Presentation on Practicals in Physical Pharmaceutics I For













- Slides: 34

Presentation on Practicals in Physical Pharmaceutics - I For Second year B. Pharm. (Sem III) Prepared by Prof. Leena K. Sawant JSPM’s Jayawantrao Sawant College of Pharmacy and Research, Hadapsar, Pune -28

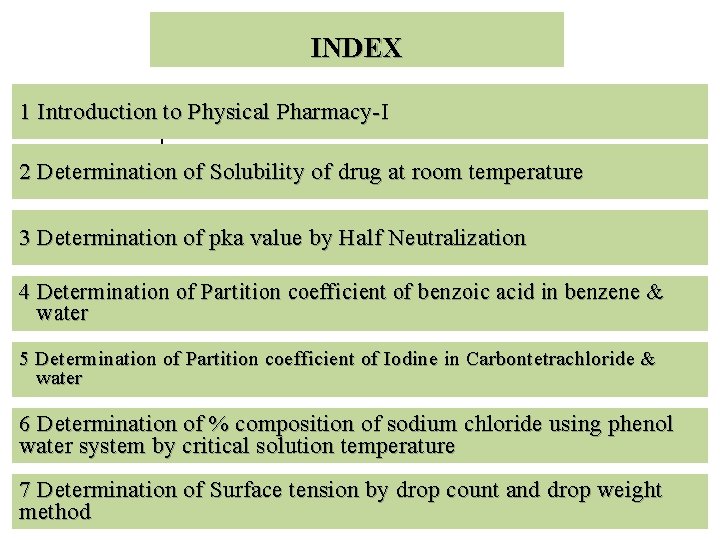
INDEX 1 Introduction to Physical Pharmacy-I 1 2 Determination of Solubility of drug at room temperature 3 Determination of pka value by Half Neutralization 4 Determination of Partition coefficient of benzoic acid in benzene & water 5 Determination of Partition coefficient of Iodine in Carbontetrachloride & water 6 Determination of % composition of sodium chloride using phenol water system by critical solution temperature 7 Determination of Surface tension by drop count and drop weight method

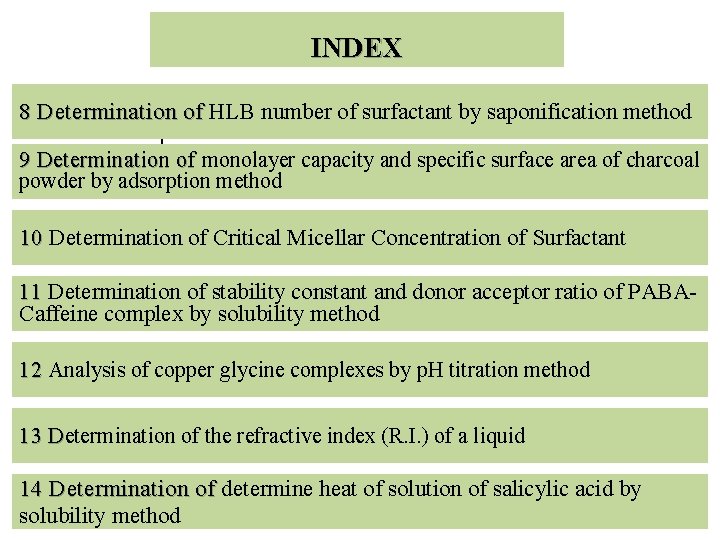
INDEX 8 Determination of HLB number of surfactant by saponification method 8 Determination of 1 9 Determination of monolayer capacity and specific surface area of charcoal 9 Determination of powder by adsorption method 10 Determination of Critical Micellar Concentration of Surfactant 10 11 Determination of stability constant and donor acceptor ratio of PABACaffeine complex by solubility method 12 Analysis of copper glycine complexes by p. H titration method 12 13 Determination of the refractive index (R. I. ) of a liquid 13 D 14 Determination of determine heat of solution of salicylic acid by 14 Determination of solubility method

1 Introduction Ø Physical pharmacy integrates knowledge of mathematics, physics and chemistry and applies them to the pharmaceutical dosage form development. Ø It is a fundamental course that leads to proper understanding of subsequent courses in Pharmaceutics and pharmaceutical technology needed for dosage form design. Ø It focuses on theories behind the phenomena needed for dosage form design. It covers many principle and mathematical models that are useful to understand processes from synthesis of molecules to its formulation. Ø These principles are helpful in studying stability of molecules and its formulation at different conditions as well as for knowing fate of a drug in body after administration. Ø It enables the pharmacist to make rational decisions on scientific basis concerning the art and technology of solutions, suspensions, emulsions, etc. Ø It provides the basis for understanding the chemical and physical phenomena that govern the in vivo and in vitro actions of pharmaceutical products.

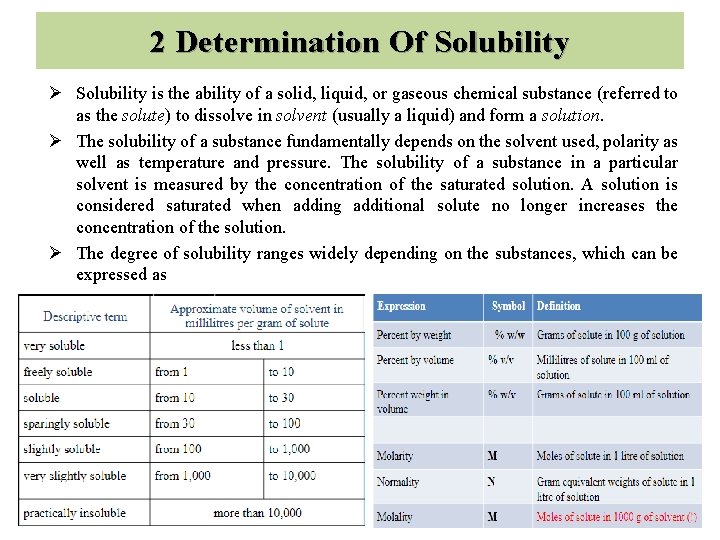
2 Determination Of Solubility Ø Solubility is the ability of a solid, liquid, or gaseous chemical substance (referred to as the solute) to dissolve in solvent (usually a liquid) and form a solution. Ø The solubility of a substance fundamentally depends on the solvent used, polarity as well as temperature and pressure. The solubility of a substance in a particular solvent is measured by the concentration of the saturated solution. A solution is considered saturated when adding additional solute no longer increases the concentration of the solution. Ø The degree of solubility ranges widely depending on the substances, which can be expressed as

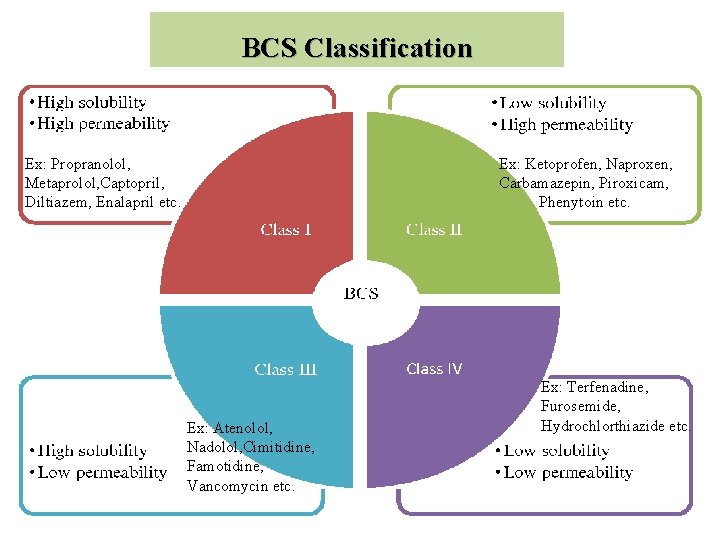
BCS Classification Ex: Propranolol, Metaprolol, Captopril, Diltiazem, Enalapril etc. Ex: Ketoprofen, Naproxen, Carbamazepin, Piroxicam, Phenytoin etc. Ex: Atenolol, Nadolol, Cimitidine, Famotidine, Vancomycin etc. Ex: Terfenadine, Furosemide, Hydrochlorthiazide etc.

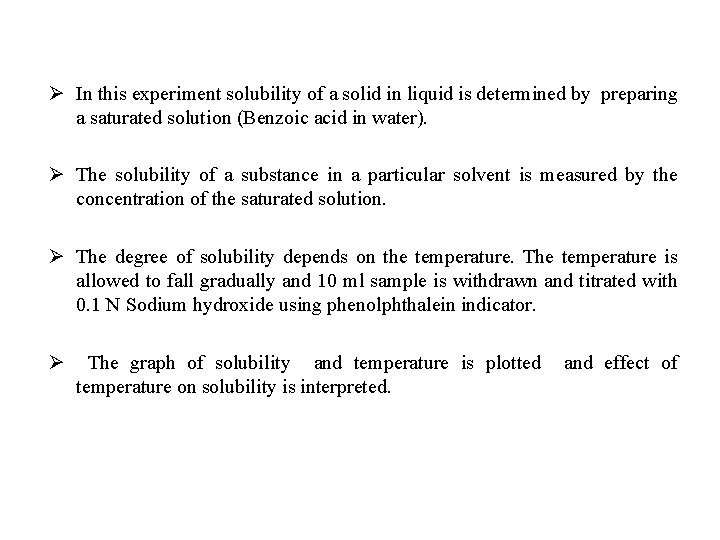
Ø In this experiment solubility of a solid in liquid is determined by preparing a saturated solution (Benzoic acid in water). Ø The solubility of a substance in a particular solvent is measured by the concentration of the saturated solution. Ø The degree of solubility depends on the temperature. The temperature is allowed to fall gradually and 10 ml sample is withdrawn and titrated with 0. 1 N Sodium hydroxide using phenolphthalein indicator. Ø The graph of solubility and temperature is plotted and effect of temperature on solubility is interpreted.

2 Determination of p. Ka Ø p. Ka is known as acid dissociation constant, which is a measure of strength of an acid. When a weak acid, HA, is dissociated in water, the following equilibrium is setup. HA ⇌ H+ + A- where, HA is the weak acid, H+ is the hydrogen ion and A- is the conjugated base. The equilibrium constant Ka for this reaction is called dissociation constant of the acid which is given as, Ka = The concentration of hydrogen ion, [H+], is determined directly by measuring the p. H. Unfortunately, calculating [HA] and [A-] is not as straight forwards. Ø The Henderson-Hasselbalch equation: p. H = p. Ka + log salt/acid ( where p. Ka = - log. Ka ), Ø A convenient method for determining Ka is to measure the p. H of a solution of the acid after a strong base has been added to half neutralize it. Thus, at half neutralization (half equivalence point), the concentration of salt is equal to acid, therefore log ( [conjugatebase] / [weakacid] ) = 1. . . . ( log 1 is zero), hence Ø p. H = p. Ka Ø The p. H at the half neutralization of a weak acid can be determined by measuring the p. H with a p. H meter.

Ø In this experiment, p. Ka of weak acid like acetic acid is determined by using p. H meter Ø The p. H of acetic acid is measured. Gradually add sodium hydroxide in increasing concentration and measure the p. H. Ø At equivalence point there is sudden rise in p. H as all the acetic acid molecules are neutralized by sodium hydroxide and any further addition of sodium hydroxide causes sharp increase in p. H. This is called as half neutralization where p. H = p. Ka. Ø The graph of Volume of Na. OH added and p. H is plotted and p. Ka is determined

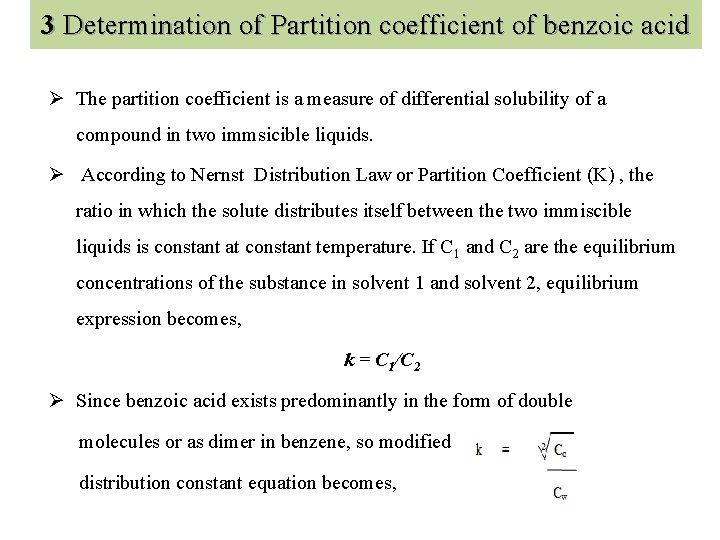
3 Determination of Partition coefficient of benzoic acid Ø The partition coefficient is a measure of differential solubility of a compound in two immsicible liquids. Ø According to Nernst Distribution Law or Partition Coefficient (K) , the ratio in which the solute distributes itself between the two immiscible liquids is constant at constant temperature. If C 1 and C 2 are the equilibrium concentrations of the substance in solvent 1 and solvent 2, equilibrium expression becomes, k = C 1/C 2 Ø Since benzoic acid exists predominantly in the form of double molecules or as dimer in benzene, so modified distribution constant equation becomes,

Ø In this experiment, partition coefficient of benzoic acid is determined by mixing different concentration with benzene and water and allowing it to stand for 30 mins. Ø The contents are transferred to separating funnel an allowed to separate into 2 layers. Ø 10 ml each of aqueous and organic are separated and titrated with sodium hydroxide. Ø The partition coefficient of benzoic acid is determined by using above mentioned formula

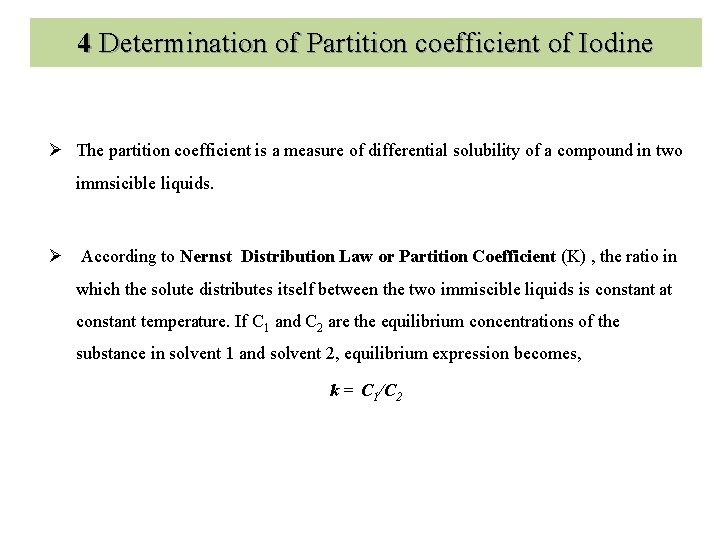
4 Determination of Partition coefficient of Iodine Ø The partition coefficient is a measure of differential solubility of a compound in two immsicible liquids. Ø According to Nernst Distribution Law or Partition Coefficient (K) , the ratio in which the solute distributes itself between the two immiscible liquids is constant at constant temperature. If C 1 and C 2 are the equilibrium concentrations of the substance in solvent 1 and solvent 2, equilibrium expression becomes, k = C 1/C 2

Ø In this experiment, partition coefficient of Iodine is determined by mixing different concentration with carbon tetrachloride and water and allowing it to stand for 30 mins. Ø The contents are allowed to separate into 2 layers in the Iodine flask. Ø 10 ml each of aqueous and organic are separated and titrated with sodium thiosulphate using starch as indicator Ø The partition coefficient of Iodine is determined by using formula k = Corg / Caq

6 Determine effect of electrolyte on CST (critical solution temperature) of phenol water system Ø The temperature at which complete miscibility is reached as the temperature is raised or in some cases lowered —of two liquids that are partially miscible under ordinary conditions is called as critical solution temperature. Ø Phenol is also soluble in water to some extent. It is due to its ability to form hydrogen bonding with water molecules. However the large part of phenol molecule is phenyl group that is non polar and hence its solubility is limited in water. Ø The concentration of phenol and water at which this occurs is 11% by weight of phenol in water. At 68. 5ºC, this is the upper consolute temperature which is the maximum temperature at which the two-phase region exists

Ø In this experiment, CST is determined by preparing different concentration of mixture of phenol and water and increasing temperature and noting the temperature at which the two immiscible liquids are completely miscible. Ø The temperature is allowed to fall till the two layers are separated Ø The procedure is repeated for different concentration of mixtures prepared and effect of sodium chloride on CST is seen Ø The graph of % of phenol and water and Temperature is plotted and CST is determined

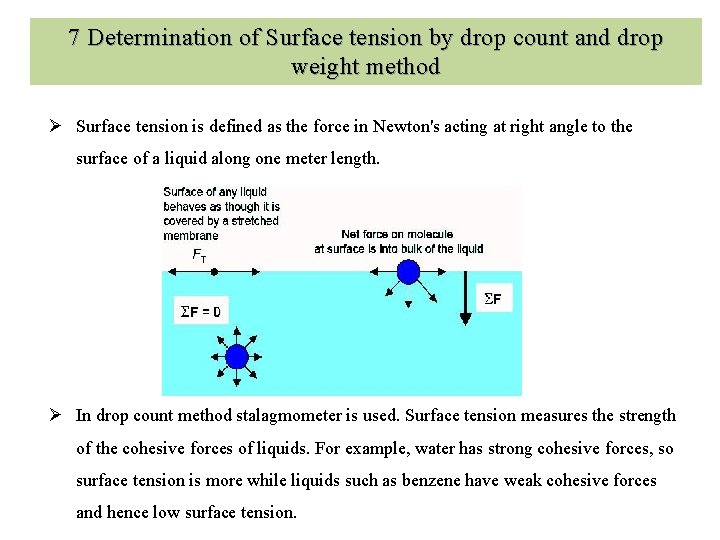
7 Determination of Surface tension by drop count and drop weight method Ø Surface tension is defined as the force in Newton's acting at right angle to the surface of a liquid along one meter length. Ø In drop count method stalagmometer is used. Surface tension measures the strength of the cohesive forces of liquids. For example, water has strong cohesive forces, so surface tension is more while liquids such as benzene have weak cohesive forces and hence low surface tension.

Ø The lower the surface tension of the liquid, the smaller the size of drops formed. Then more number of drops are formed for the same volume of a liquid. Hence, simply counting the number of drops for an unknown liquid and water is sufficient to calculate surface tension. Ø The drop weight method is based on the principle that the weight of liquid falling from a capillary tube is approximately proportional to the surface tension of the liquid.

Ø In this experiment , stalagmometer is filled with purified water and the number of drops falling down between two markings are counted Ø Density of liquid sample is determined using pycnometer Ø Surface tension can be calculated using following formula Ø Surface tension of liquid= Ƴl = (dl/dw)×(nw/nl)× Ƴw Ƴl = Surface tension of sample liquid Ƴw = Surface tension of water dl = Density of liquid sample dw = Density of water nw = Number of drops of water nl = Number of drops of liquid sample

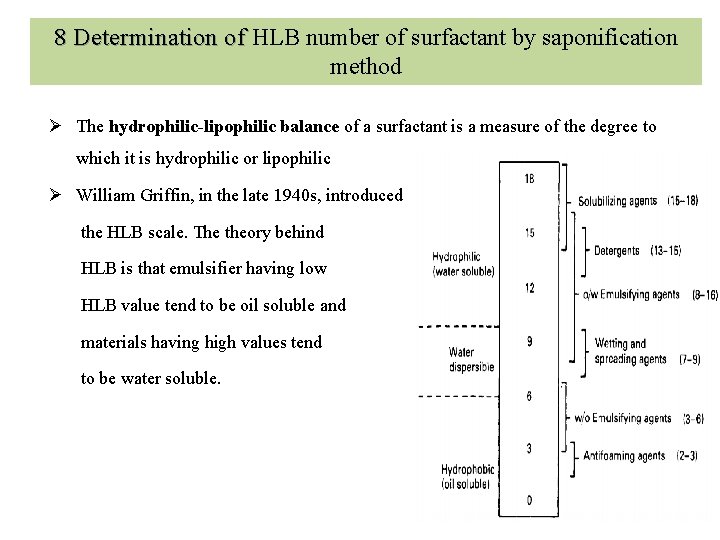
8 Determination of HLB number of surfactant by saponification 8 Determination of method Ø The hydrophilic-lipophilic balance of a surfactant is a measure of the degree to which it is hydrophilic or lipophilic Ø William Griffin, in the late 1940 s, introduced the HLB scale. The theory behind HLB is that emulsifier having low HLB value tend to be oil soluble and materials having high values tend to be water soluble.

Ø In this experiment , Saponification number and acid number was first determined and the HLB was calculated using the formula Ø HLB = 20 X (1 -S/A) where, S is Saponification number A is acid number

9 Determine monolayer capacity and specific surface area of charcoal powder by adsorption method • Adsorption is a surface phenoemenon in which adsorbate is adhered to the surface of adsorbent. When a solution of acetic acid in water is shaken in presence of charcoal, a part of acetic acid is adsorbed on charcoal. As a result, the concentration of acetic acid in solution is decreased. The quantitative relationship between the amount of adsorbate adsorbed at equilibrium pressure and at constant temperature is referred to as an adsorption isotherm. Freundlich adsorption equation: • • • Y = w/m = w is concentration of acetic acid adsorbed m is weight of adsorbent used p is equilibrium pressure k & b are constants • • •

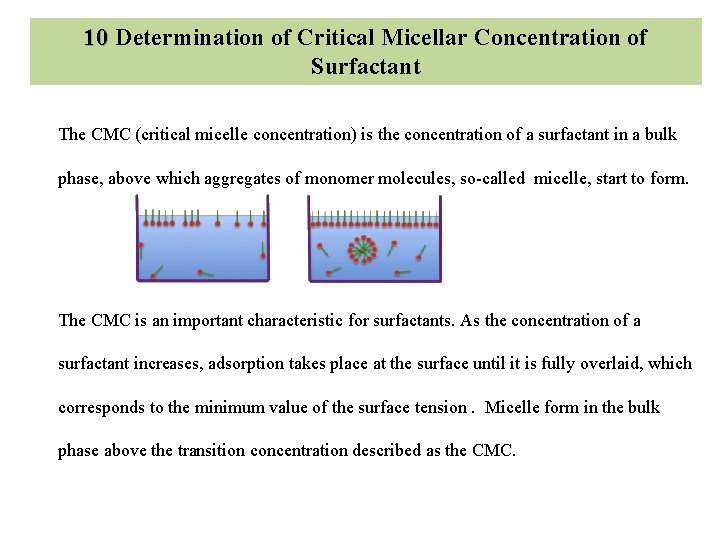
Ø In this experiment , five different mixtures of different concentration of acetic acid and water is prepared and 1 gm charcoal powder is added to each and kept in constant temperature water bath for 1 hour. Ø 10 ml sample is withdrawn from each mixture and titrated with 0. 1 N sodium hydroxide and concentration of acetic acid adsorbed on charcoal is calculated.

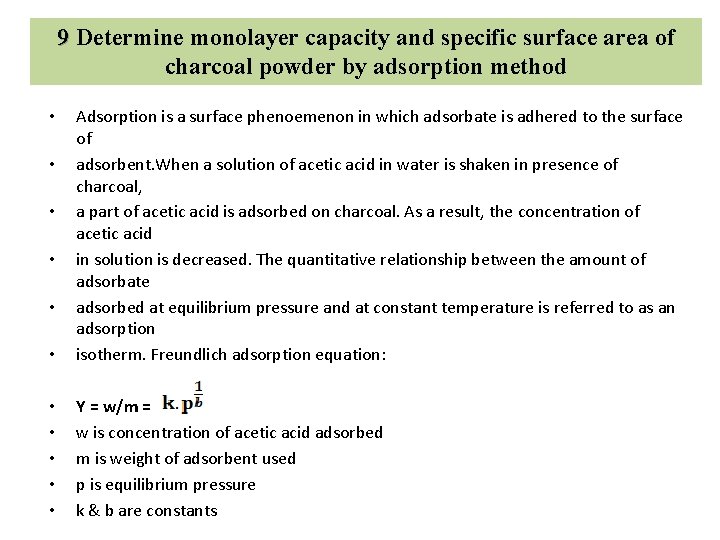
10 Determination of Critical Micellar Concentration of Surfactant The CMC (critical micelle concentration) is the concentration of a surfactant in a bulk phase, above which aggregates of monomer molecules, so-called micelle, start to form. The CMC is an important characteristic for surfactants. As the concentration of a surfactant increases, adsorption takes place at the surface until it is fully overlaid, which corresponds to the minimum value of the surface tension. Micelle form in the bulk phase above the transition concentration described as the CMC.

Ø In this experiment , five different mixtures of different concentration of acetic acid and water is prepared and 1 gm charcoal powder is added to each and kept in constant temperature water bath for 1 hour. Ø 10 ml sample is withdrawn from each mixture and titrated with 0. 1 N sodium hydroxide and concentration of acetic acid adsorbed on charcoal is calculated.

Applications of CMC Ø Improve solubility of poorly soluble drug (example: Pacletaxel, Docetaxel, Griseofulvin) Ø Protection of drug from degradation hydrolysis Ø Targeted drug delivery (example: Doxorubicin to tumor) Ø Polymeric micelle in Diagnostic imaging Ø Improve stability

11 Determination of stability constant and donor acceptor ratio of PABA-Caffeine complex by solubility method Complexes or coordination compounds, result from donor acceptor mechanism or Lewis acid-base reaction between two or more different chemical constituents. Any non-metallic atom or ion whether free or contained in an ionic compound, that can donate an electron pair can serve as the donor. The acceptor or constituents that accepts a share in the pair of electrons is usually a metallic ion or a neutral atom

Ø In this experiment , different concentrations of caffiene are prepared and equal quantity of PABA is added to each flask and shaken for a period of 30 minutes and filtered through whatman filter paper. Ø 10 ml sample is withdrawn from each flask and titrated with 0. 025 N sodium hydroxide using phenolphthalein indicator Ø The phase solubility graph is plotted by taking concentration of caffeine on X axis and concentration of PABA on Y axis. Then calculate the Stoichiometric ratio and Complex stability constant by using formula Ø Stoichoimetric ratio = [Caffiene in complex] / [PABA in complex Ø Stability constant = [PABA - Caffiene] / [PABA] [Caffiene]

12 Analysis of copper glycine complexes by p. H titration method Ø This is one of the most reliable methods and can be used whenever the complexation is effected by a change in p. H. For example the chelation of the cupric ion by glycine molecules can be represented by following equation: Ø Cu 2+ + 2 NH 3 + 2 CH 2 COO- Cu(NH 2 COO-)2 + 2 H+ Ø Since two protons are formed in the reaction , the addition of glycine to a solution containing cupric ions should result in a decrease in p. H. Therefore, p. H titration method is suitable for the analysis of these complexes. titration curves can be obtained by adding a strong base to a solution of glycine and to another solution containing glycine and a copper salt and plotting the p. H against the equivalents of base added. The curve for the metal glycine mixture is well below that for the glycine alone and the decrease in p. H shows that complexation is occuring throughout most of the neutralisation range.

Ø In this experiment , 75 ml of glycine hydrochloride solution is taken into a beaker and the p. H is measured. Ø 0. 25 N Na. OH is added gradually to this glycine solution and the p. H is measured Ø Then 75 ml of complex solution (i. e. glycine hydrochloride and cupric chloride) is taken into a beaker and the p. H is measured. Ø 0. 25 N Na. OH is added gradually to this complex solution and the p. H is measured Ø Identify the range where in sudden increase in p. H is obtained in the complex solution. (For example, sudden increase in p. H is observed between 5 to 6 ml) Ø The graph of the volume of sodium hydroxide added on x-axis and p. H on the y-axis is plotted

13 Determination of the refractive index (R. I. ) of a liquid 13 D Ø When a ray of light passes from one medium to another, it shows refraction i. e change of direction. If it passes from a rarer to a denser medium, it bends towards the normal. The angle of incidence (i) is more than angle of refraction (r). According to Snell’s law, refractive index is given as Ø n = sin i/ sin r Ø The velocity of light decreases, when it passes through a dense medium. Hence, the ratio can be written as Ø where, v 1 & v 2 is velocity of light in air and in medium. Refractive index is greater than 1 for substances denser than air, because the velocity of light in air is always higher compared to the value in denominator.

Ø In this experiment, Abbe's refractometer is used to measure the refractive index of the given organic liquid. Using a particular monochromatic light source, the apparatus is calibrated with water as the liquid. Ø Adjust the micrometer screw to focus the boundary between the bright and dark regions. Adjust the refractometer scale to place the cross wire of the telescope exactly on the boundary between the bright and dark regions. Ø Repeat the same process for different organic liquids after the equipment is calibrated

14 Determination of heat of solution of salicylic acid by solubility method Ø The Heat of Solution is the amount of heat energy absorbed (endothermic) or released (exothermic) when a specific amount of solute dissolves in a solvent. Ø Molar heat of solution is the amount of energy absorbed or released per one mole of the solute. Ø Enthalpy of solution, or heat of solution, is expressed in k. J/mol, and it is the amount of heat energy that is released or absorbed when a solution is formed. Ø There are three steps in solvation: the breaking of bonds between solute molecules, the breaking of intermolecular attractions between solvent molecules, and the formation of new solute-solvent attractive bonds. Energy is absorbed during the first two steps, and it is released during the last step. Ø Depending on the relative amounts of energy required to break bonds initially, as well as how much is released upon solute-solvent bond formation, the overall heat of solution can either be endothermic or exothermic.

Ø In this experiment , salicylic acid is added to the beaker containing water and stir it properly. Ø The temperature is increased to 85 C with continuous stirring, maintain the same for few minutes and then cool to any temperature lower than 85 C say 70 C (T 1) Ø Transfer 10 ml of the sample solution to beaker or porcelain dish. Ø Allow the temperature to fall further T 2 say 30 C and withdraw 10 ml of sample by pipette and transfer to beaker or porcelain dish. Ø Evaporate these solutions either by direct heat or on hot plate or in hot air oven. Ø Dry these contents till a constant weight is obtained for each sample , when weighed on a sensitive analytical balance.

THANK YOU Jayawantrao Sawant College of Pharmacy and Research