Physician role in risk monitoring for delayed onset






















- Slides: 57

Physician role in risk monitoring for delayed onset and progressive hearing loss Dr. Jessica Stich-Hennen, Au. D. , PASC Doctor of Audiology Specialty Certification in Pediatric Audiology Elks Hearing & Balance Center - Boise St. Luke’s Pediatric Otolaryngology - Boise 208 -489 -4999 jstich@elksrehab. org

Learner Goals • Understand the importance of early detection and intervention of childhood hearing loss • Understand risk indicators for delayed onset hearing loss in children • Understand the pediatric audiologist role in newborn hearing screening and risk indicator monitoring for delayed onset hearing loss • Understand the physician/medical home role in newborn hearing screening and risk indicator monitoring for delayed onset hearing loss

EARLY DETECTION & INTERVENTION OF CHILDHOOD HEARING LOSS

Importance of Newborn Hearing Screening • Hearing loss is invisible • 3/1000 newborn infants are identified with permanent hearing loss – 1/1000 babies have profound hearing loss – 2/1000 have lesser degrees of hearing loss • If a child with hearing loss is identified early and given appropriate services (educational, medical, and audiological), over $400, 000 can be saved in special education costs.

Effects of Early Identification q Intervention by 6 month old is key q Children identified < 6 months develop significantly better language, vocabulary and social skills (Yoshinaga-Itano) q Lack of appropriate intervention results in the re-organization of auditory tissue in the brain q In the absence of sound, the brain begins to reorganize itself to receive information from other senses (i. e. vision).

Idaho Sound Beginnings • Hearing screening by 1 month old • Audiological testing by 3 months old • Early intervention services by 6 months old

Idaho Sound Beginnings • Idaho is 1 of 7 states without legislation for newborn hearing screening • In 2012, 98. 7% of newborns were screened in Idaho – ~4% of infants referred to audiology – 3/1000 infants diagnosed with hearing loss at birth

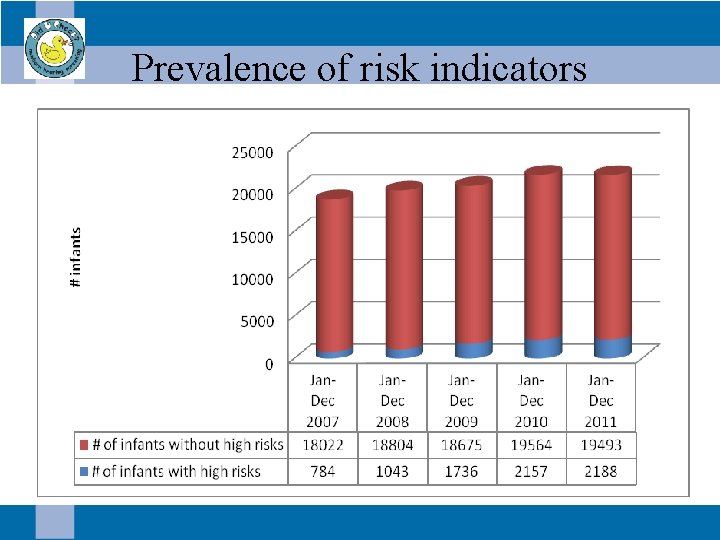
Idaho Sound Beginnings • In 2007 -2011, 3. 5/10, 000 infants were diagnosed with a delayed onset hearing loss. – 1/10, 000 infants had no risk indicators – 2. 5/10, 000 infants had risk indicators – 75% of these infants had multiple risk indicators reported

RISK INDICATORS FOR DELAYED ONSET HEARING LOSS IN CHILDREN

Joint Committee on Infant Hearing (JCIH) Established in 1969 Comprised of: • American Academy of Pediatrics • American Academy of Ophthalmology and Otolaryngology • American Speech & Hearing Association

JCIH 1972 Position Statement • High risk criteria • Family history of childhood hearing loss • Intrauterine fetal infection (Rubella) • Defects of ear, nose or throat (atresia, cleft lip/palate) • Low birth weight (<1500 grams) • High bilirubin levels

JCIH 1982 Position Statement –High risk criteria » Bacterial meningitis, severe asphyxia (i. e. low APGAR) were added

JCIH 1990 Position Statement –High risk criteria additions: » Ototoxic medications » Prolonged mechanical ventilation » Physical findings of syndromes » Parent/caregiver concerns » Head trauma » Neurodegenerative disorders » Infectious diseases associated with hearing loss

JCIH 1994 Position Statement –Studies has shown that only 50% of all hearing loss were being identified using the High Risk Register

JCIH 2007: Risk indicators for delayed onset hearing loss ¨ Caregiver concerns (re: hearing, speech, language, or developmental delay) ¨ Family history of permanent childhood hearing loss ¨ Neonatal Intensive Care (NICU) of more than 5 days or any of the following regardless of length of stay: ECMO, assisted ventilation, exposure to ototoxic medications (gentimycin and tobramycin) or loop diuretics (furosemide, Lasix), and hyperbilirubinemia that requires exchange transfusion. ¨ In-utero infections ¨ Craniofacial anomalies ¨ Known physical findings associated with a syndrome ¨ Syndromes associated with hearing loss, progressive hearing loss or late-onset hearing loss neurodegenerative disorders ¨ Culture-positive postnatal infections associated with hearing loss ¨ Head trauma, especially basal skull/temporal bone, requiring hospitalization ¨ Chemotherapy

Most frequently occurring risk factors • Ototoxic Medications (>70%) • Severe Asphyxia (>50%) • Mechanical Ventilation less than 5 days (>25%) • Low birth weight (>20%) • Parental/Physician concerns (>15%) • ECMO (>10%) (Cone-Wesson, et al. , 2000; Van Riper & Kileny, 2002, Hall, 2007)

Least frequently occurring risk factors (<10%) • Hyperbilirubinemia • Craniofacial anomalies • Family history • Congenital infections • Bacterial meningitis • Substance abuse (maternal) • Neurodegenerative disorders (Cone-Wesson, et al. , 2000; Van Riper & Kileny, 2002, Hall, 2007)

Frequency of hearing loss among high risk indicators • Craniofacial anomalies (>50%) • ECMO treatments (>20%) • Severe Asphyxia/ Mechanical ventilation (>15%) • Congenital infections (>15%) • Family History (>15%) • Bacterial meningitis (>10%) • Other risk indicators (<10%) (Cone-Wesson, et al. , 2000; Fligor, 2008; Van Riper & Kileny, 2002, Hall, 2007)

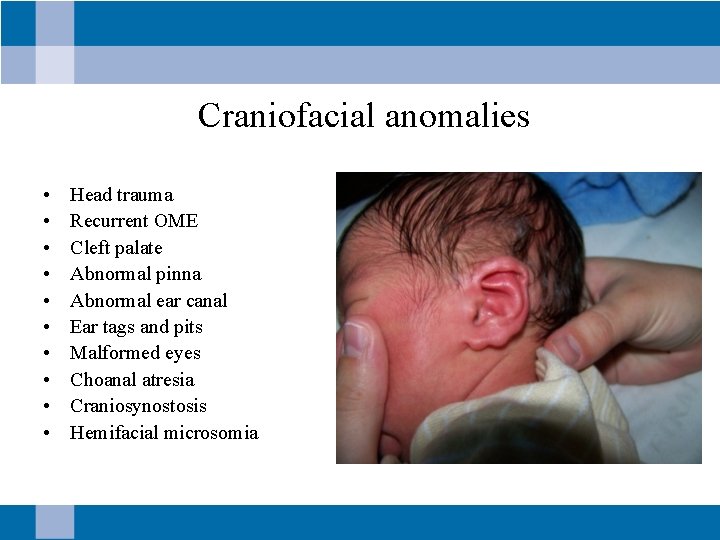
Craniofacial anomalies • • • Head trauma Recurrent OME Cleft palate Abnormal pinna Abnormal ear canal Ear tags and pits Malformed eyes Choanal atresia Craniosynostosis Hemifacial microsomia

Incidence of hearing loss in cleft palate patients • Children’s Hospital of Philadelphia (1972 -1976) • n = 70 (2 - 21 year olds) with cleft palate • 50% conductive hearing loss (microtia, OME, tympanosclerosis, cerumen impactions, external ear deformities ) • Viswanathan et al (2008) • n = 90 infants with cleft palate • 82% (74) hearing loss (varying from mild to severe) » 7 mixed hearing loss » 1 unilateral SNHL » 66 conductive hearing loss • 18% (16) normal hearing

Idaho Cleft Palate & Craniofacial Deformities team • Chart review (October 2007 -February 2010) • N = 210 – 104 (Normal hearing) = 50% • At least 50% of these children have a history of OME and PE tubes – 94 (Conductive hearing loss) = 45% • 2 bilateral microtia – 4 (Mixed hearing loss) = 1% – 8 (Sensorineural hearing loss) = 4% • 3 unilateral – profound left ear • 5 bilateral

ECMO treatments • Expracorporeal Membrane Oxygenation (ECMO)- is an aggressive treatment that is used for the life support in infants with respiratory or cardiopulmonary failure (Fligor, 2008)

Mechanical ventilation ** Estimates 1/56 children with permanent hearing loss at age 1, had the following risk factors: Respiratory distress syndrome Bronchiopulmonary dysplasia Mechanical Ventlitation >36 days Cone-Wesson et. al (2000)

Infections Congenital Infections • Cytomegalovirus (CMV) • Herpes • Rubella • Syphilis • Toxoplasmosis Postnatal infections • Bacterial or viral meningitis

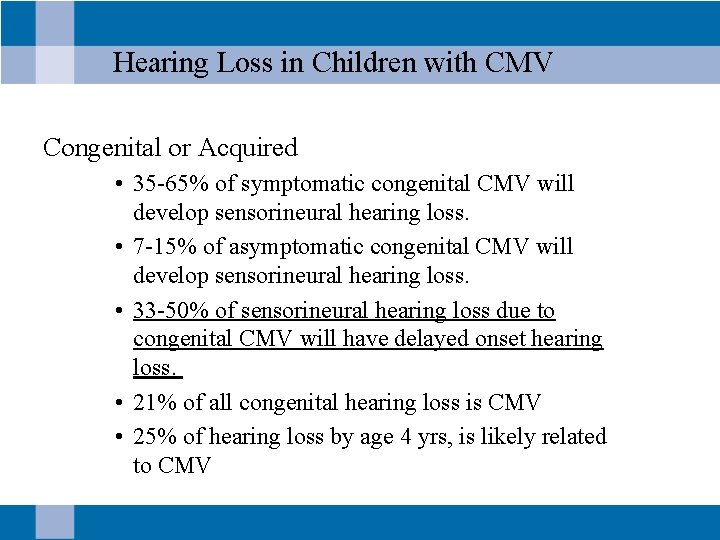
Hearing Loss in Children with CMV Congenital or Acquired • 35 -65% of symptomatic congenital CMV will develop sensorineural hearing loss. • 7 -15% of asymptomatic congenital CMV will develop sensorineural hearing loss. • 33 -50% of sensorineural hearing loss due to congenital CMV will have delayed onset hearing loss. • 21% of all congenital hearing loss is CMV • 25% of hearing loss by age 4 yrs, is likely related to CMV

Hearing Loss in Children with CMV Configuration and degree of hearing loss – Unilateral or Bilateral – Mild to profound degree of hearing loss • Mild hearing loss at birth may progress to profound hearing loss – Unpredictable configuration (i. e. rising, sloping, or flat)

Hearing Loss in Children with CMV Stable or progressive • 50% of children with sensorineural hearing loss related to CMV will have progressive hearing loss. • Recommendation- hearing evaluations at minimum every 6 months. Every 3 months during times when hearing is changing.

Family History • A family member with a congenital hearing loss congenital or hearing loss acquired during childhood. • Family history of hearing loss is the most common risk indicator found in healthy newborns (Hall 2007).

Extended NICU stay • National Perinatal Research Center (NPIC) (Quality Analytic Services (QAS) ~ made the recommendation regarding NICU stay for JCIH 2007 – Approximately 25% of NICU infants are considered “LOW” risk and discharged by 5 days old. – The remaining approximately 75% of NICU infants, who are hospitalized for greater than 5 days, are considered the “TARGET” population to rule out neural hearing loss.

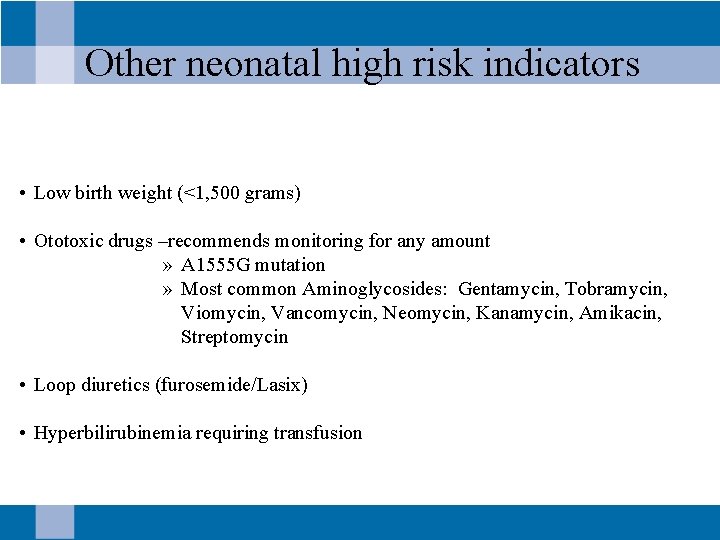
Other neonatal high risk indicators • Low birth weight (<1, 500 grams) • Ototoxic drugs –recommends monitoring for any amount » A 1555 G mutation » Most common Aminoglycosides: Gentamycin, Tobramycin, Viomycin, Vancomycin, Neomycin, Kanamycin, Amikacin, Streptomycin • Loop diuretics (furosemide/Lasix) • Hyperbilirubinemia requiring transfusion

Syndromes associated with hearing loss • • • Waardenburg syndrome • Congenital sensorineural hearing loss Branchio-Oto-Renal (BOR) syndrome • Mild-to-profound conductive, sensorineural or mixed hearing loss Stickler syndrome • Hearing loss variable (sometimes progressive) CHARGE syndrome • Ear anomalies and/or deafness (mixed sensorineural and conductive) Neurofibromatosis Type II • Hearing loss, tinnitus, balance disorders • • • Downs syndrome • Conductive hearing loss Treacher Collins syndrome • Conductive hearing loss Usher syndrome • Congenital bilateral mild-toprofound hearing loss or progressive hearing loss Pendred syndrome • Congenital or late onset. Hearing loss can be progressive. Possible vestibular dysfunction Alport syndrome • Hearing loss is never congenital but can be detected in late childhood or early adulthood

Data collected by referral forms

Prevalence of risk indicators



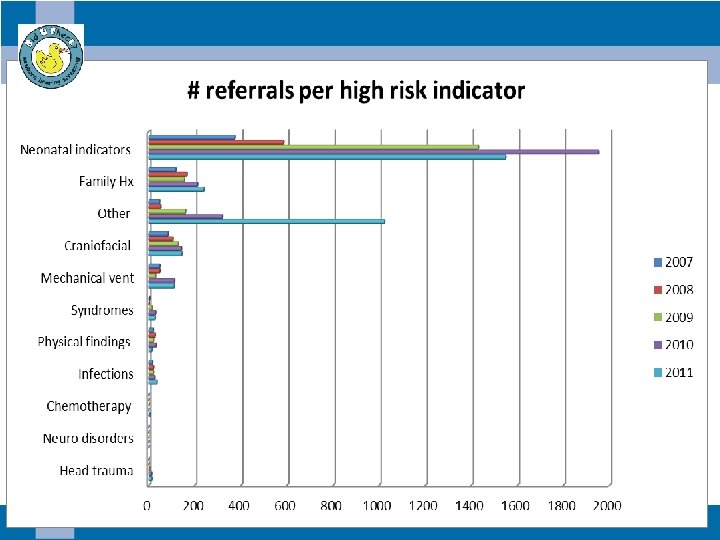
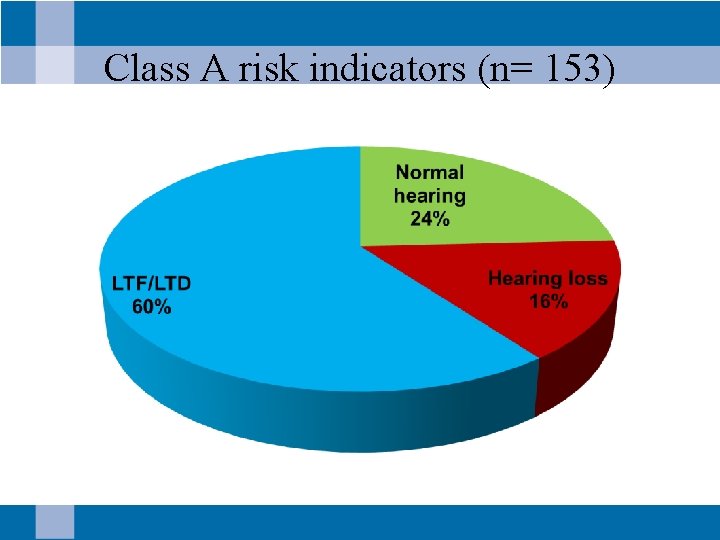
Class A risk indicators (n= 153)

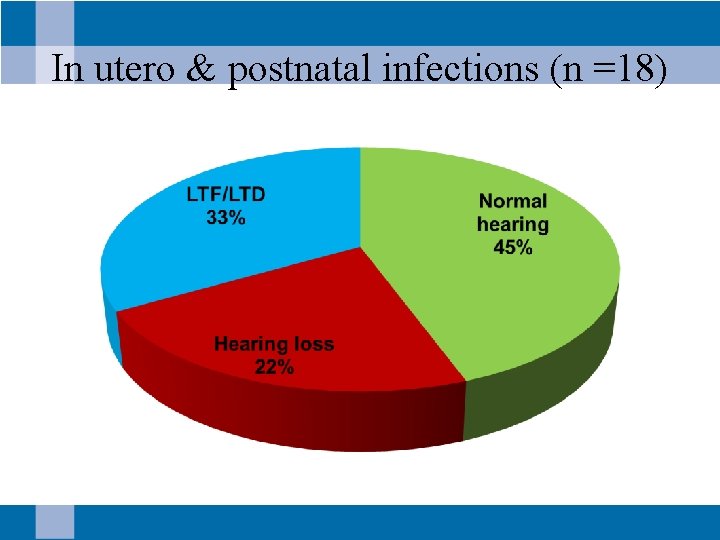
In utero & postnatal infections (n =18)

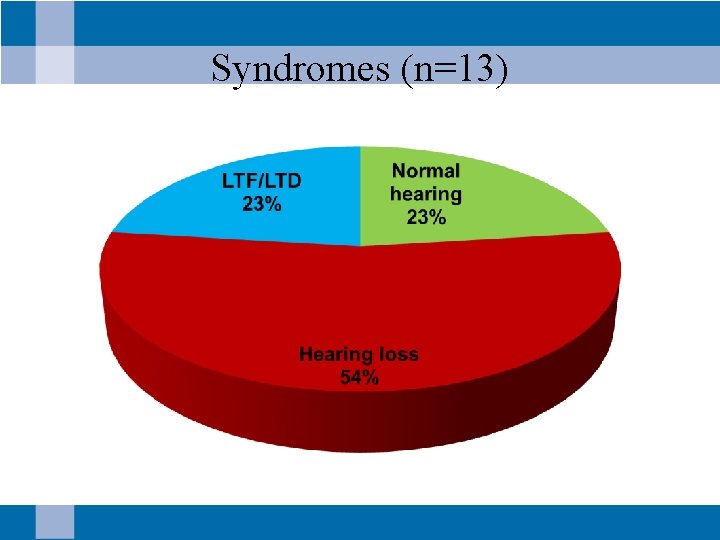
Syndromes (n=13)

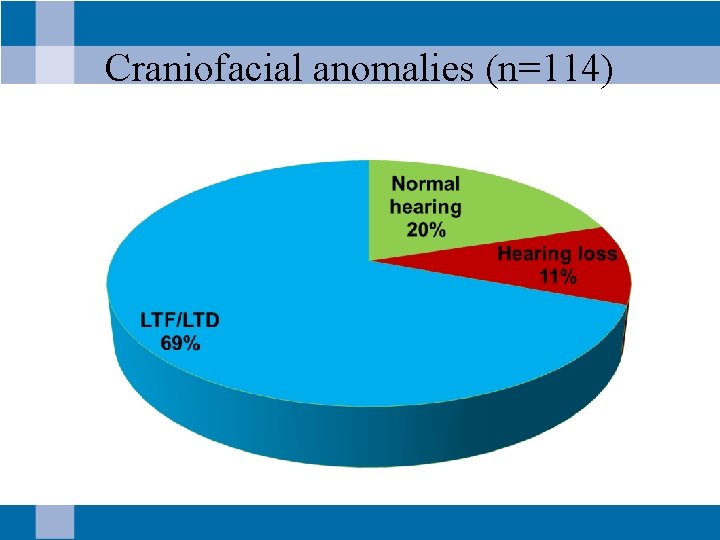
Craniofacial anomalies (n=114)

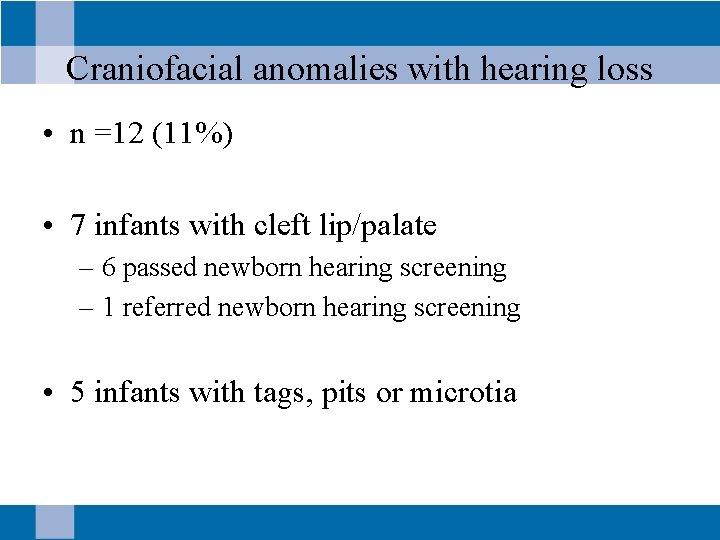
Craniofacial anomalies with hearing loss • n =12 (11%) • 7 infants with cleft lip/palate – 6 passed newborn hearing screening – 1 referred newborn hearing screening • 5 infants with tags, pits or microtia

PEDIATRIC AUDIOLOGIST ROLE IN NEWBORN HEARING SCREENING AND RISK INDICATOR MONITORING FOR DELAYED ONSET HEARING LOSS

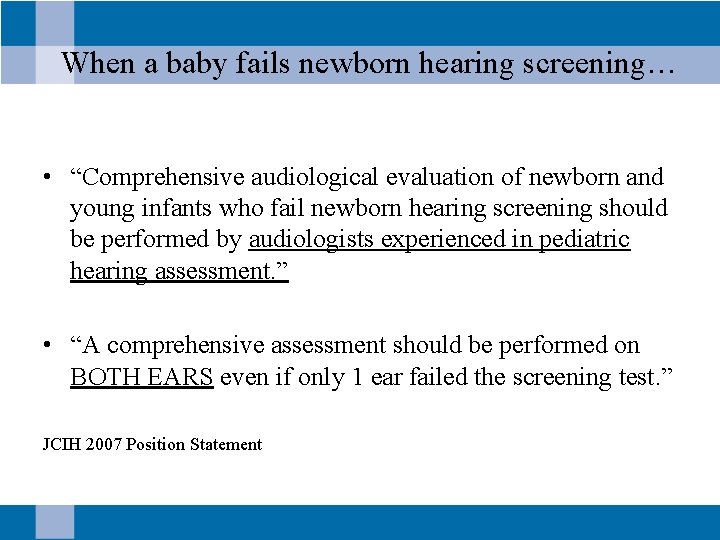
When a baby fails newborn hearing screening… • “Comprehensive audiological evaluation of newborn and young infants who fail newborn hearing screening should be performed by audiologists experienced in pediatric hearing assessment. ” • “A comprehensive assessment should be performed on BOTH EARS even if only 1 ear failed the screening test. ” JCIH 2007 Position Statement

What about children who have risk indicators… “Infants with risk factors for hearing loss should have at least one diagnostic evaluation by 24 -30 months of age. ” JCIH 2007 Position Statement

Pediatric Audiology Assessment (Birth to 6 months) • Child and family history • Frequency specific ABR using tone bursts • Click evoked ABR testing using condensation and rarefaction single-polarity stimulus • Otoacoustic Emissions • 1000 Hz tympanometry • “Behavioral observation alone is not adequate in this age group, and it is not adequate for the fitting of amplification. ”

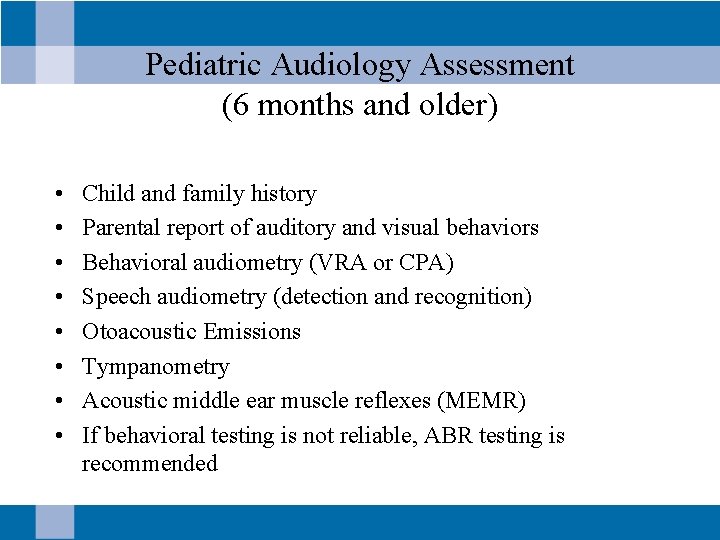
Pediatric Audiology Assessment (6 months and older) • • Child and family history Parental report of auditory and visual behaviors Behavioral audiometry (VRA or CPA) Speech audiometry (detection and recognition) Otoacoustic Emissions Tympanometry Acoustic middle ear muscle reflexes (MEMR) If behavioral testing is not reliable, ABR testing is recommended

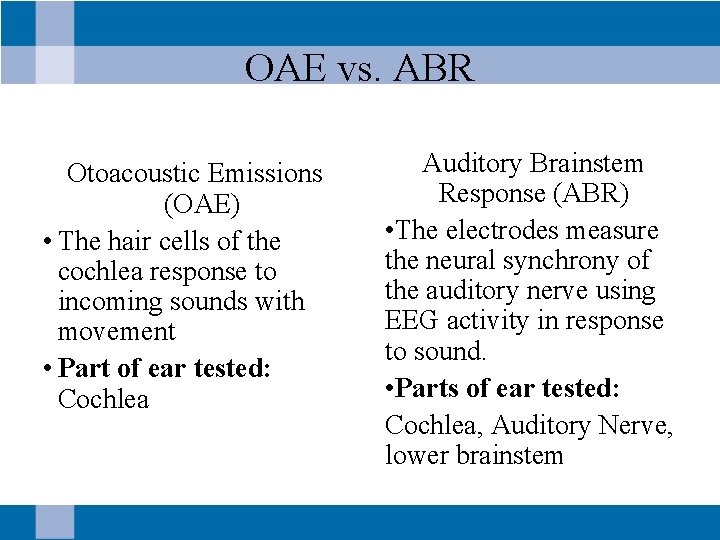
OAE vs. ABR Otoacoustic Emissions (OAE) • The hair cells of the cochlea response to incoming sounds with movement • Part of ear tested: Cochlea Auditory Brainstem Response (ABR) • The electrodes measure the neural synchrony of the auditory nerve using EEG activity in response to sound. • Parts of ear tested: Cochlea, Auditory Nerve, lower brainstem

If hearing loss is diagnosed… • Child should be referred back to medical home for recommendations, specialist referrals and additional testing – Urine CMV viral culture • Child should return for audiological testing at minimum every 3 months in the first year of life • Family should be given appropriate intervention options by Pediatric Audiologist based on the family’s communication choice

PHYSICIAN ROLE IN NEWBORN HEARING SCREENING AND RISK INDICATOR MONITORING FOR DELAYED ONSET HEARING LOSS

“Medical Home” • Defined as “a philosophy of care that emphasizes the role of the primary care physician in the care of all children, including children who have special needs. ” – Primary medical care – Family support – Coordination of specialty medical care – Referrals for various services

2012 Idaho Physician Survey regarding newborn hearing screening

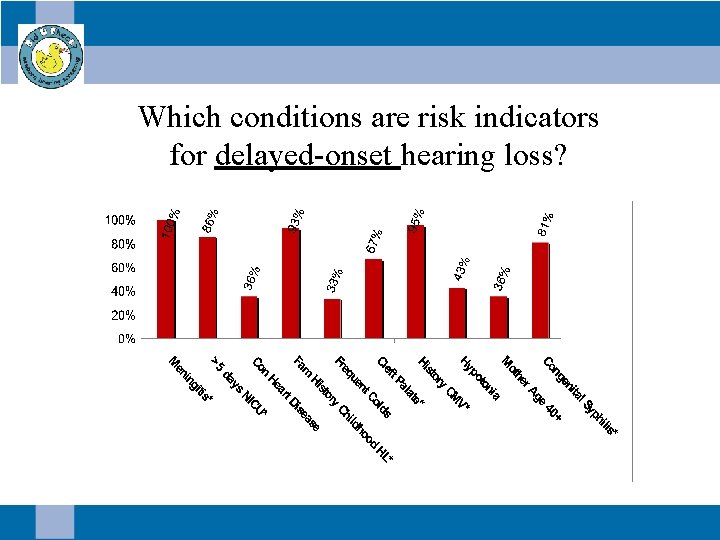
Which conditions are risk indicators for delayed-onset hearing loss?

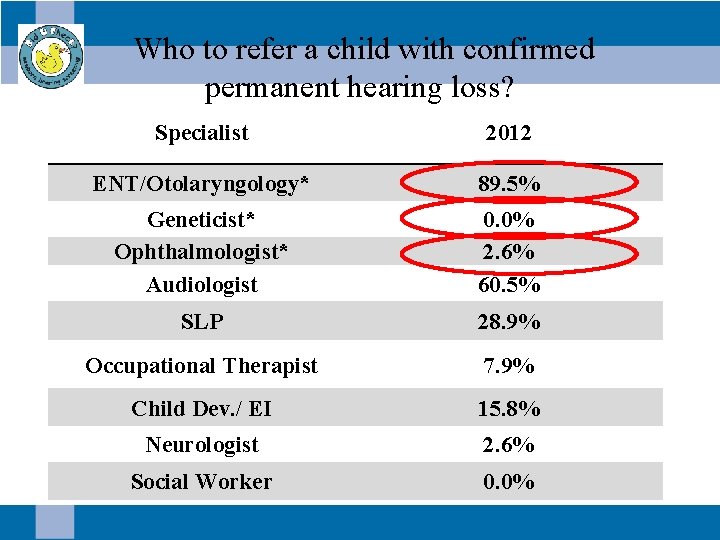
Who to refer a child with confirmed permanent hearing loss? Specialist 2012 ENT/Otolaryngology* 89. 5% Geneticist* Ophthalmologist* Audiologist 0. 0% 2. 6% 60. 5% SLP 28. 9% Occupational Therapist 7. 9% Child Dev. / EI 15. 8% Neurologist 2. 6% Social Worker 0. 0%

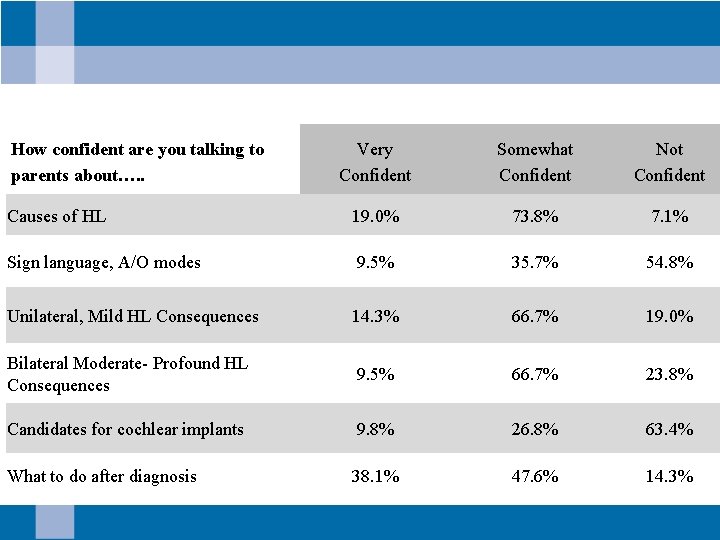
How confident are you talking to parents about…. . Very Confident Somewhat Confident Not Confident Causes of HL 19. 0% 73. 8% 7. 1% Sign language, A/O modes 9. 5% 35. 7% 54. 8% Unilateral, Mild HL Consequences 14. 3% 66. 7% 19. 0% Bilateral Moderate- Profound HL Consequences 9. 5% 66. 7% 23. 8% Candidates for cochlear implants 9. 8% 26. 8% 63. 4% What to do after diagnosis 38. 1% 47. 6% 14. 3%

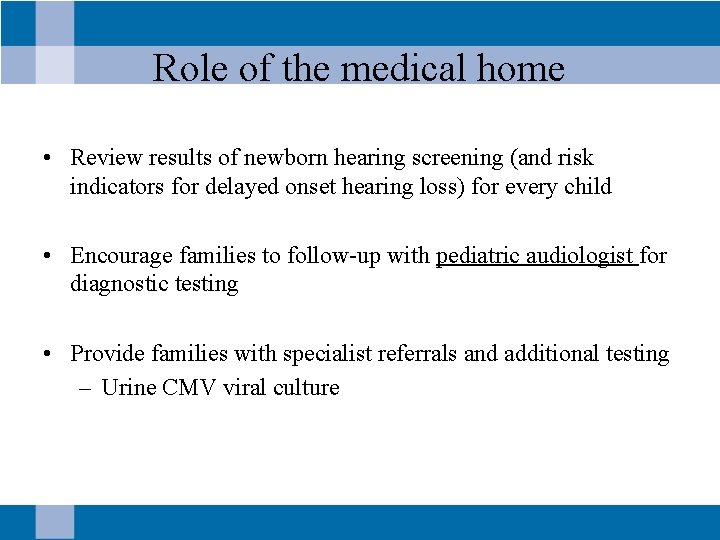
Role of the medical home • Review results of newborn hearing screening (and risk indicators for delayed onset hearing loss) for every child • Encourage families to follow-up with pediatric audiologist for diagnostic testing • Provide families with specialist referrals and additional testing – Urine CMV viral culture


Concluding points • Importance of early detection and intervention of childhood hearing loss • Importance of audiological monitoring for children with risk indicators for delayed onset hearing loss • Understand the pediatric audiologist role in newborn hearing screening and risk indicator monitoring for delayed onset hearing loss • Understand the physician role in newborn hearing screening and risk indicator monitoring for delayed onset hearing loss

References • Cone-Wesson et. al. (2000). Identification of neonatal hearing impairment: Infants with hearing impairment. Ear and Hearing, 21, 488 -507. • Fligor, B. (2008). Hearing outcomes in the most critically ill neonate population. Audiology Today, 20 (5), 9 -16. • Hall (2007). New Handbook of Auditory Evoked Potentials. • Hi-Track data from Idaho Sound Beginnings Program (2007 -2011). • Joint Committee on Infant Hearing (2007). Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Invention Programs. Pediatrics, 120, 898 -921. • NCHAM e. Book (2013). A Resource guide for Early Hearing Detection & Intervention. http: //www. ncham. org • Van Riper & Kileny (2002). ABR hearing screening for high-risk infants. Neonatal Intensive Care, 15. 47 -54.
 Liquidity measures
Liquidity measures Summary of great gatsby chapter 3
Summary of great gatsby chapter 3 Sugar coated tablet example
Sugar coated tablet example Delayed offsides hockey
Delayed offsides hockey Average ball size
Average ball size Delayed differentiation and modular design
Delayed differentiation and modular design Hertrosexual meaning
Hertrosexual meaning Causes of delayed bone age
Causes of delayed bone age Delayed afterdepolarization
Delayed afterdepolarization Approach to child with delayed walking
Approach to child with delayed walking Delayed coker unit process+flow diagram
Delayed coker unit process+flow diagram Delayed sequence intubation
Delayed sequence intubation Delayed column generation
Delayed column generation Delayed column generation
Delayed column generation Dr sears delayed vaccine schedule 2021
Dr sears delayed vaccine schedule 2021 Descriptive lead in journalism
Descriptive lead in journalism What does product and service design do?
What does product and service design do? Startling statement lead
Startling statement lead Sip early offer vs delayed offer
Sip early offer vs delayed offer Advance sunrise and delayed sunset is due to
Advance sunrise and delayed sunset is due to Complications of blood transfusion
Complications of blood transfusion What is a controlled intersection
What is a controlled intersection How to write lead
How to write lead Hygiene hypothesis
Hygiene hypothesis Delayed type hypersensitivity reactions
Delayed type hypersensitivity reactions Program common stimuli aba
Program common stimuli aba Caries control restoration
Caries control restoration Multiple baseline vs multiple probe design
Multiple baseline vs multiple probe design Delayed expansion of amalgam
Delayed expansion of amalgam Delayed differentiation and modular design
Delayed differentiation and modular design Delayed submission
Delayed submission Delayed submission
Delayed submission Ambisilbisch
Ambisilbisch Consonat clusters
Consonat clusters Food poisoning onset
Food poisoning onset Voice onset time
Voice onset time Was ist ein silbenkern
Was ist ein silbenkern Minimal pair in english
Minimal pair in english Manner of articulation
Manner of articulation Marginalization probability example
Marginalization probability example Insulin peak and duration
Insulin peak and duration Food poisoning onset
Food poisoning onset Anaphylaxis onset
Anaphylaxis onset Lenisierung beispiel
Lenisierung beispiel Ryzodeg onset peak duration
Ryzodeg onset peak duration Onset coda and nucleus
Onset coda and nucleus Labour normal
Labour normal Webtopings
Webtopings Abrupt stormy onset
Abrupt stormy onset Onset and coda examples
Onset and coda examples Maximal onset principle example
Maximal onset principle example Early onset scoliosis classification
Early onset scoliosis classification The sound patterns of language
The sound patterns of language Types of insulin
Types of insulin Onset offset trial
Onset offset trial Web role in azure
Web role in azure Rollenmodell
Rollenmodell Statuses and their related roles determine
Statuses and their related roles determine