Systemic lupus erythematosus desperate search for a therapy



- Slides: 28

Systemic lupus erythematosus: desperate search for a therapy 22 nd AUI Grindelwald Dr. Denis Comte Associate Physician, PD et MER Division of Immunology and Allergy, CHUV 31. 01. 2020

Systemic lupus erythematosus SLE

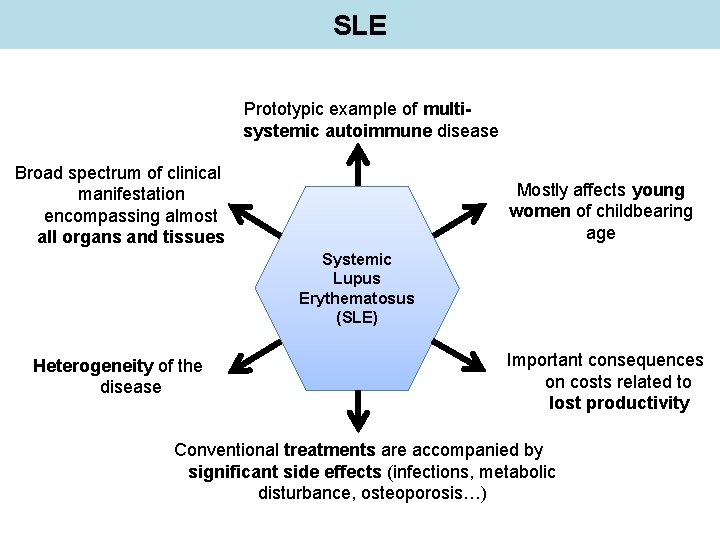
SLE Prototypic example of multisystemic autoimmune disease Broad spectrum of clinical manifestation encompassing almost all organs and tissues Mostly affects young women of childbearing age Systemic Lupus Erythematosus (SLE) Heterogeneity of the disease Important consequences on costs related to lost productivity Conventional treatments are accompanied by significant side effects (infections, metabolic disturbance, osteoporosis…)

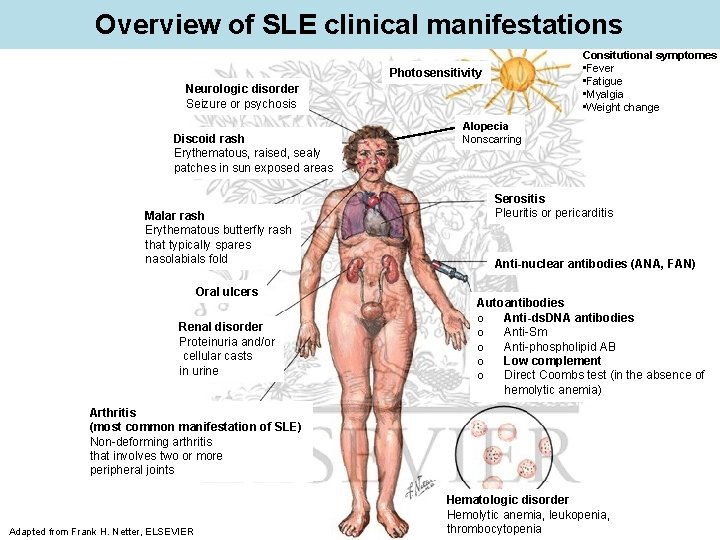
Overview of SLE clinical manifestations Consitutional symptomes • Fever • Fatigue • Myalgia • Weight change Photosensitivity Neurologic disorder Seizure or psychosis Discoid rash Erythematous, raised, sealy patches in sun exposed areas Malar rash Erythematous butterfly rash that typically spares nasolabials fold Oral ulcers Renal disorder Proteinuria and/or cellular casts in urine Alopecia Nonscarring Serositis Pleuritis or pericarditis Anti-nuclear antibodies (ANA, FAN) Autoantibodies o Anti-ds. DNA antibodies o Anti-Sm o Anti-phospholipid AB o Low complement o Direct Coombs test (in the absence of hemolytic anemia) Arthritis (most common manifestation of SLE) Non-deforming arthritis that involves two or more peripheral joints Adapted from Frank H. Netter, ELSEVIER Hematologic disorder Hemolytic anemia, leukopenia, thrombocytopenia

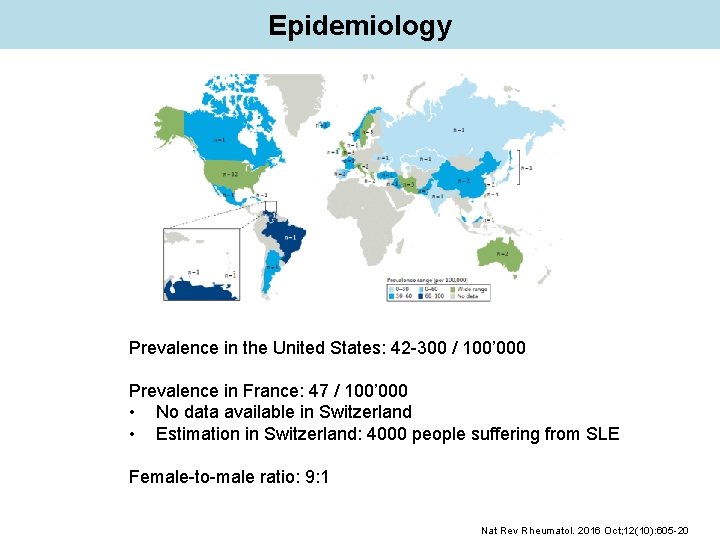
Epidemiology Prevalence in the United States: 42 -300 / 100’ 000 Prevalence in France: 47 / 100’ 000 • No data available in Switzerland • Estimation in Switzerland: 4000 people suffering from SLE Female-to-male ratio: 9: 1 Nat Rev Rheumatol. 2016 Oct; 12(10): 605 -20

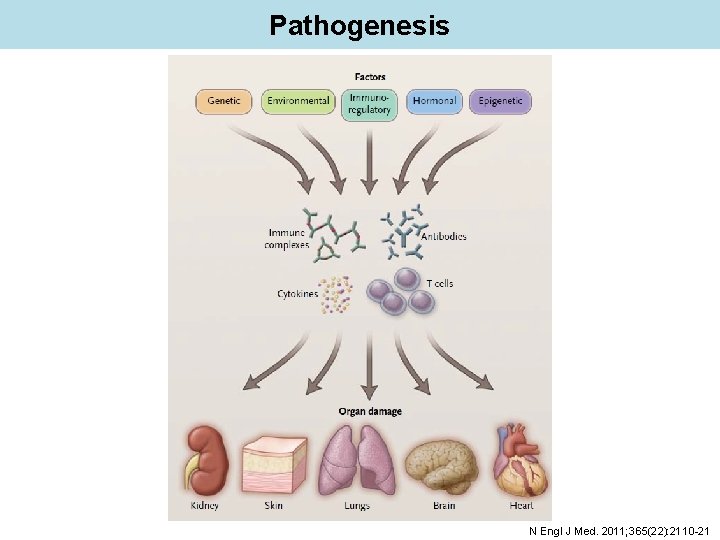
Pathogenesis N Engl J Med. 2011; 365(22): 2110 -21

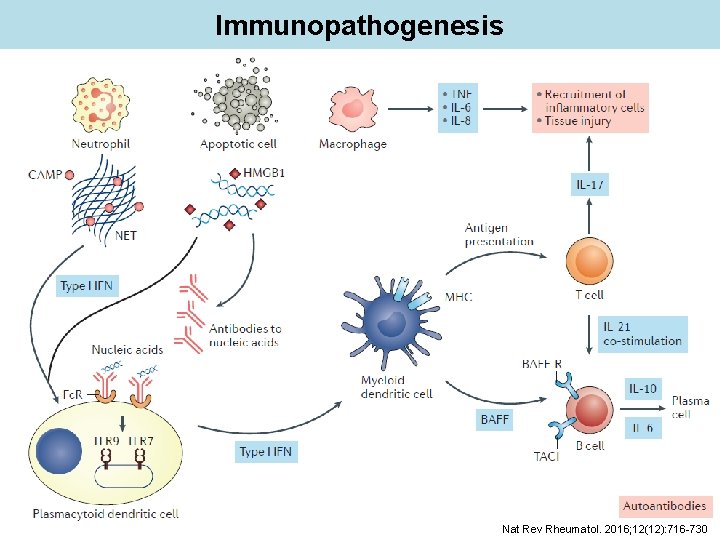
Immunopathogenesis Nat Rev Rheumatol. 2016; 12(12): 716 -730

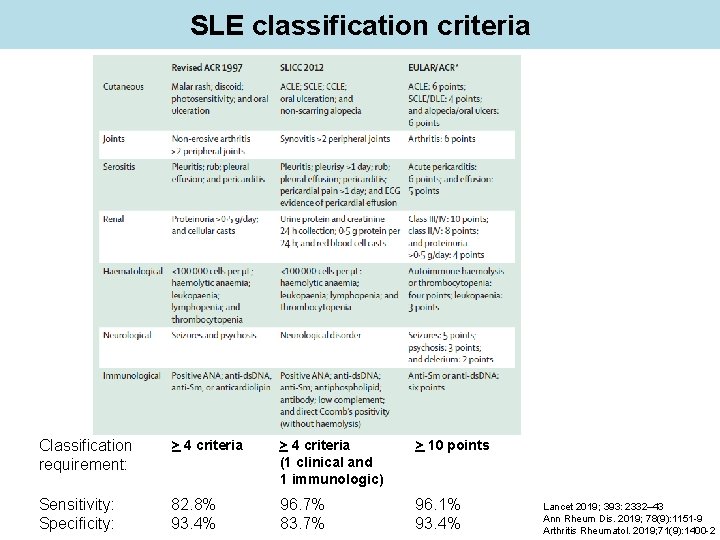
SLE classification criteria Classification requirement: > 4 criteria (1 clinical and 1 immunologic) > 10 points Sensitivity: Specificity: 82. 8% 93. 4% 96. 7% 83. 7% 96. 1% 93. 4% Lancet 2019; 393: 2332– 43 Ann Rheum Dis. 2019; 78(9): 1151 -9 Arthritis Rheumatol. 2019; 71(9): 1400 -2

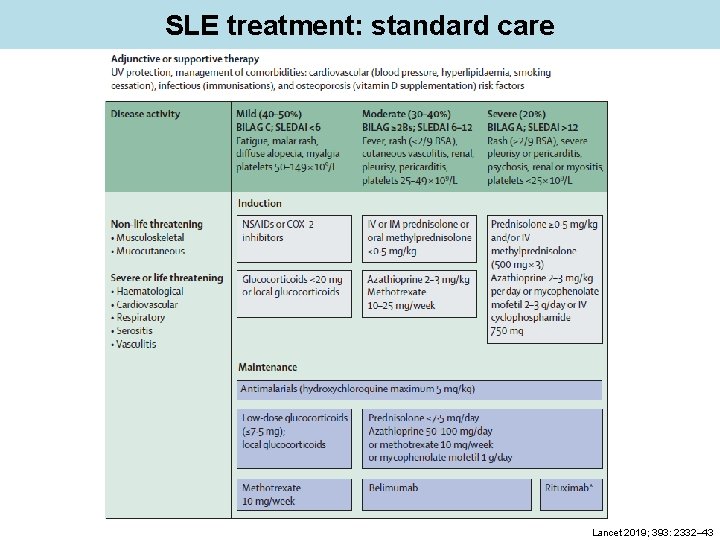
SLE treatment: standard care Lancet 2019; 393: 2332– 43

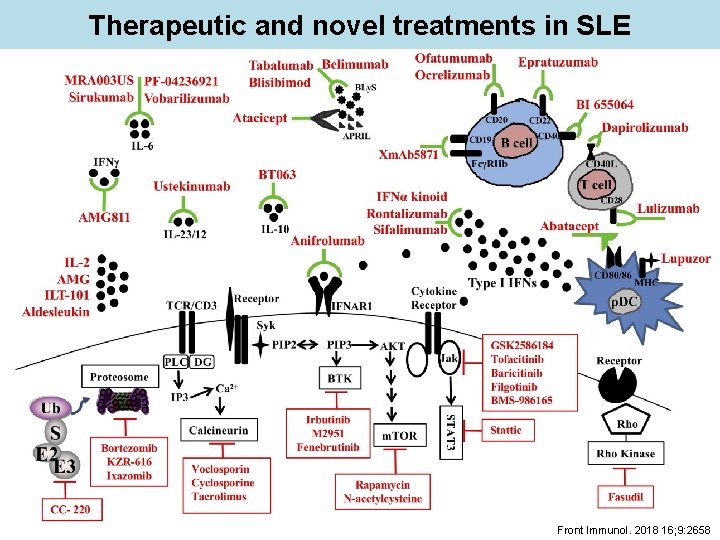
Therapeutic and novel treatments in SLE Front Immunol. 2018 16; 9: 2658

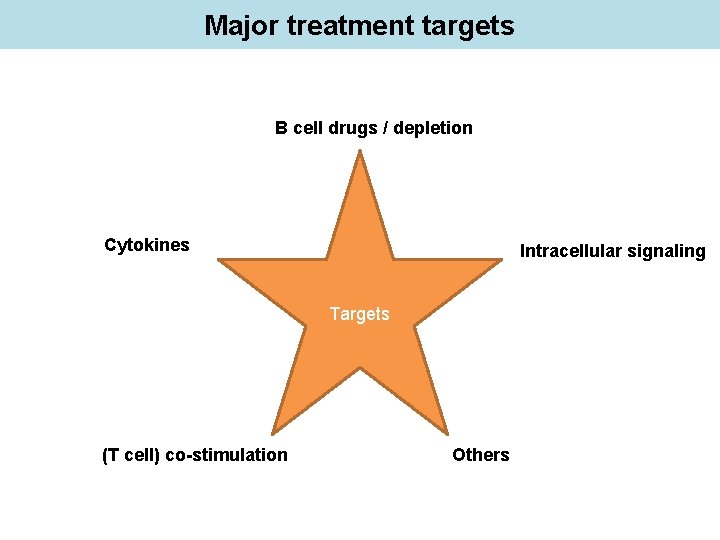
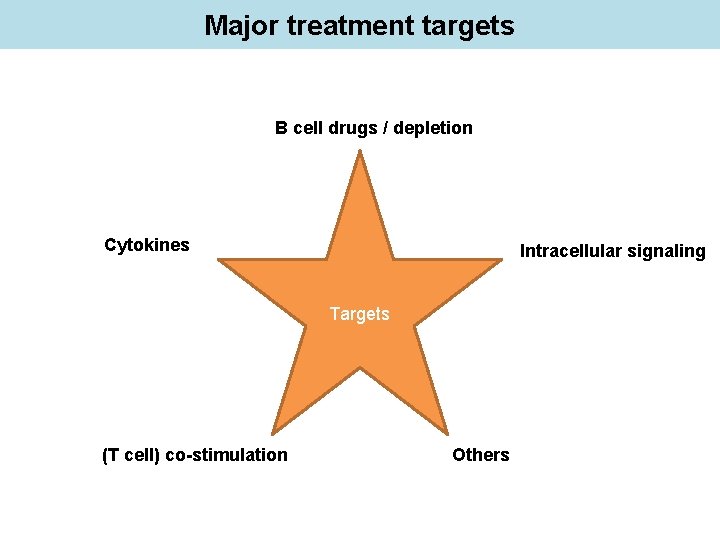
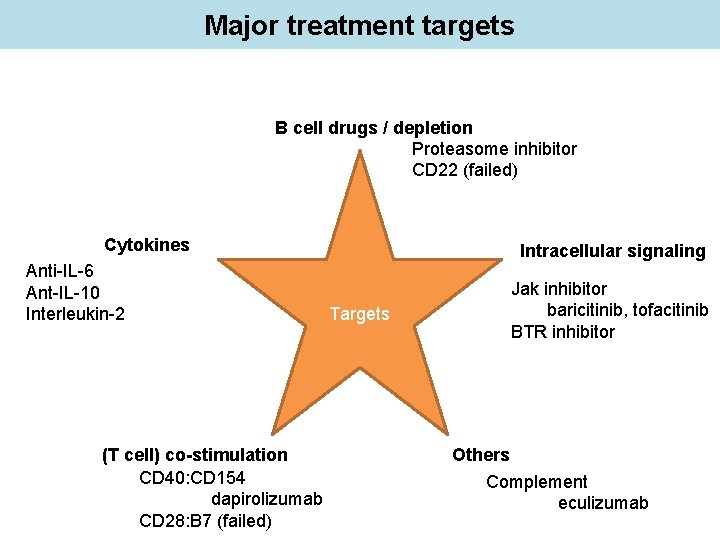
Major treatment targets B cell drugs / depletion Cytokines Intracellular signaling Targets (T cell) co-stimulation Others

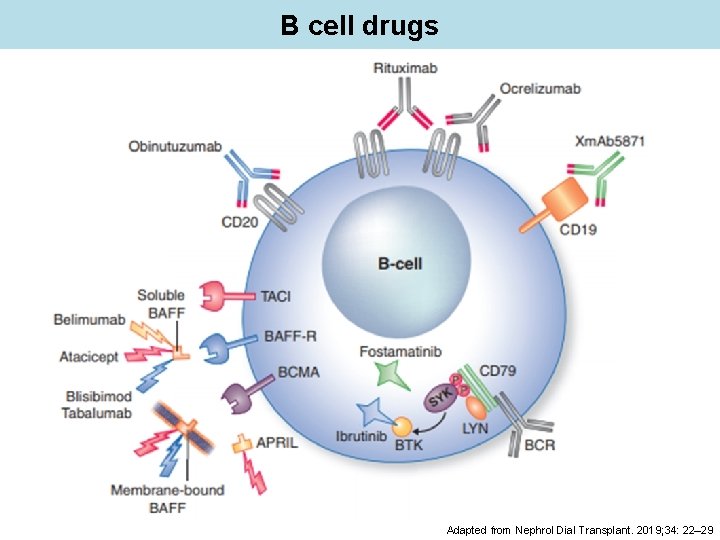
B cell drugs Adapted from Nephrol Dial Transplant. 2019; 34: 22– 29

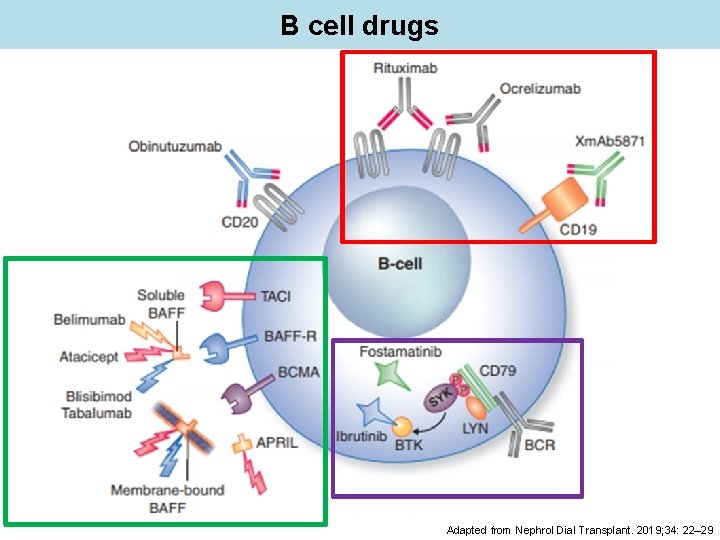
B cell drugs Adapted from Nephrol Dial Transplant. 2019; 34: 22– 29

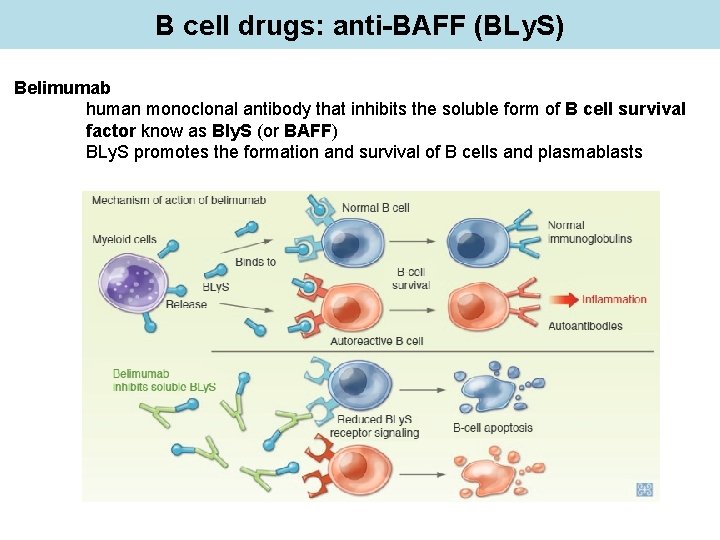
B cell drugs: anti-BAFF (BLy. S) Belimumab human monoclonal antibody that inhibits the soluble form of B cell survival factor know as Bly. S (or BAFF) BLy. S promotes the formation and survival of B cells and plasmablasts

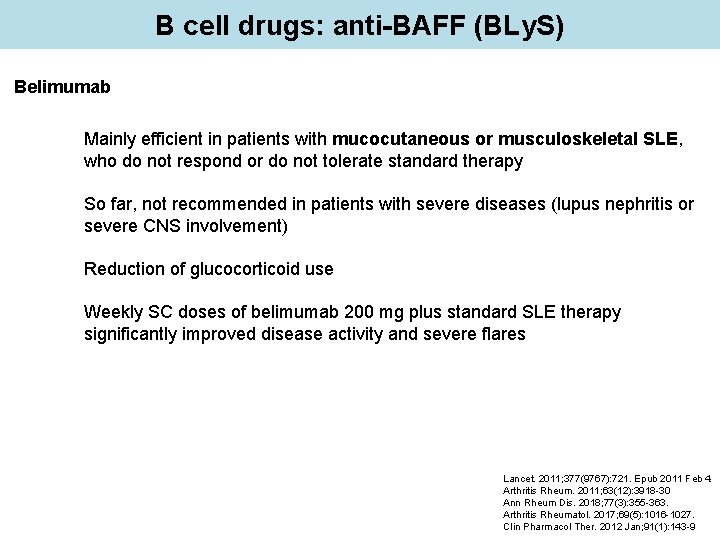
B cell drugs: anti-BAFF (BLy. S) Belimumab Mainly efficient in patients with mucocutaneous or musculoskeletal SLE, who do not respond or do not tolerate standard therapy So far, not recommended in patients with severe diseases (lupus nephritis or severe CNS involvement) Reduction of glucocorticoid use Weekly SC doses of belimumab 200 mg plus standard SLE therapy significantly improved disease activity and severe flares Lancet. 2011; 377(9767): 721. Epub 2011 Feb 4. Arthritis Rheum. 2011; 63(12): 3918 -30 Ann Rheum Dis. 2018; 77(3): 355 -363. Arthritis Rheumatol. 2017; 69(5): 1016 -1027. Clin Pharmacol Ther. 2012 Jan; 91(1): 143 -9

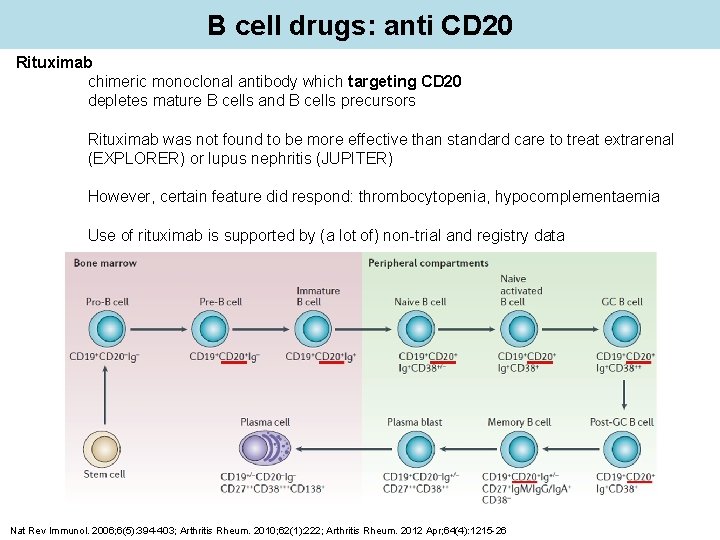
B cell drugs: anti CD 20 Rituximab chimeric monoclonal antibody which targeting CD 20 depletes mature B cells and B cells precursors Rituximab was not found to be more effective than standard care to treat extrarenal (EXPLORER) or lupus nephritis (JUPITER) However, certain feature did respond: thrombocytopenia, hypocomplementaemia Use of rituximab is supported by (a lot of) non-trial and registry data Nat Rev Immunol. 2006; 6(5): 394 -403; Arthritis Rheum. 2010; 62(1): 222; Arthritis Rheum. 2012 Apr; 64(4): 1215 -26

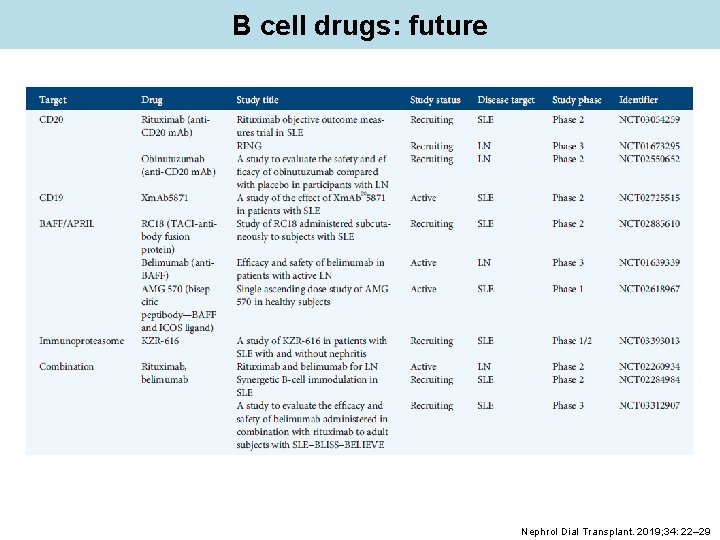
B cell drugs: future Nephrol Dial Transplant. 2019; 34: 22– 29

Major treatment targets B cell drugs / depletion Cytokines Intracellular signaling Targets (T cell) co-stimulation Others

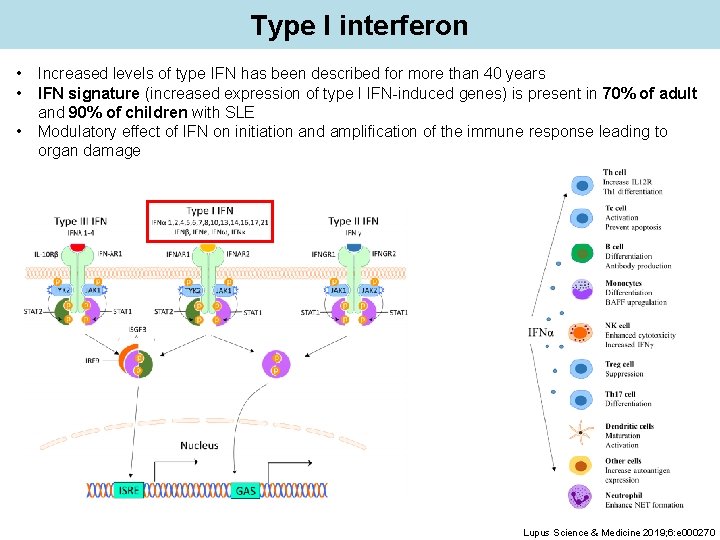
Type I interferon • • • Increased levels of type IFN has been described for more than 40 years IFN signature (increased expression of type I IFN-induced genes) is present in 70% of adult and 90% of children with SLE Modulatory effect of IFN on initiation and amplification of the immune response leading to organ damage Lupus Science & Medicine 2019; 6: e 000270

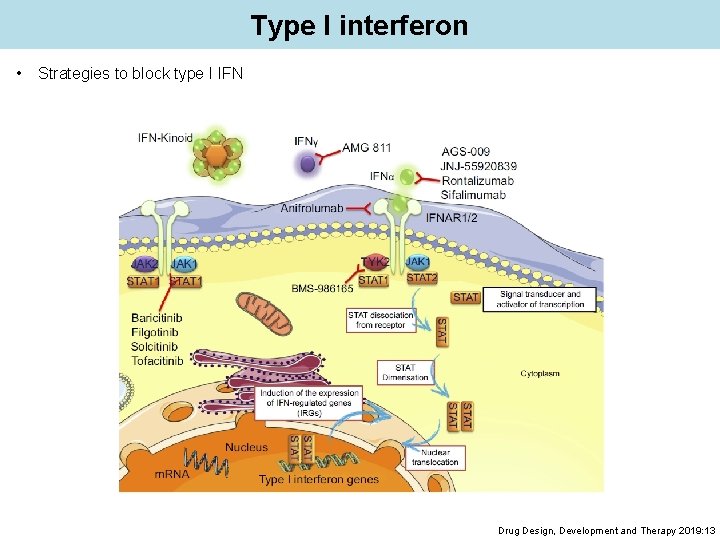
Type I interferon • Strategies to block type I IFN Drug Design, Development and Therapy 2019: 13

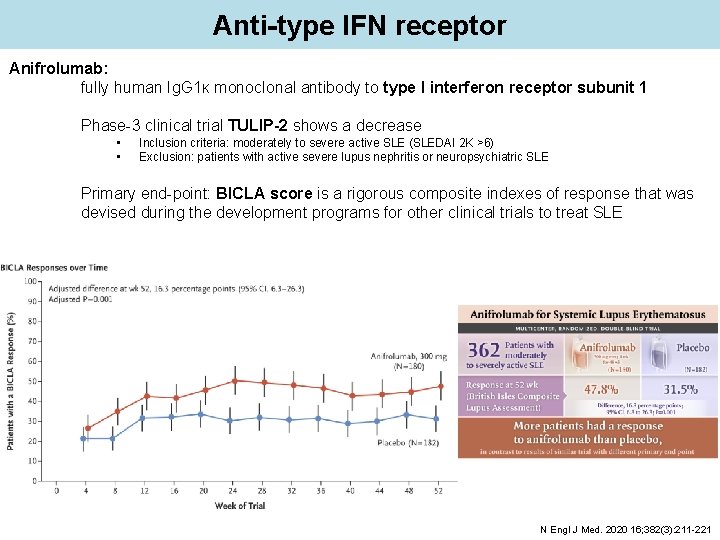
Anti-type IFN receptor Anifrolumab: fully human Ig. G 1κ monoclonal antibody to type I interferon receptor subunit 1 Phase-3 clinical trial TULIP-2 shows a decrease • • Inclusion criteria: moderately to severe active SLE (SLEDAI 2 K >6) Exclusion: patients with active severe lupus nephritis or neuropsychiatric SLE Primary end-point: BICLA score is a rigorous composite indexes of response that was devised during the development programs for other clinical trials to treat SLE N Engl J Med. 2020 16; 382(3): 211 -221

Anti-type IFN receptor Anifrolumab: fully human Ig. G 1κ monoclonal antibody to type I interferon receptor subunit 1 Phase-3 clinical trial TULIP-2 shows a decrease • • Inclusion criteria: moderately to severe active SLE (SLEDAI 2 K >6) Exclusion: patients with active severe lupus nephritis or neuropsychiatric SLE Primary end-point met: BICLA score is a rigorous composite indexes of response that was devised during the development programs for other clinical trials to treat SLE Major sides effects: Bronchitis and upper respiratory tract infection Herpes zoster “In a protocol amendment, before the unblinding of trial data but after completion of the first phase 3 trial, the primary end point was changed to BICLA response from SRI (Systemic Lupus Erythematosus Responder Index). This change was informed by information from the first phase 3 trial…” N Engl J Med. 2020 16; 382(3): 211 -221 Lancet Rheumatol 2019; 1(4): e 208 -e 219

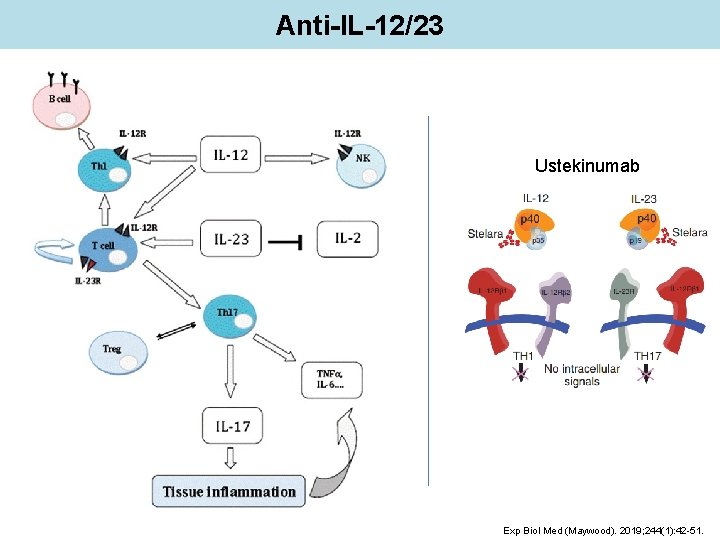
Anti-IL-12/23 Ustekinumab Exp Biol Med (Maywood). 2019; 244(1): 42 -51.

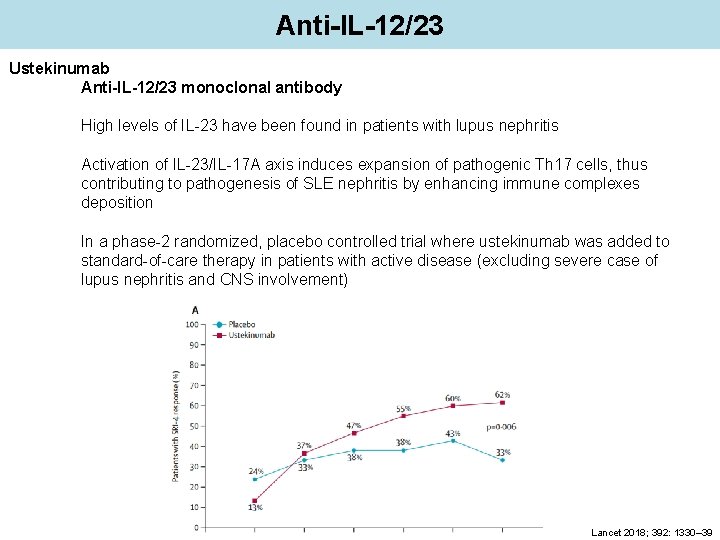
Anti-IL-12/23 Ustekinumab Anti-IL-12/23 monoclonal antibody High levels of IL-23 have been found in patients with lupus nephritis Activation of IL-23/IL-17 A axis induces expansion of pathogenic Th 17 cells, thus contributing to pathogenesis of SLE nephritis by enhancing immune complexes deposition In a phase-2 randomized, placebo controlled trial where ustekinumab was added to standard-of-care therapy in patients with active disease (excluding severe case of lupus nephritis and CNS involvement) Lancet 2018; 392: 1330– 39

Major treatment targets B cell drugs / depletion Proteasome inhibitor CD 22 (failed) Cytokines Anti-IL-6 Ant-IL-10 Interleukin-2 (T cell) co-stimulation CD 40: CD 154 dapirolizumab CD 28: B 7 (failed) Intracellular signaling Targets Jak inhibitor baricitinib, tofacitinib BTR inhibitor Others Complement eculizumab

Future Lancet 2019; 393: 2332– 43

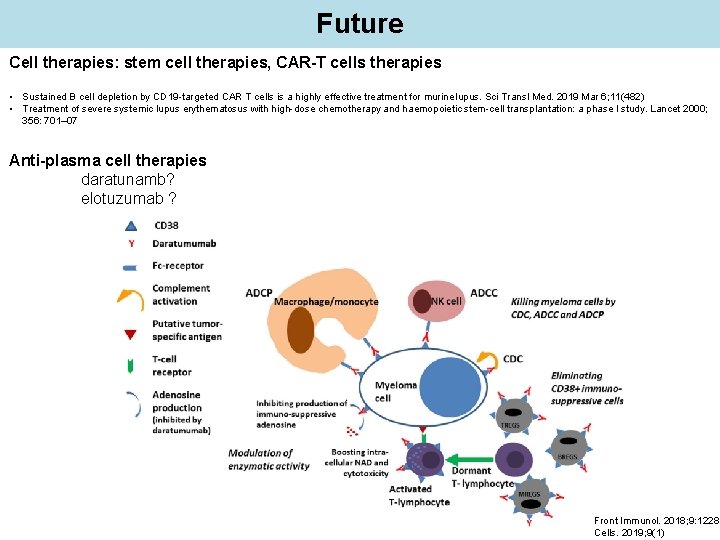
Future Cell therapies: stem cell therapies, CAR-T cells therapies • Sustained B cell depletion by CD 19 -targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019 Mar 6; 11(482) • Treatment of severe systemic lupus erythematosus with high-dose chemotherapy and haemopoietic stem-cell transplantation: a phase I study. Lancet 2000; 356: 701– 07 Anti-plasma cell therapies daratunamb? elotuzumab ? Front Immunol. 2018; 9: 1228 Cells. 2019; 9(1)

Acknowledgement Research team Morgane Humbel Florence Bellanger Immunology Lab Giuseppe Pantaleo Craig Fenwick Madeleine Suffiotti Matthieu Perreau Line Leuenberger Navina Rajah Alex Farina Camillo Ribi Alice Horisberger Swiss SLE cohort steering committee George Tsokos Maria Karampetsou
 Case study 87 systemic lupus erythematosus
Case study 87 systemic lupus erythematosus Systemic lupus erythematosus
Systemic lupus erythematosus Systemic family therapy techniques
Systemic family therapy techniques Iv site complications
Iv site complications Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness bits cost
Bioness bits cost Humanistic therapies aim to boost
Humanistic therapies aim to boost Hold thy desperate hand
Hold thy desperate hand The desperate man
The desperate man Whom do the onondaga credit with the creation of earth
Whom do the onondaga credit with the creation of earth Warm up sentence
Warm up sentence Desperate houseknives
Desperate houseknives Romeo and juliet quotes act 4
Romeo and juliet quotes act 4 Romeo o romeo
Romeo o romeo Thou detestable maw thou womb of death
Thou detestable maw thou womb of death Why is the community so desperate to get jonas back?
Why is the community so desperate to get jonas back? Out your
Out your A desperate deed
A desperate deed Lupus eritematoso
Lupus eritematoso Fedro lupus et agnus
Fedro lupus et agnus Apa itu doktrin
Apa itu doktrin Calsium
Calsium Lupus presentation powerpoint
Lupus presentation powerpoint Lupus research alliance
Lupus research alliance Nekrotizan papillit nedir
Nekrotizan papillit nedir Raynaud's disease lupus
Raynaud's disease lupus Jaccoud's arthropathy lupus
Jaccoud's arthropathy lupus Tiopentano
Tiopentano Padrao full house lupus
Padrao full house lupus


















































