Physical Pharmacy Frank M Etzler LECOM Fall 2012

















- Slides: 63

Physical Pharmacy Frank M. Etzler LECOM Fall 2012

Introduction • Instructor Contact Info – Room A 4 -354 – 814 -860 -5184 – fetzler@lecom. edu • Exams – 2 Exams (100 pts ea. ) – 1 Final Exam (100 pts) • Classroom conduct – Distractions from cell phones, computers, newspapers, etc. are disrespectful to the instructor and your classmates.

Textbook

Suggested Reading

Purpose • Provide a basic knowledge of physical pharmacy, pharmaceutics and biopharmaceutical principles as they apply to the development and assessment of various types of drug delivery systems. • Develop critical thinking and problem solving required to address related to dosage form design and effective use. • Acquire technical vocabulary to discuss pharmaceutical problems.

Physical Pharmacy Fall 2012 REVIEW OF BASIC CONCEPTS

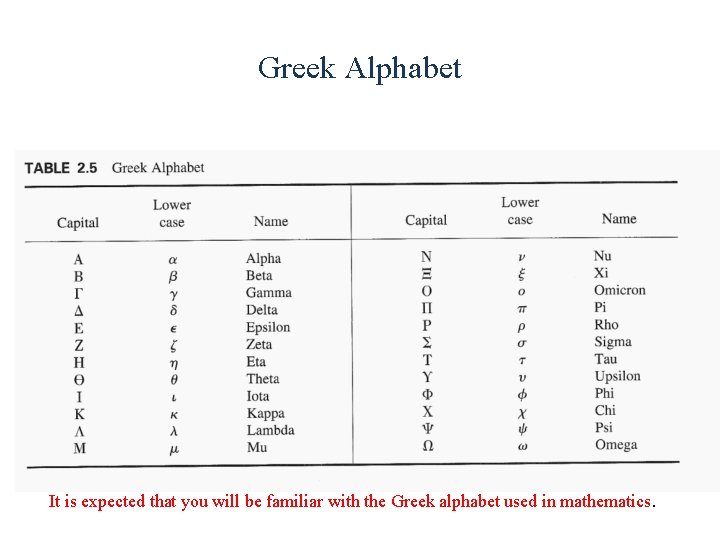
Greek Alphabet It is expected that you will be familiar with the Greek alphabet used in mathematics.

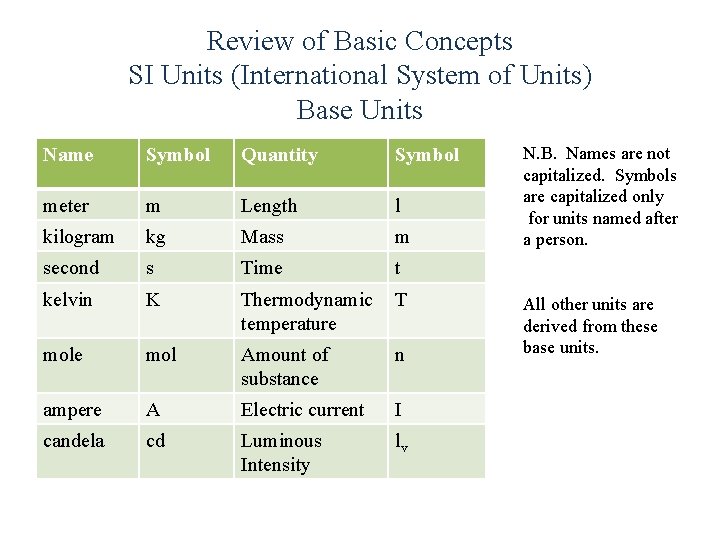
Review of Basic Concepts SI Units (International System of Units) Base Units Name Symbol Quantity Symbol meter m Length l kilogram kg Mass m second s Time t kelvin K Thermodynamic temperature T mole mol Amount of substance n ampere A Electric current I candela cd Luminous Intensity lv N. B. Names are not capitalized. Symbols are capitalized only for units named after a person. All other units are derived from these base units.

Review of Basic Concepts SI Units (International System of Units) Prefixes Prefix Factor c centi 10 -2 k kilo 103 m mili 10 -3 M mega 106 µ micro 10 -6 G giga 109 n nano 10 -9 T Tera 1012 p pico 10 -12

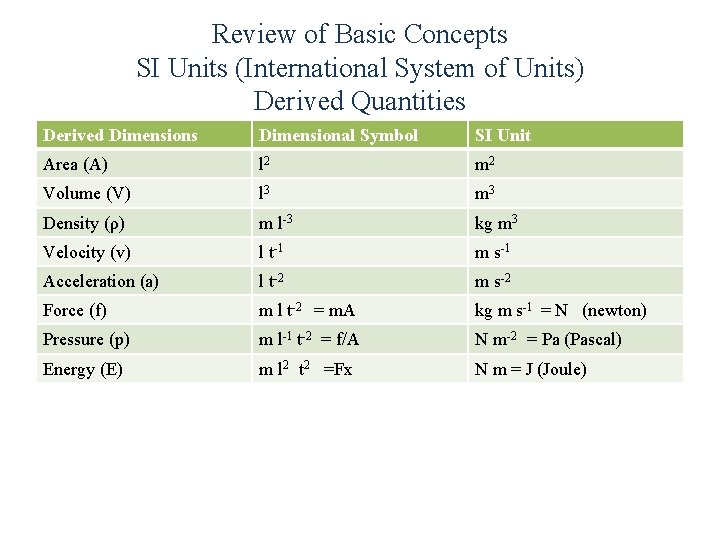
Review of Basic Concepts SI Units (International System of Units) Derived Quantities Derived Dimensions Dimensional Symbol SI Unit Area (A) l 2 m 2 Volume (V) l 3 m 3 Density (ρ) m l-3 kg m 3 Velocity (v) l t-1 m s-1 Acceleration (a) l t-2 m s-2 Force (f) m l t-2 = m. A kg m s-1 = N (newton) Pressure (p) m l-1 t-2 = f/A N m-2 = Pa (Pascal) Energy (E) m l 2 t 2 =Fx N m = J (Joule)

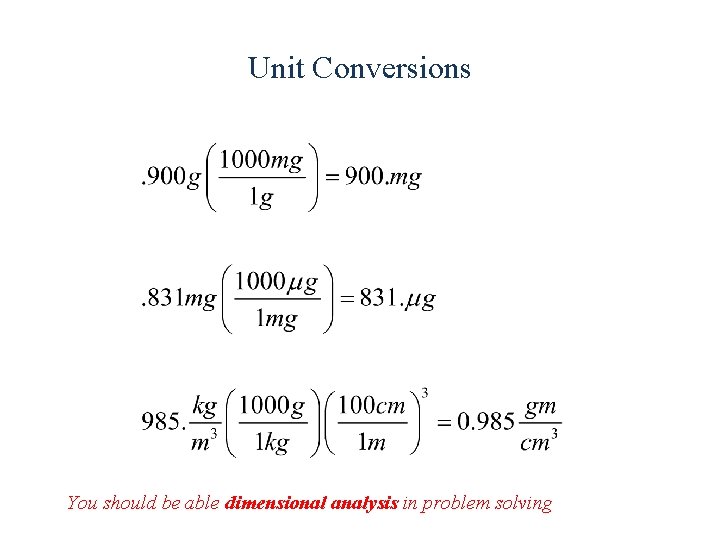
Unit Conversions You should be able dimensional analysis in problem solving

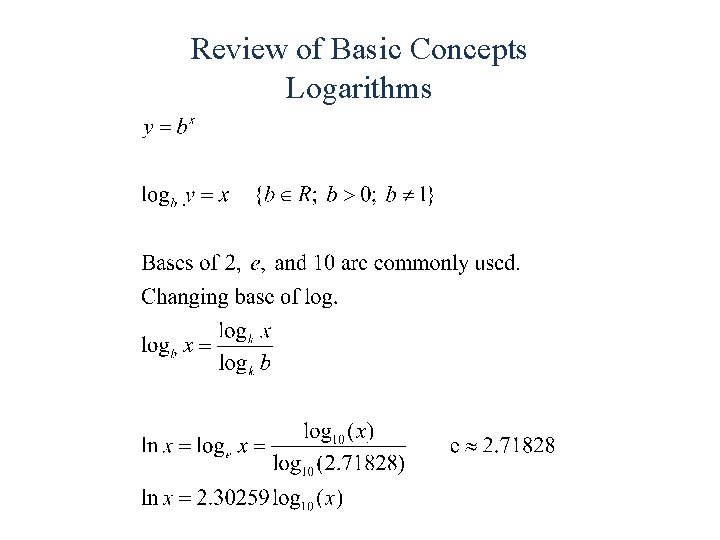
Review of Basic Concepts Logarithms

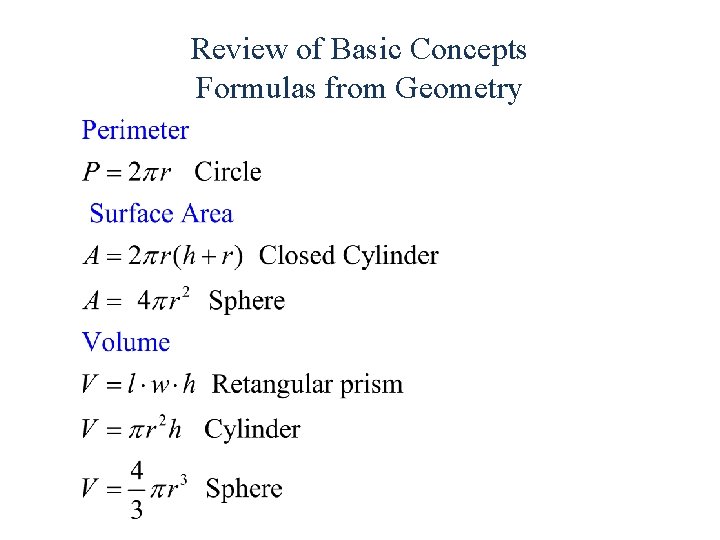
Review of Basic Concepts Formulas from Geometry

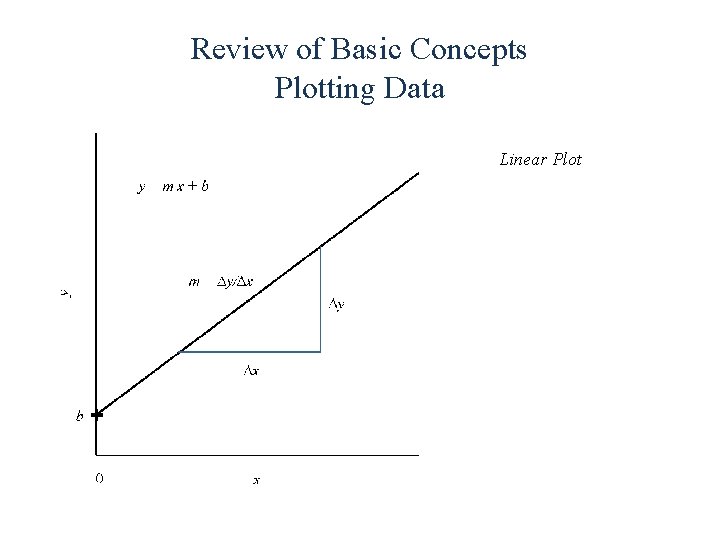
Review of Basic Concepts Plotting Data Linear Plot

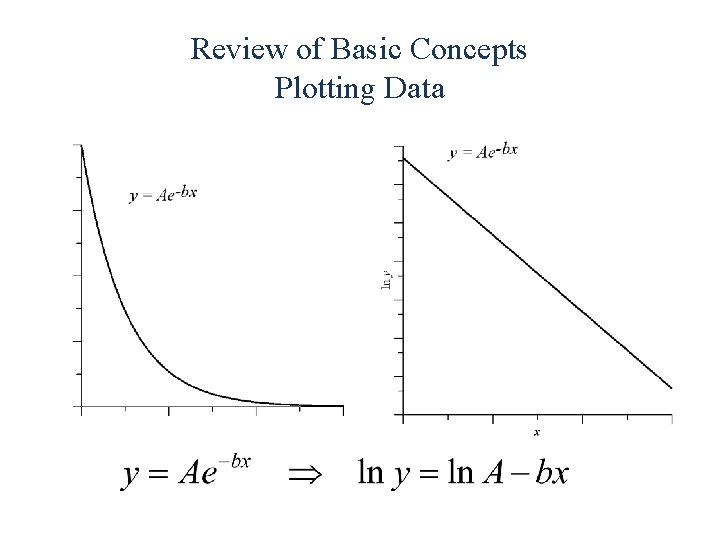
Review of Basic Concepts Plotting Data

Log Graph Paper

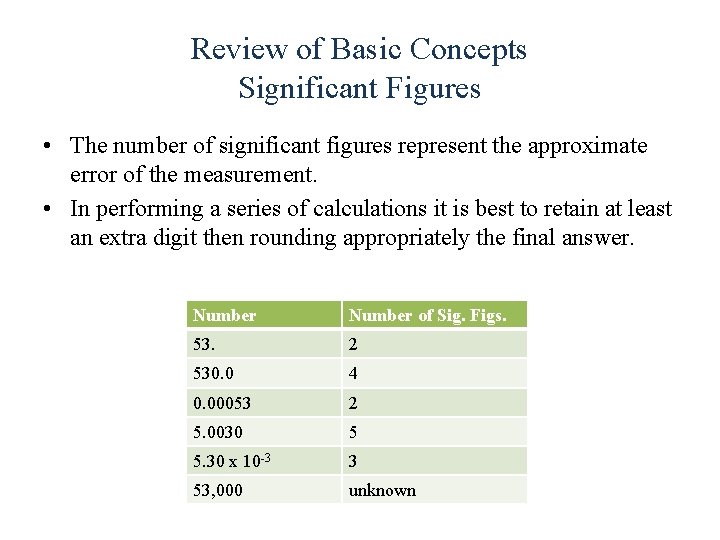
Review of Basic Concepts Significant Figures • The number of significant figures represent the approximate error of the measurement. • In performing a series of calculations it is best to retain at least an extra digit then rounding appropriately the final answer. Number of Sig. Figs. 53. 2 530. 0 4 0. 00053 2 5. 0030 5 5. 30 x 10 -3 3 53, 000 unknown

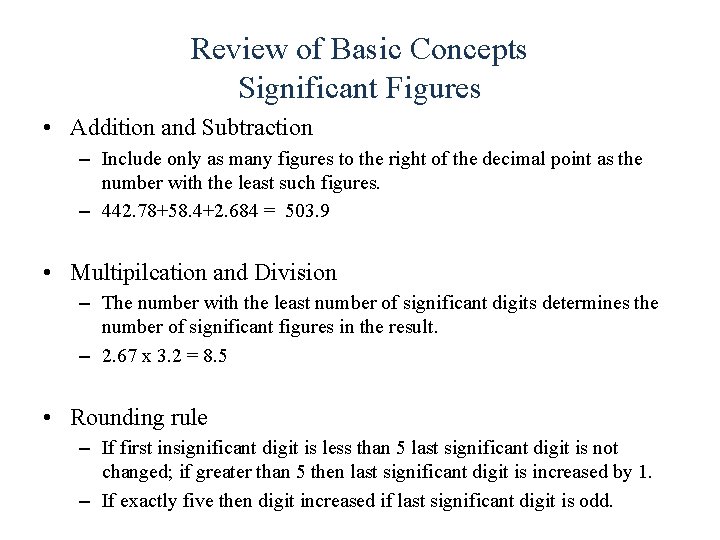
Review of Basic Concepts Significant Figures • Addition and Subtraction – Include only as many figures to the right of the decimal point as the number with the least such figures. – 442. 78+58. 4+2. 684 = 503. 9 • Multipilcation and Division – The number with the least number of significant digits determines the number of significant figures in the result. – 2. 67 x 3. 2 = 8. 5 • Rounding rule – If first insignificant digit is less than 5 last significant digit is not changed; if greater than 5 then last significant digit is increased by 1. – If exactly five then digit increased if last significant digit is odd.

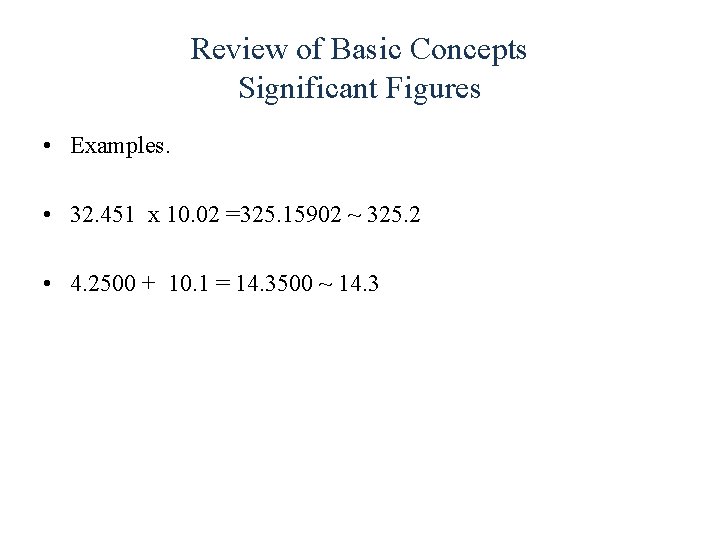
Review of Basic Concepts Significant Figures • Examples. • 32. 451 x 10. 02 =325. 15902 ~ 325. 2 • 4. 2500 + 10. 1 = 14. 3500 ~ 14. 3

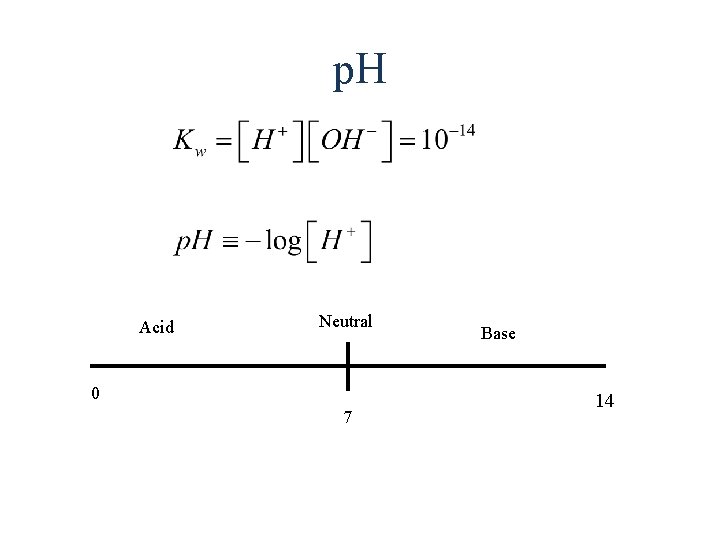
p. H Acid Neutral 0 7 Base 14

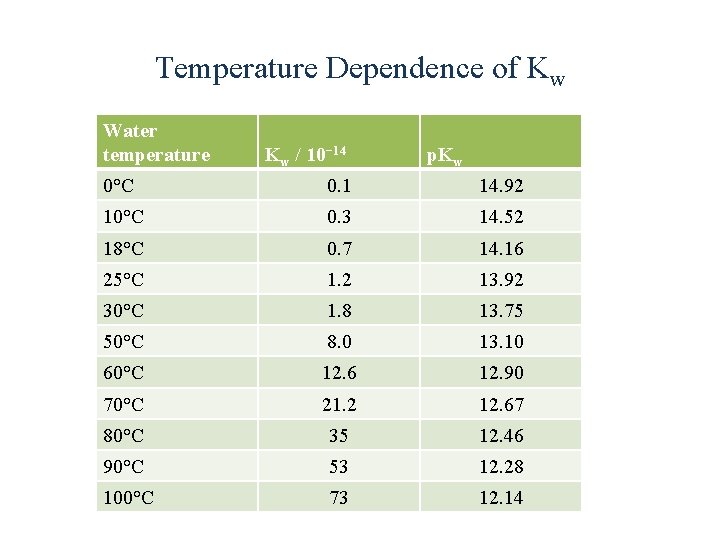
Temperature Dependence of Kw Water temperature Kw / 10− 14 p. Kw 0°C 0. 1 14. 92 10°C 0. 3 14. 52 18°C 0. 7 14. 16 25°C 1. 2 13. 92 30°C 1. 8 13. 75 50°C 8. 0 13. 10 60°C 12. 6 12. 90 70°C 21. 2 12. 67 80°C 35 12. 46 90°C 53 12. 28 100°C 73 12. 14

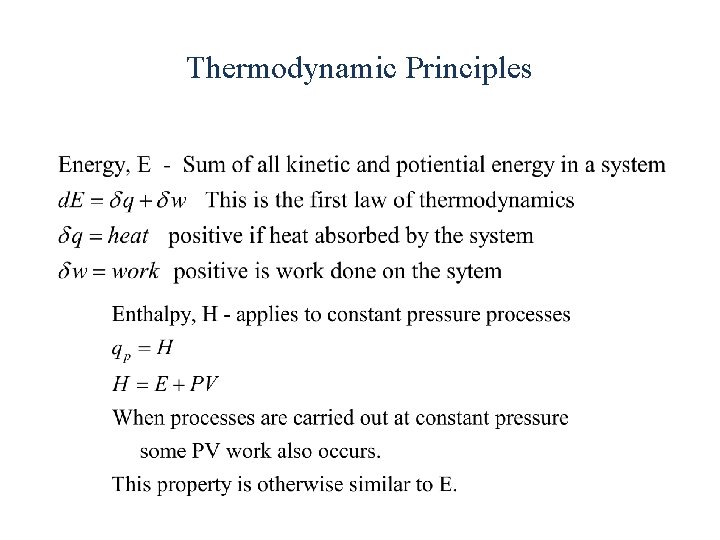
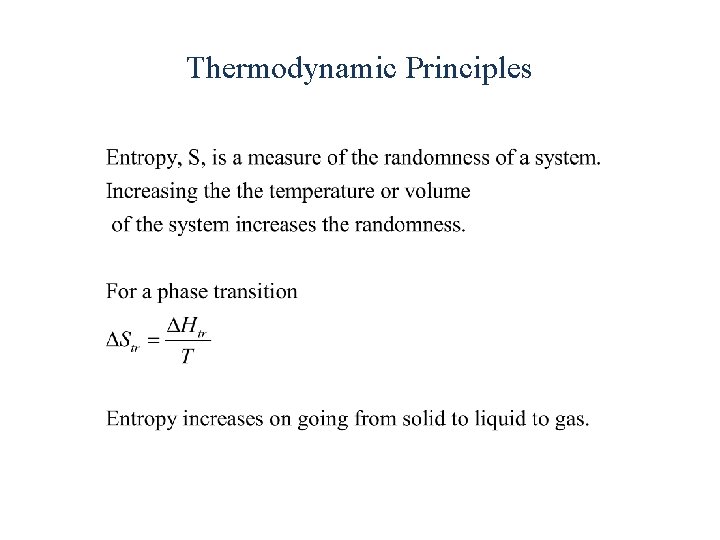
Thermodynamic Principles

Thermodynamic Principles

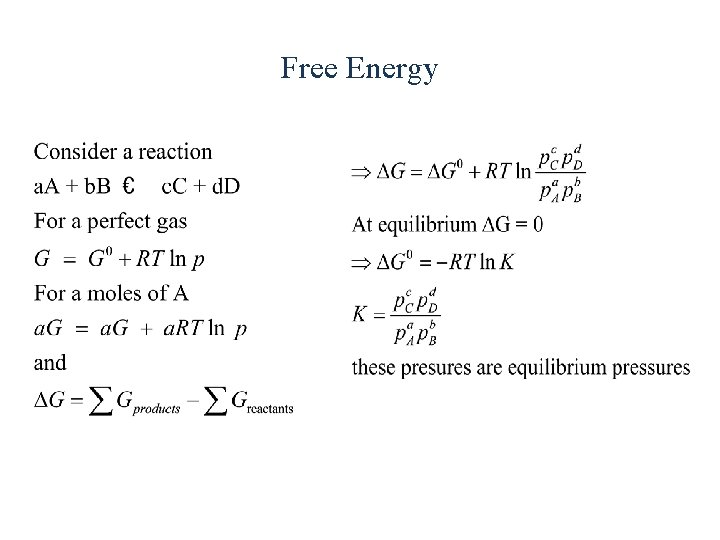
Free Energy

Free Energy

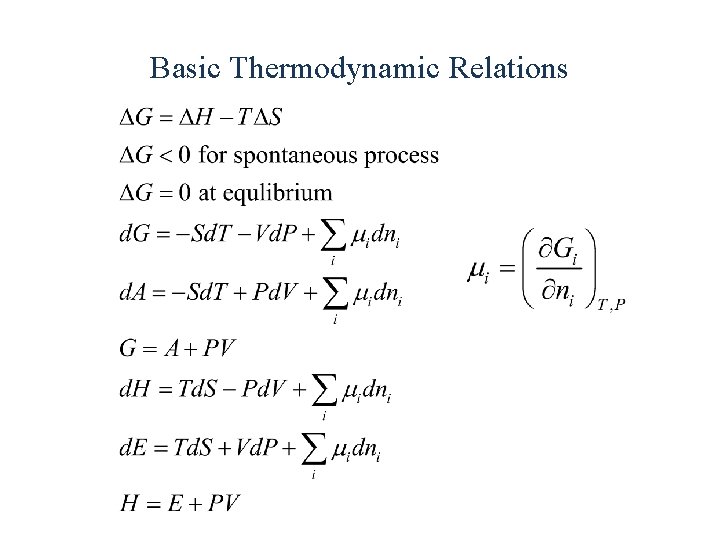
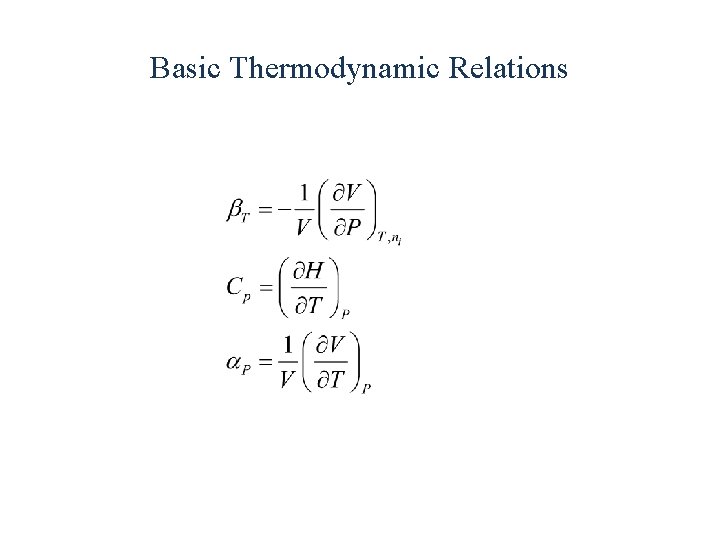
Basic Thermodynamic Relations

Basic Thermodynamic Relations

Things you need to know • • Recognize greek characters SI units / perform unit conversions Logarithms - define and convert between bases Significant figures p. H and Kw definitions Define basic thermodynamic functions E, H, S and G Know the value of ΔG for and equlibrium and spontaneous process. • The relation between ΔG and K

Physical Pharmacy Fall 2011 CHAPTER 1 - SOLIDS

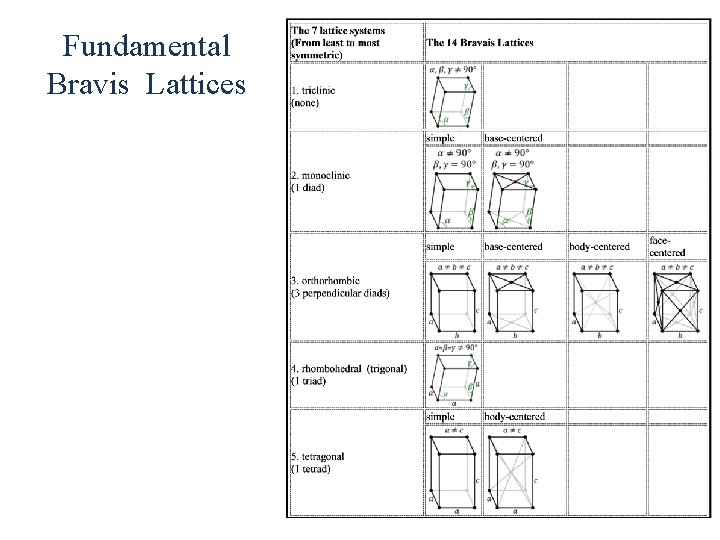
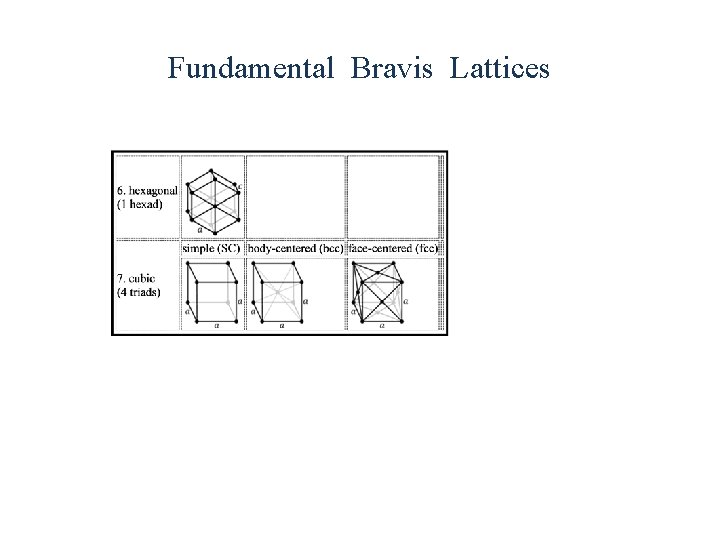
Crystal Structure • All crystalline materials composed of repeating units called unit cells. • There are 7 types of primitive unit cells. Some of these cells can be divided into sub classes bringing the total number of types of cells to 14. • Various planes in the crystal are described by Miller indices

Fundamental Bravis Lattices

Fundamental Bravis Lattices

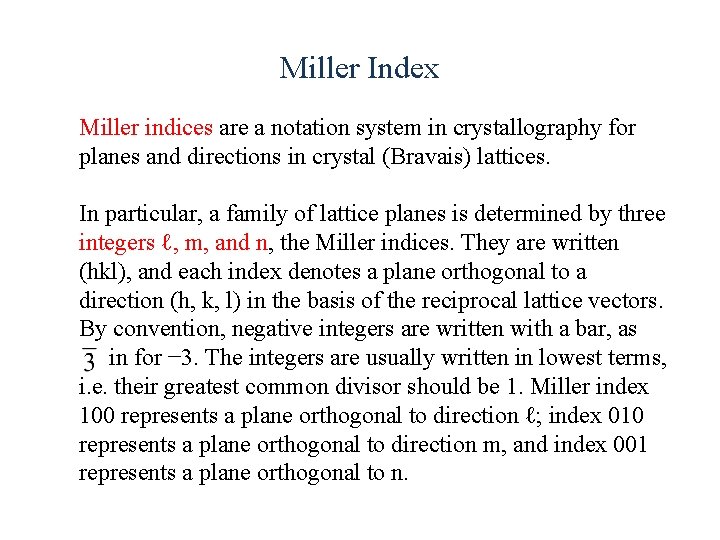
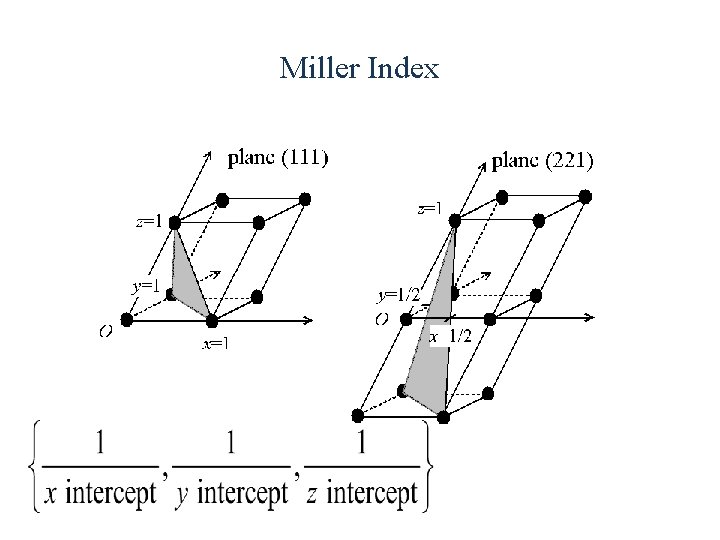
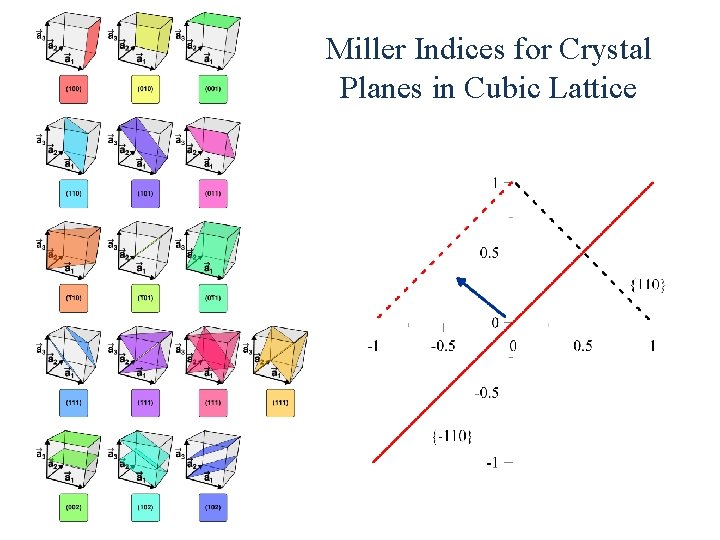
Miller Index Miller indices are a notation system in crystallography for planes and directions in crystal (Bravais) lattices. In particular, a family of lattice planes is determined by three integers ℓ, m, and n, the Miller indices. They are written (hkl), and each index denotes a plane orthogonal to a direction (h, k, l) in the basis of the reciprocal lattice vectors. By convention, negative integers are written with a bar, as in for − 3. The integers are usually written in lowest terms, i. e. their greatest common divisor should be 1. Miller index 100 represents a plane orthogonal to direction ℓ; index 010 represents a plane orthogonal to direction m, and index 001 represents a plane orthogonal to n.

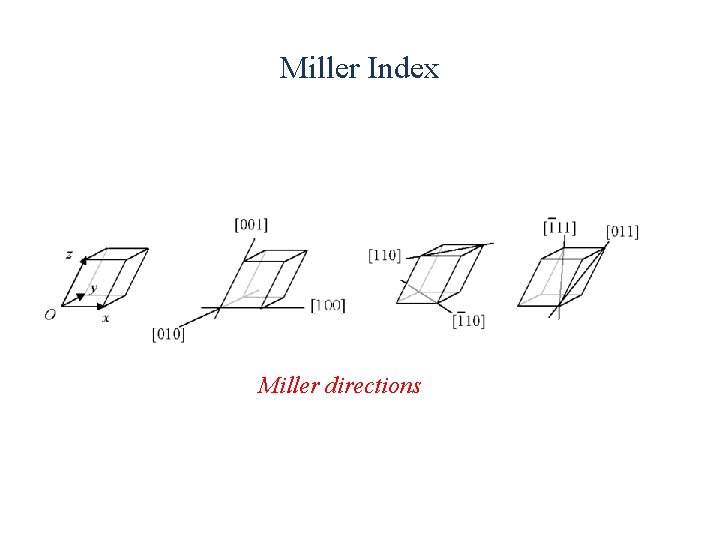
Miller Index Miller directions

Miller Index

Miller Indices for Crystal Planes in Cubic Lattice

Crystal Habit In nature perfect crystals are rare. The faces that develop on a crystal depend on the space available for the crystals to grow. If crystals grow into one another or in a restricted environment, it is possible that no well -formed crystal faces will be developed. However, crystals sometimes develop certain forms more commonly than others, although the symmetry may not be readily apparent from these common forms. The term used to describe general shape of a crystal is habit.

Some Common Crystal Habits Some common crystal habits are as follows. • Cubic - cube shapes • Octahedral - shaped like octahedrons, as described above • Tabular - rectangular shapes. • Equant - a term used to describe minerals that have all of their boundaries of approximately equal length. • Fibrous - elongated clusters of fibers. • Acicular - long, slender crystals. • Prismatic - abundance of prism faces. • Bladed - like a wedge or knife blade • Dendritic - tree-like growths • Botryoidal - smooth bulbous shape

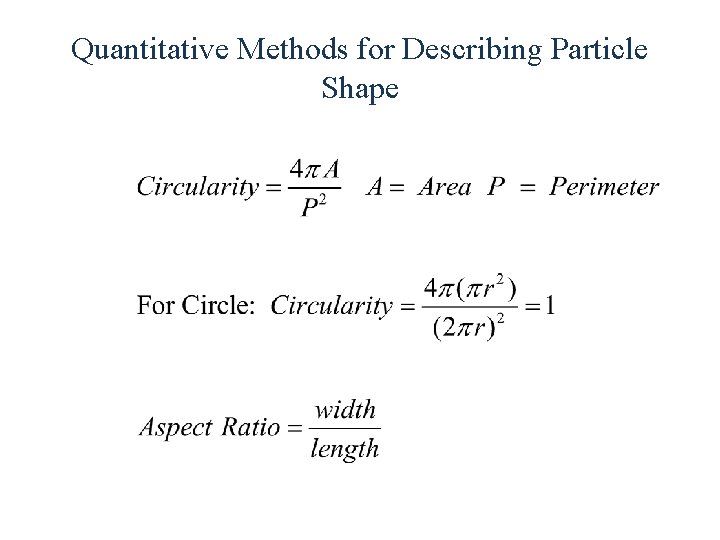
Quantitative Methods for Describing Particle Shape

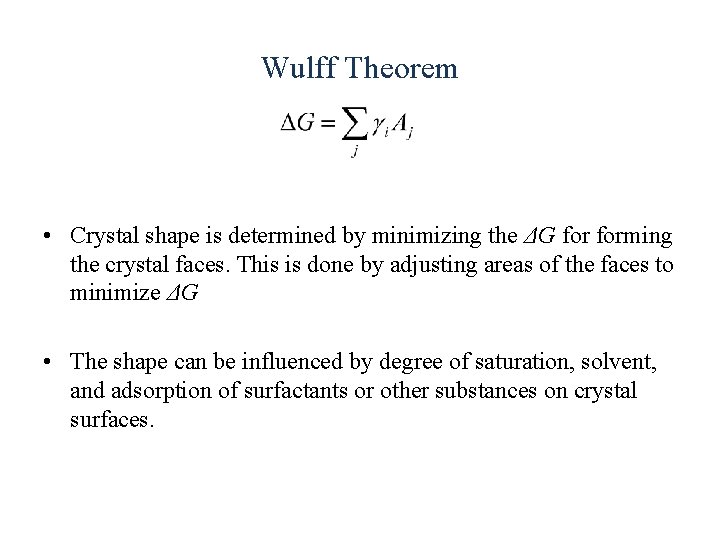
Wulff Theorem • Crystal shape is determined by minimizing the ΔG forming the crystal faces. This is done by adjusting areas of the faces to minimize ΔG • The shape can be influenced by degree of saturation, solvent, and adsorption of surfactants or other substances on crystal surfaces.

What is Particle Size? r The size of a sphere can be described by a single number, r

What is Particle Size? Irregular Particles Size no longer described by single number. Equivalent sphere diameter used to describe size. Equivalent sphere diameters may be based on volume, surface area, mass or linear dimension. Various calculated equivalent diameters are only equal for spheres These diameters differ to a greater degree when the particle shape deviates more from that of a sphere.

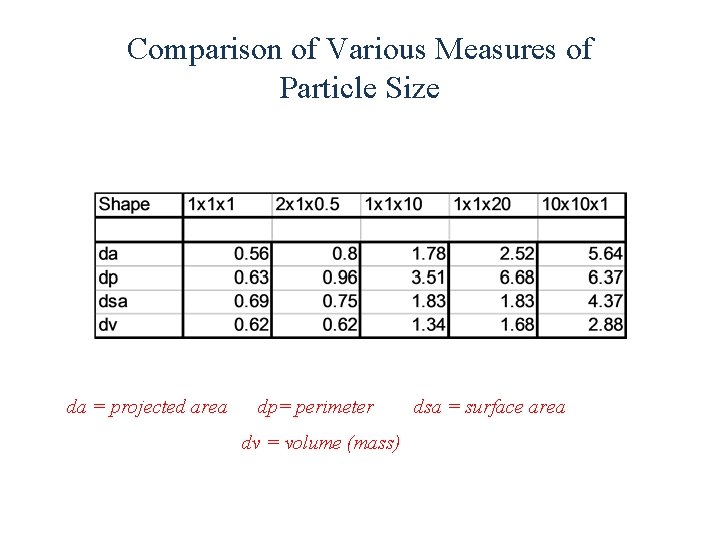
Comparison of Various Measures of Particle Size da = projected area dp= perimeter dv = volume (mass) dsa = surface area

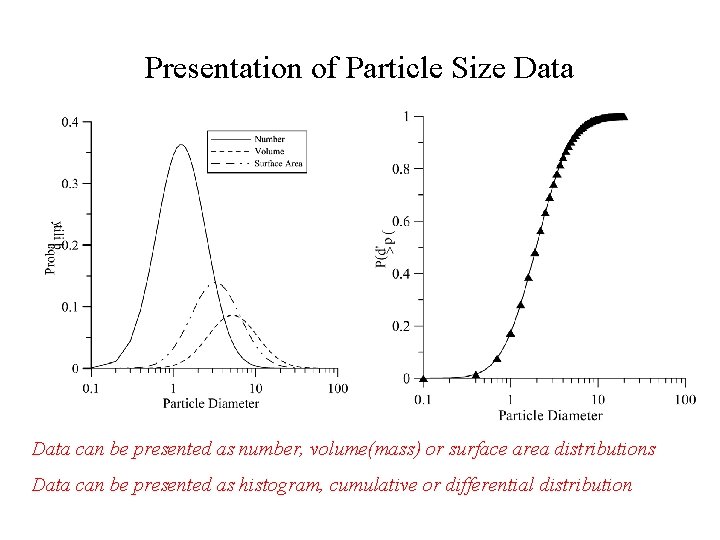
Presentation of Particle Size Data can be presented as number, volume(mass) or surface area distributions Data can be presented as histogram, cumulative or differential distribution

Particle Size Analysis • Particle size is expressed as an equivalent spherical diameter. • There a number of different ways to calculate equivalent diameters each giving a different result. • Particle size distributions may be number, surface area or volume (mass) weighted. • Various methods for determining particle size exist. These are divided into two classes ensemble methods (e. g. sieves, light scattering) and number counting methods ( e. g. microscopy) • When comparing particle sizes the same type of distribution and method must be used.

Pharmaceutical Importance of Particle Size and Shape • Particle size and shape influence a number of parmaceutical processes. – – Powder flow (smaller size worse flow) Aerosolization (dry powder inhalers) Dissolution (small size better) Mixing and blending.

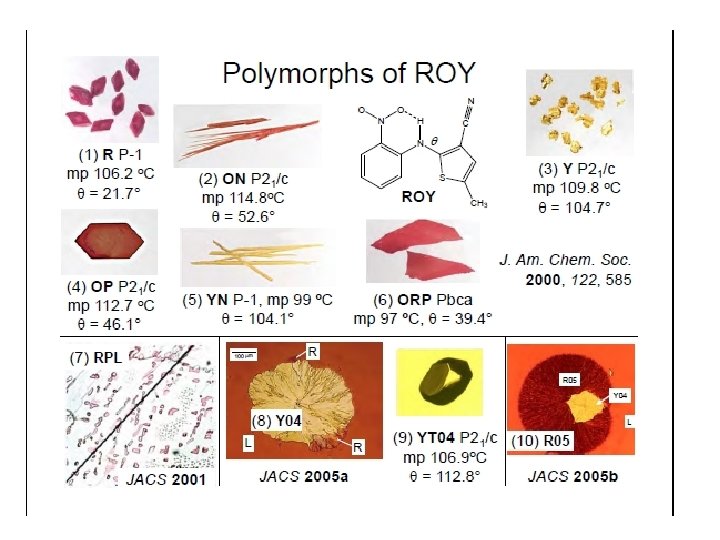
Crystal Forms and Polymorphism – The ability of a solid to exist with more than one crystal structure. (e. g. ROY) Pseudopolymorphs- hydrates or solvates that have their own crystal structure. Allotropes – solid chemical elements which exist in different crystalline forms. ( diamond, graphite and fullerenes are allotropes of carbon)

Crystal Forms and Polymorphism • Other crystal forms – Salts ( often exhibit improved solubility) – co-crystals - crystalline solids composed of at least two components that form a unique crystal structure. Salts differ from cocrystals in the complete proton transfer occurs in the case of salts.


Factors Affecting Which Polymorph is Formed • Various factors affect which polymorph is formed. • These factors include: – – – Choice of solvent Level of supersaturation Presence of impurities Temperature Stirring conditions.

Pharmaceutical Importance of Polymorphism • Polymorphs have different properties including melting point and solubility, dissolution rate, bioavailability and mechanical properties. The most stable polymorph has the lowest solubility. • Upon storage or handling a polymorph may convert to another form. • Polymorphic forms are patentable. • A polymorph initially formed may dissappear and never again be made in a given facility. If this occurs after production starts a product may have to be withdrawn.

Surface Free Energy (Surface Tension) • Surface free energy is the extra free energy resulting from creation of a surface. • When liquids are studied surface free energy is referred to as surface tension.

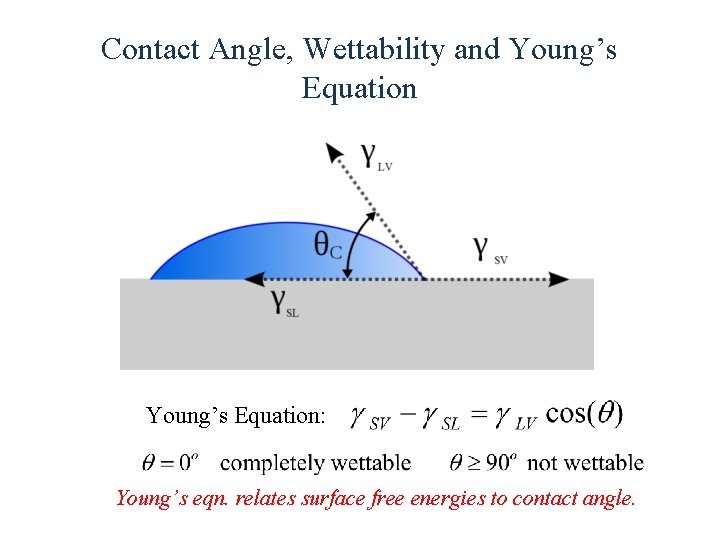
Contact Angle, Wettability and Young’s Equation: Young’s eqn. relates surface free energies to contact angle.

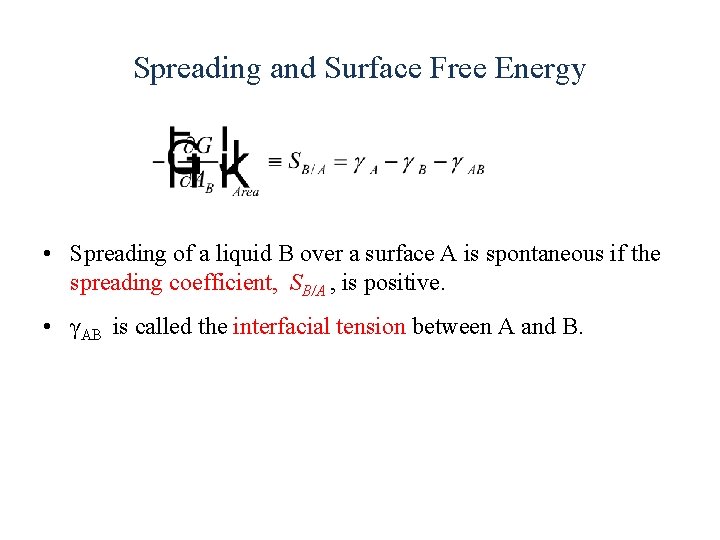
Spreading and Surface Free Energy • Spreading of a liquid B over a surface A is spontaneous if the spreading coefficient, SB/A , is positive. • γAB is called the interfacial tension between A and B.

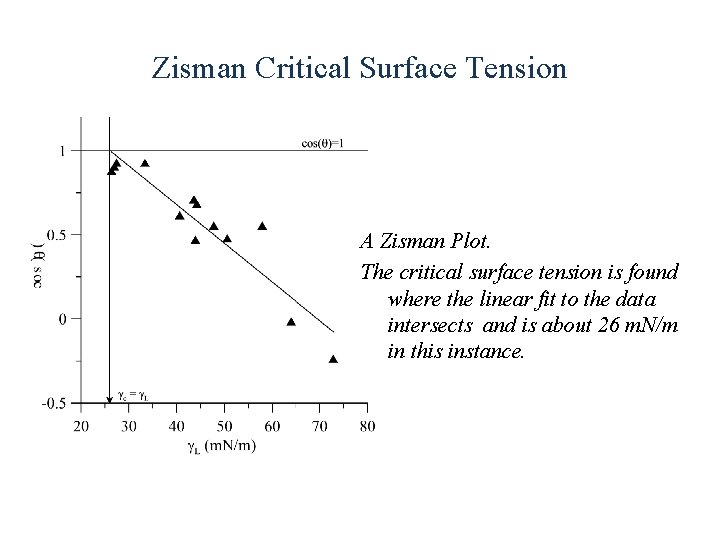
Zisman Critical Surface Tension A Zisman Plot. The critical surface tension is found where the linear fit to the data intersects and is about 26 m. N/m in this instance.

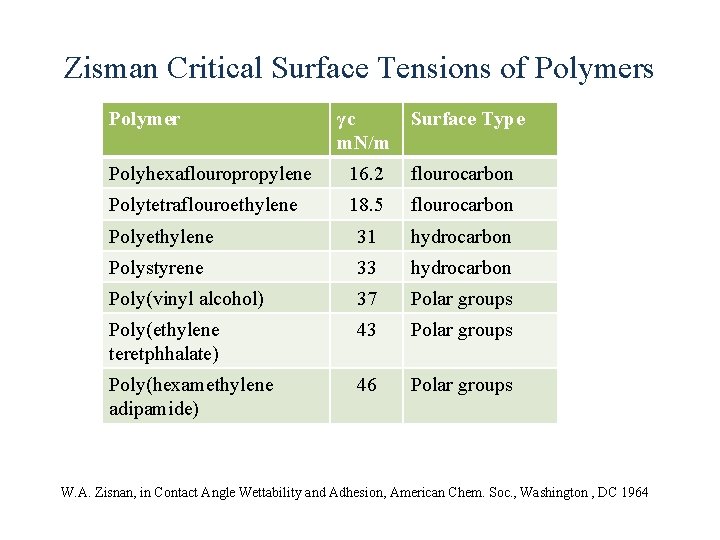
Zisman Critical Surface Tensions of Polymers Polymer γc m. N/m Surface Type Polyhexaflouropropylene 16. 2 flourocarbon Polytetraflouroethylene 18. 5 flourocarbon Polyethylene 31 hydrocarbon Polystyrene 33 hydrocarbon Poly(vinyl alcohol) 37 Polar groups Poly(ethylene teretphhalate) 43 Polar groups Poly(hexamethylene adipamide) 46 Polar groups W. A. Zisnan, in Contact Angle Wettability and Adhesion, American Chem. Soc. , Washington , DC 1964

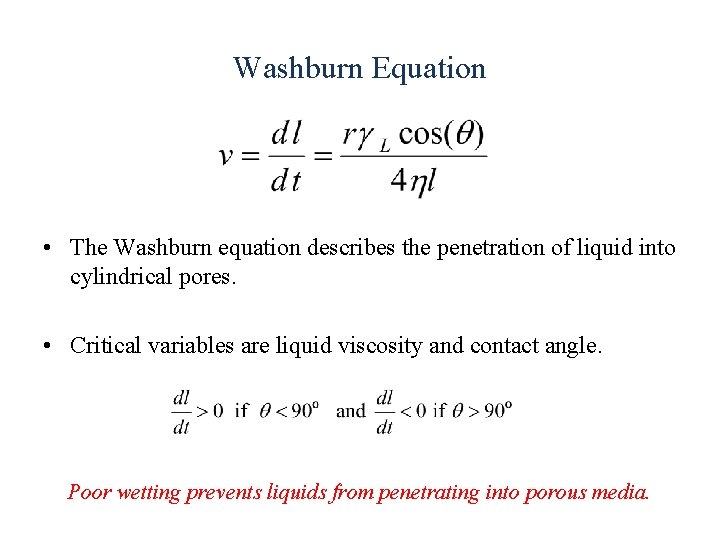
Washburn Equation • The Washburn equation describes the penetration of liquid into cylindrical pores. • Critical variables are liquid viscosity and contact angle. Poor wetting prevents liquids from penetrating into porous media.

Washburn Equation High contact angle thus no liquid penetration Water Drop Hydrophobic Sand

Some Pharmaceutical Consequences of Wetting • Good wetting is required for dispersion of powders in liquid media and for the penetration of liquid into tablets. • Wetting problems can often be solved by the inclusion of surfactants into formulations. • Surface free energies of particles along with the mechanical properties of the particles determines the hardness of tablets. • Adhesion of powders is in part influenced by surface free energy.

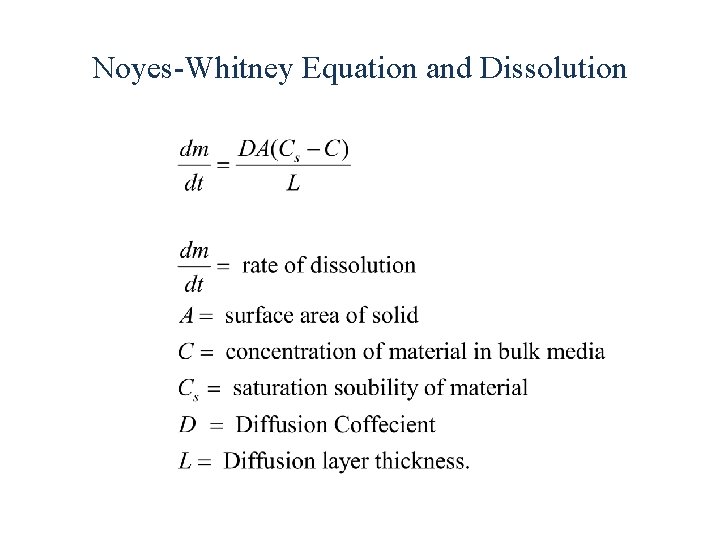
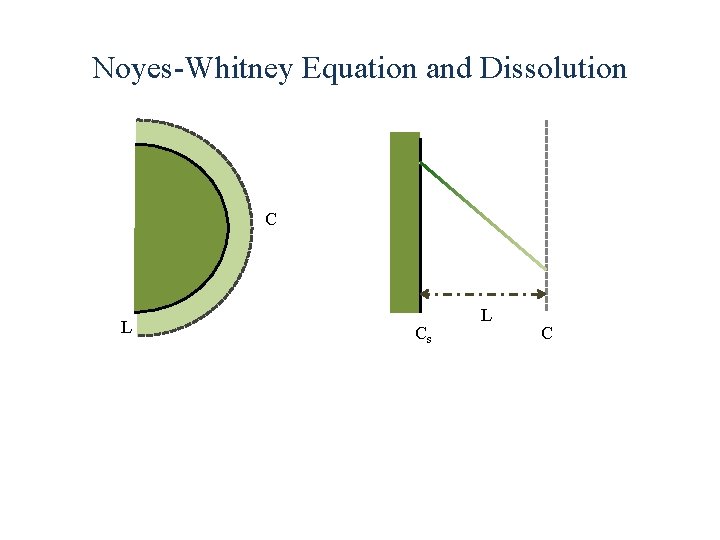
Noyes-Whitney Equation and Dissolution

Noyes-Whitney Equation and Dissolution C L Cs L C

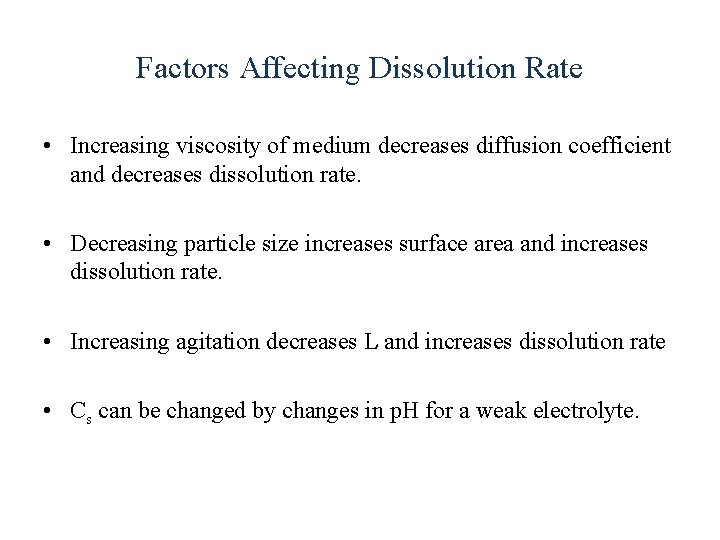
Factors Affecting Dissolution Rate • Increasing viscosity of medium decreases diffusion coefficient and decreases dissolution rate. • Decreasing particle size increases surface area and increases dissolution rate. • Increasing agitation decreases L and increases dissolution rate • Cs can be changed by changes in p. H for a weak electrolyte.

What you need to know • • • Recognize 7 types of unit cells. Miller indices – identify crystal planes for cubic lattice Crystal habit –factors affecting Particle size – volume and number weighting. Crystal forms –polymorphs, pseudopolymorphs, allotropes, salts, co-crystals • Contact angle – Zisman critical surface tension, Washburn equation. Wulff theorem • Noyes-Whitney equation – factors affecting dissolution rate.