CH 2 Pharmacy Procedures and Dosage Calculations PHARMACY















- Slides: 21

CH 2 Pharmacy Procedures and Dosage Calculations

PHARMACY VS. PHARMACOLOGY • ____________: the study of how drugs work • ____________: the art and science of preparing and dispensing medications • LVTs must know how to prepare and administer their patient’s medications and be aware of the effects that the medication will have on the body.

HOW TO AVOID PHARMACY DISASTERS: • The drug order or prescription has to come from a ______. • The order or prescription has to be ________ and interpreted correctly. • The LVT must correctly __________ the patient’s dose and dispense it in the correct dosage form. This includes dispensing the proper amount of medication to last the duration of treatment. • The LVT must administer the correct drug, via the correct route, to the correct patient, at the correct interval. • The LVT must be able to instruct the _________ on how to administer the medication.

DRUG ORDER VS. PRESCRIPTION • _______________: a request by a veterinarian to dispense or administer a drug within a hospital – May be verbal or written, but should always be noted in the patient’s chart – “Send Chili home with 500 mg Cephalexin to take by mouth twice daily for 14 days. ” In Chili’s chart under today’s date write: Cephalexin 500 mg PO BID x 14 d

DRUG ORDER VS. PRESCRIPTION • ______________ _: a drug order sent from a veterinarian to a pharmacy to be filled by a pharmacist – It is illegal to fill a prescription from another veterinary practice. – A prescription is a legal document. – In addition to the info that must be included on labeled dispensed medication, prescriptions must also include Rx, sig, and the veterinarian’s signature

CONTROLLED SUBSTANCES • AKA ____________ drugs • Drugs that have potential for physical addiction, psychological addiction, and/or abuse • Regulated by the ______. • The original container must have a capital ______ and ______________________ indicating its schedule • A veterinarian must be registered with the DEA to purchase, dispense, or prescribe controlled substances. – Registration # must be on all CS prescriptions and order forms. – Registration is valid for three years

CONTROLLED SUBSTANCE RESTRICTIONS • C-______ drugs cannot be prescribed to animals. • C-______ drugs must have written prescriptions, a special form is required, and the script cannot include refills. • Controlled substances must be dispensed in child-proof containers that read “Caution: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed. ” • Expired or damaged controlled substances should be disposed of via “reverse distributors”.

CONTROLLED SUBSTANCES §C-I: extreme potential for abuse, no approved veterinary purpose (heroin, LSD, marijuana, ecstasy) §C-II: high potential for abuse/dependence (hydrocodone, cocaine, morphine, pentobarbital, Ritalin, Adderall, Demerol, Oxy. Contin) §C-III: some potential for abuse/low moderate dependence (ketamine, Tylenol with codeine, anabolic steroids) §C-IV: low potential for abuse/limited dependence (butorphanol, Valium, Tramadol, Ambien, Xanax) §C-V: lowest potential for abuse. Antitussives and Antidiarrheals (Lomotil, Robitussin AC, Lyrica)

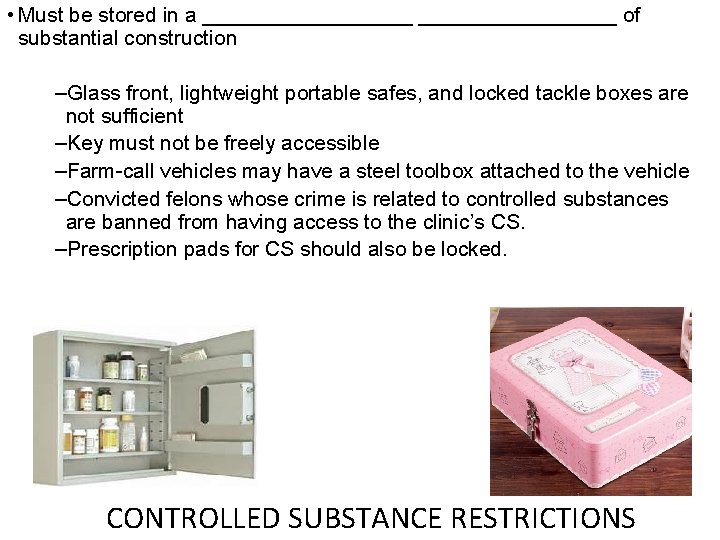
• Must be stored in a _________ of substantial construction –Glass front, lightweight portable safes, and locked tackle boxes are not sufficient –Key must not be freely accessible –Farm-call vehicles may have a steel toolbox attached to the vehicle –Convicted felons whose crime is related to controlled substances are banned from having access to the clinic’s CS. –Prescription pads for CS should also be locked. CONTROLLED SUBSTANCE RESTRICTIONS

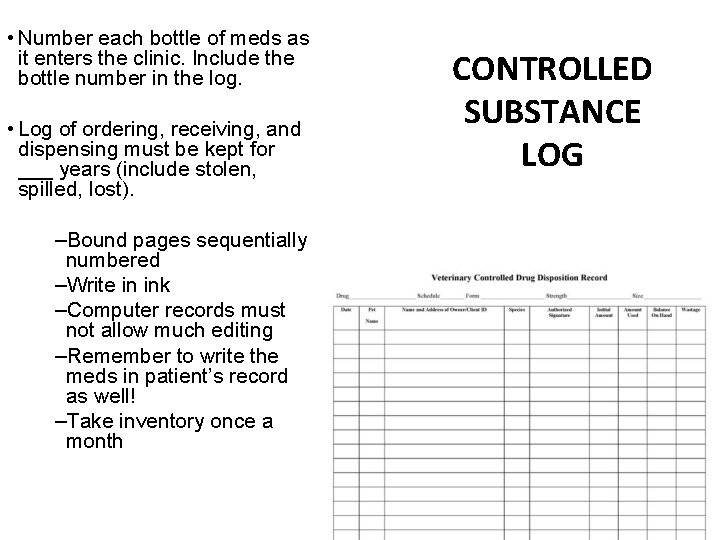
• Number each bottle of meds as it enters the clinic. Include the bottle number in the log. • Log of ordering, receiving, and dispensing must be kept for ___ years (include stolen, spilled, lost). –Bound pages sequentially numbered –Write in ink –Computer records must not allow much editing –Remember to write the meds in patient’s record as well! –Take inventory once a month CONTROLLED SUBSTANCE LOG

Dose vs Dosage §DOSAGE is used to determine the DOSE. §________= mass of drug needed per unit of weight of animal. § 15 mg/kg, 10 g/lb § A range is often listed, such as 20 -50 mg/kg §________= amount of drug administered to a patient at one time. Stated in units of mass (mg, g, gr, etc. ), NOT tablets or milliliters. § 50 mg

Dosage continued • Dosage _________: mass + route + interval + duration • Dosage _________: is the physical form of the drug to be administered. • Dose _________: time between doses or number of doses per day

SYSTEMS OF MEASUREMENT • ___________ – Gram, meter, liter • ___________ – Teaspoon, tablespoon, cup, pint, gallon, pound • ___________ – Grain, dram, minim

COMPOUNDING §DEFINITION: ___________________________________________ §Examples: §Flavoring to improve palatability §Diluting drugs with saline §Crushing a tablet into a liquid §Mixing drugs together in a syringe or vial

AMDUCA rules of compounding • • • A valid VCPR must exist The animal’s health or life is threatened without compounding No FDA approved drug exists in the desired formulation Can only be done by using FDA-approved drugs Can only be done by a veterinarian or pharmacist The compounded drug must meet safety and efficacy standards A new withdrawal time must be established if the compounded drug is given to food animals Cannot put the general public at risk Drug must be fully labeled Cannot be made for anyone outside the practice Records must be maintained Must be done for a specific patient, as needed

Labels from veterinary hospitals should contain: • Name of prescribing veterinarian • Name, address, and phone number of clinic • Name of patient or ID of patient with and client’s last name • Drug name, concentration, and number of units dispensed • Date (also thorough to have expiration date) • Refills • Dose, frequency, route of administration, duration of treatment • Cautionary statements • Withdrawal or discard times (food animals)

Containers and Storage §Childproof containers vs. Pill envelopes §Temperature of storage environment must be observed § Cold: below 46 F § Cool: 46 - 59 F § Room Temp 59 - 86 F § Warm 86 - 104 F § Excessive Heat: above 104 F §Amber bottles §Silica packets §Reconstituted meds and bacterial growth

CYTOTOXIC AND HAZARDOUS DRUGS § Cytotoxic drugs- antineoplastic and antifungal drugs that destroy mammalian cells § ____________/___________ effects- birth defects (not only in the patient, but also in the person dispensing the drug) § _______________ effects- cancerous or preneoplastic changes § OSHA has guidelines for the safe use, storage, and disposal of these drugs

Cytotoxic drug exposure routes: • Absorption through the skin when drug spills/drips • Inhalation of aerosolized drug when needle is removed from pressurized bottle, when tablet is being crushed/broken, or when ampules are broken. • Ingestion of food contaminated with the drug • REMEMBER: • Store your lunch in a refrigerator that only contains food! • Don‘t place lunch on a counter where meds are placed. • Wash your hands! • Every hazardous material should have an MSDS, package insert, and a hospital policy procedure sheet for spills and disposal of equipment.

Cytotoxic drug safety §Properly train all staff on the safe handling and storage of hazardous drugs §Store cytotoxic drugs separately from other drugs and clearly label them. §Prepare the drug just prior to administration in a low traffic, well-ventilated area. §Wear protective gear: mask (not surgical), gloves (multiple pairs if latex), gown with long sleeves and cuffs, goggles.

Cytotoxic drug safety § Use screw-on syringes and IV lines. §Recheck calculations. §Insure catheter placement is correct. §Place all equipment in sealable plastic bags immediately after use and into a leak and puncture proof hazardous waste container. §Clean up properly after use. Do not allow maintenance staff to handle cleaning § Chemotherapy spill kits are available