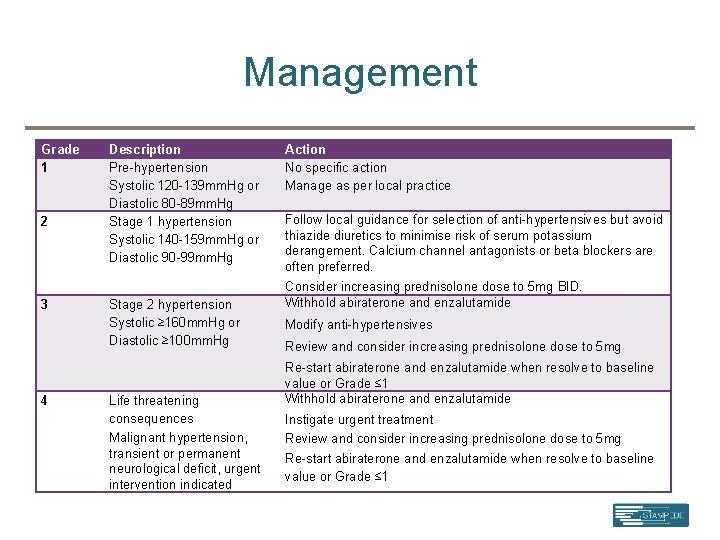
Management Grade 1 2 3 4 Description Pre-hypertension Systolic 120 -139 mm. Hg or Diastolic 80 -89 mm. Hg Stage 1 hypertension Systolic 140 -159 mm. Hg or Diastolic 90 -99 mm. Hg Stage 2 hypertension Systolic ≥ 160 mm. Hg or Diastolic ≥ 100 mm. Hg Life threatening consequences Malignant hypertension, transient or permanent neurological deficit, urgent intervention indicated Action No specific action Manage as per local practice Follow local guidance for selection of anti-hypertensives but avoid thiazide diuretics to minimise risk of serum potassium derangement. Calcium channel antagonists or beta blockers are often preferred. Consider increasing prednisolone dose to 5 mg BID. Withhold abiraterone and enzalutamide Modify anti-hypertensives Review and consider increasing prednisolone dose to 5 mg Re-start abiraterone and enzalutamide when resolve to baseline value or Grade ≤ 1 Withhold abiraterone and enzalutamide Instigate urgent treatment Review and consider increasing prednisolone dose to 5 mg Re-start abiraterone and enzalutamide when resolve to baseline value or Grade ≤ 1

Hypertension Summary STAMPEDE Protocol guidance for hypertension management has been updated to reflect CTCAE v. 4. 03 Key points: • Representative BP values (e. g. those obtained on home monitoring) should be used and recorded in source documentation • Abiraterone and enzalutamide should be paused if grade 3 (≥ 160 or 90 mm. Hg) hypertension occurs • If treatment is paused, prednisolone should continue and be weaned if treatment is to stop • If symptoms of mineralocorticoid excess (hypertension, hypokalaemia or peripheral oedema) occur consider increasing the prednisolone dose to 5 mg BD • Re-start treatment once blood pressure is controlled (returned to baseline or resolved to Grade ≤ 1)

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 60

Arlen Wilcox OTHER PROTOCOL V 19 UPDATES Nov-18 STAMPEDE: Protocol V 19. 0 Training 61

Other Updates • Metformin Sample size has increased to align the statistical power of the comparison with the others. • Protocol & PIS has been updated to reflect the new general data protection regulation • A planned comparison (“rucaparib comparison”, protocol v 18) is no longer being implemented. Nov-18 STAMPEDE: Protocol V 19. 0 Training 62

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 63

Mazna Anjum PROTOCOL ACTIVATION Nov-18 STAMPEDE: Protocol V 19. 0 Training 64

Activation Checklist The following needs to be completed & returned to the STAMPEDE Trial Team prior to activation: 1. PI, Research Team & Pharmacy Amendment Acknowledgments 2. Delegation and Personnel Log Version 4. 0 3. CVs of Randomising Clinicians (Dated & signed within 2 years) 4. GCP Certificates of Randomising Clinicians (Dated within 2 years) 5. 6. 7. 8. 9. 10. 11. Completed ISF Checklist (November 2018) Completed PSF Checklist (November 2018) Localised version of all PISs Localised version of CF Investigator’s Statement (protocol v 19. 0) Signed SECs Document Signed Deviations and Concerns Log Nov-18 Sites can access all updated documents via the STAMPEDE website – www. stampedetrial. org A member of site staff must have attended this training session, preferably a research team member and the PI of your site. Following the training, please ensure a member of staff notify the STAMPEDE trial team of who attended this session – mrcctu. stampede@ucl. ac. uk STAMPEDE: Protocol V 19. 0 Training 65

Activation Documents Randomising Sites Nov-18 Non Randomising Sites STAMPEDE: Protocol V 19. 0 Training 66

ISF & PSF Checklists The STAMPEDE ISF and PSF checklists have had a significant update, and will be mandatory to complete and return to the STAMPEDE Trial Team. All documents current and historic are available to download from the STAMPEDE website to ensure 100% adherence to the checklist and GCP. We recommend that each site take this as an opportunity to review their ISF & PSF and ensure the files are updated and in an orderly manner – e. g. divided and ordered as described on the checklist. Nov-18 STAMPEDE: Protocol V 19. 0 Training www. stampedetrial. org 67

Delegation Log v 4. 0 The Delegation Log & the Personnel Signature List have been combined into a new single form replacing both. Please ensure Row 1 is completed by the Principal Investigator. Please ensure all staff working on STAMPEDE have appropriate documentation and are signed off by the PI for their delegated tasks. Nov-18 STAMPEDE: Protocol V 19. 0 Training 68

Green Light Next steps: Ø Sites should return all documentation & Activation Checklist to the STAMPEDE Trial Team Ø STAMPEDE Trial Team will provide confirmation that your site has received the green light for protocol version 19 Ø Protocol v 19. 0 should be implemented at site NB: This protocol cannot be implemented at site without this confirmation. Nov-18 STAMPEDE: Protocol V 19. 0 Training 69

Questions? Nov-18 STAMPEDE: Protocol V 19. 0 Training 70

Agenda Item Presenter “Comparison Closure” Joanna Calvert SOC Abiraterone Arlen Wilcox IMP Reminders Mazna Anjum & Arlen Wilcox Eligibility & Randomisation Arlen Wilcox CRF Updates Hannah Sweeney Pharmacovigilance Michelle Buckner Sub Studies Mazna Anjum & Aminata Sy Hypertension Breach Follow Up Arlen Wilcox Other Protocol 19 Updates Arlen Wilcox Protocol Activation Mazna Anjum Nov-18 STAMPEDE: Protocol V 19. 0 Training 71

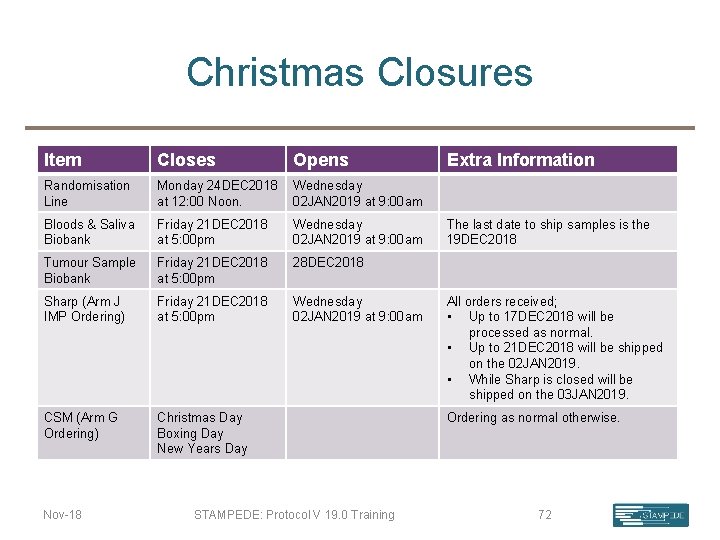
Christmas Closures Item Closes Randomisation Line Monday 24 DEC 2018 Wednesday at 12: 00 Noon. 02 JAN 2019 at 9: 00 am Bloods & Saliva Biobank Friday 21 DEC 2018 at 5: 00 pm Wednesday 02 JAN 2019 at 9: 00 am Tumour Sample Biobank Friday 21 DEC 2018 at 5: 00 pm 28 DEC 2018 Sharp (Arm J IMP Ordering) Friday 21 DEC 2018 at 5: 00 pm Wednesday 02 JAN 2019 at 9: 00 am CSM (Arm G Ordering) Christmas Day Boxing Day New Years Day Nov-18 Opens STAMPEDE: Protocol V 19. 0 Training Extra Information The last date to ship samples is the 19 DEC 2018 All orders received; • Up to 17 DEC 2018 will be processed as normal. • Up to 21 DEC 2018 will be shipped on the 02 JAN 2019. • While Sharp is closed will be shipped on the 03 JAN 2019. Ordering as normal otherwise. 72

More Information For more detailed information, please see the STAMPEDE protocol, the FAQ section of the website, or contact the trial team mrcctu. STAMPEDE@ucl. ac. uk Nov-18 STAMPEDE: Protocol V 19. 0 Training 73