Selected Topics In Physical Pharmacy Fifth Year 2017





![Pseudo plastic flow behaviour Shear thinning Example: Paint 1. 0 120 100 h [Pa·s] Pseudo plastic flow behaviour Shear thinning Example: Paint 1. 0 120 100 h [Pa·s]](https://slidetodoc.com/presentation_image/46ace0375c013b6a8846f2e8b2e063cd/image-19.jpg)
![Shear thickening Example: PVC-Plastisol 10 3000 Viscosity Curve 2000 h [Pa·s] t [Pa] 2500 Shear thickening Example: PVC-Plastisol 10 3000 Viscosity Curve 2000 h [Pa·s] t [Pa] 2500](https://slidetodoc.com/presentation_image/46ace0375c013b6a8846f2e8b2e063cd/image-25.jpg)

![450 400 t [Pa] A quantitative measurement of thixotropy is done by measuring the 450 400 t [Pa] A quantitative measurement of thixotropy is done by measuring the](https://slidetodoc.com/presentation_image/46ace0375c013b6a8846f2e8b2e063cd/image-29.jpg)


- Slides: 36

Selected Topics In Physical Pharmacy Fifth Year, 2017 -2018 Introduction to Rheology and Viscometry

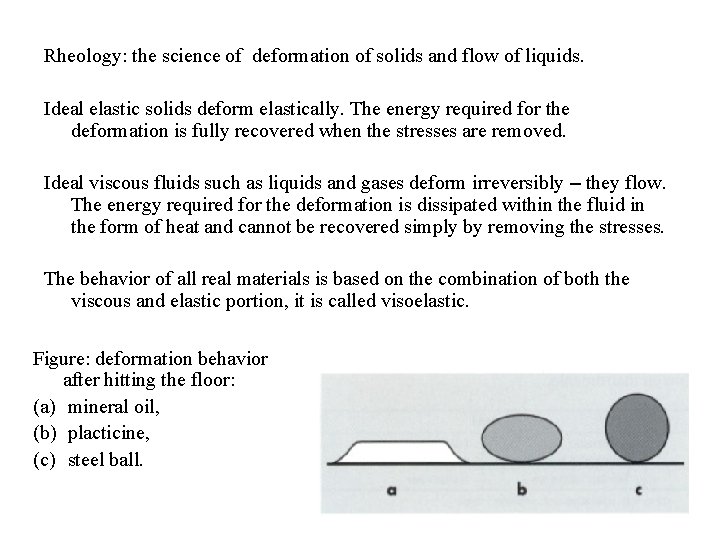
Rheology: the science of deformation of solids and flow of liquids. Ideal elastic solids deform elastically. The energy required for the deformation is fully recovered when the stresses are removed. Ideal viscous fluids such as liquids and gases deform irreversibly – they flow. The energy required for the deformation is dissipated within the fluid in the form of heat and cannot be recovered simply by removing the stresses. The behavior of all real materials is based on the combination of both the viscous and elastic portion, it is called visoelastic. Figure: deformation behavior after hitting the floor: (a) mineral oil, (b) placticine, (c) steel ball.

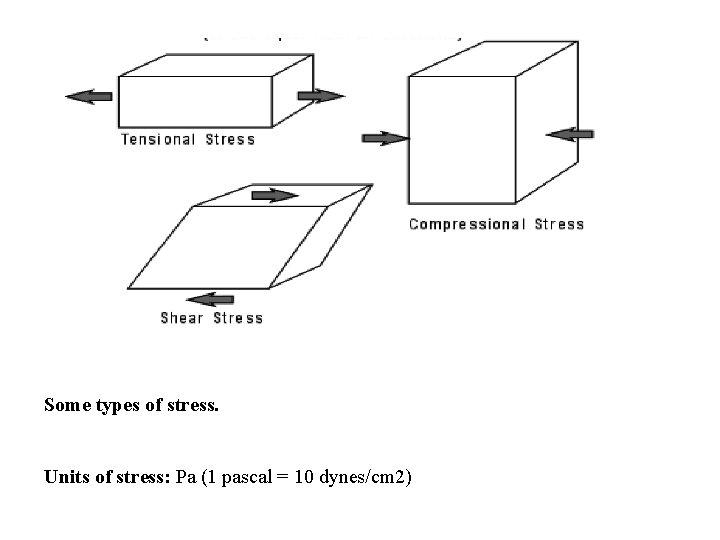
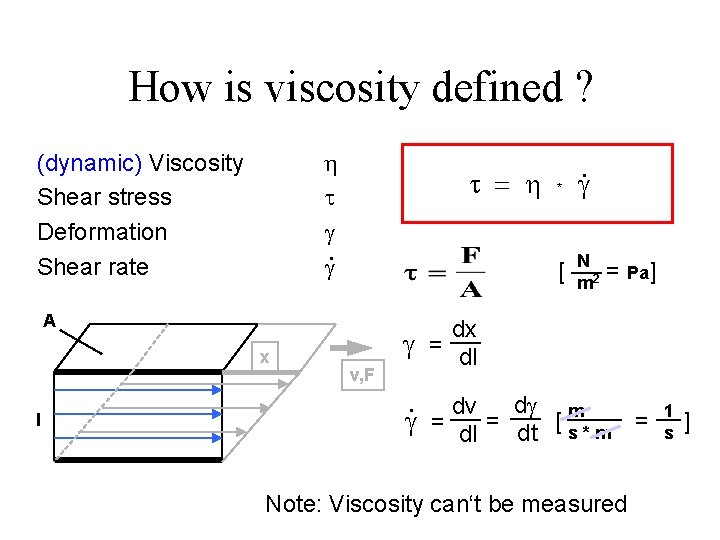
Basic Concepts Rheological properties of liquids can be described in terms of at least three parameters: Stress, Shear Rate and Time. Stress: force per unit area required to cause the deformation, F/A, N/m 2.

Some types of stress. Units of stress: Pa (1 pascal = 10 dynes/cm 2)

Example: hanging 1 -g weight in a rubber band (loop) of 0. 049 cm 2 cross sectional area. Find the stress on the rubber band. Effective surface area of the loop = 2 x cross sectional area. Force = mass x gravity acceleration. Stress = force/area = 981 dyne/2 x 0. 049 cm 2 = 1 x 10 E 4 dynes /cm 2 = 1 x 10 E 4 dynes. E+1 cm E-2 x 0. 1 Pa dyne. E-1 cm. E+2 = 1 x 10 E 3 Pa = 1 KPa

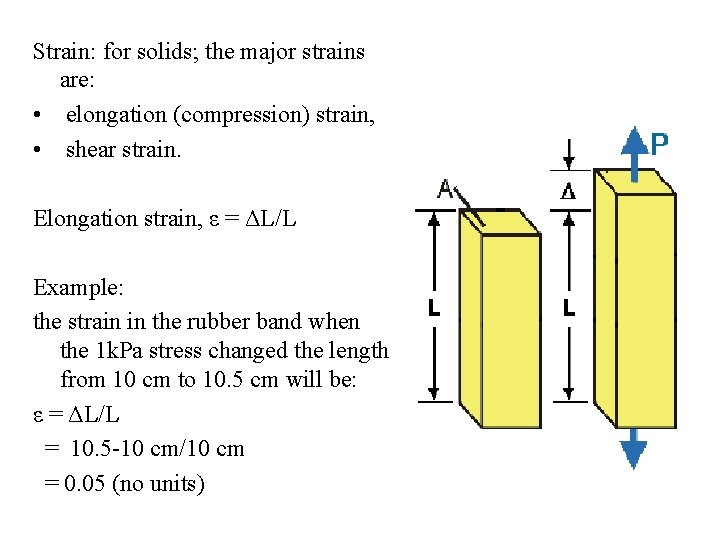
Strain: for solids; the major strains are: • elongation (compression) strain, • shear strain. Elongation strain, ε = ΔL/L Example: the strain in the rubber band when the 1 k. Pa stress changed the length from 10 cm to 10. 5 cm will be: ε = ΔL/L = 10. 5 -10 cm/10 cm = 0. 05 (no units)

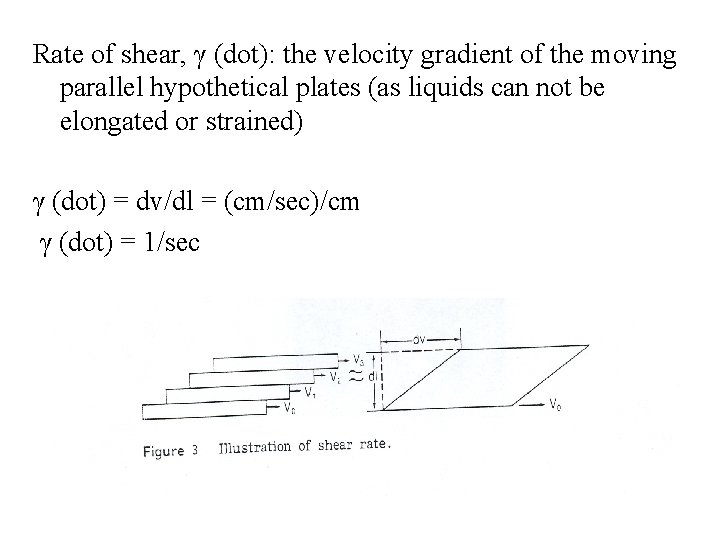
Rate of shear, γ (dot): the velocity gradient of the moving parallel hypothetical plates (as liquids can not be elongated or strained) γ (dot) = dv/dl = (cm/sec)/cm γ (dot) = 1/sec

How is viscosity defined ? h . (dynamic) Viscosity Shear stress Deformation Shear rate = h [ A x l * v, F . N m 2 = Pa] dx = dl . d m dv = dl = dt [ s * m Note: Viscosity can‘t be measured = 1 s ]

Viscosity: is a measure of the resistance of a liquid to flow. • The SI units for viscosity is Pascal-second. • In cgs system, it is poise, P. • 1 Pas-sec = 10 poise = 10 dyne sec cm 2 A shear rate of 100/sec is a little more than the shear rate of pouring water from a bottle.

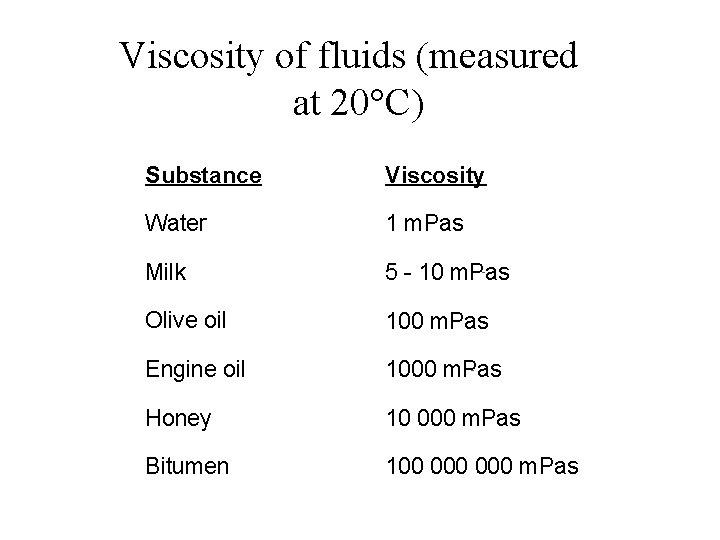
Viscosity of fluids (measured at 20°C) Substance Viscosity Water 1 m. Pas Milk 5 - 10 m. Pas Olive oil 100 m. Pas Engine oil 1000 m. Pas Honey 10 000 m. Pas Bitumen 100 000 m. Pas

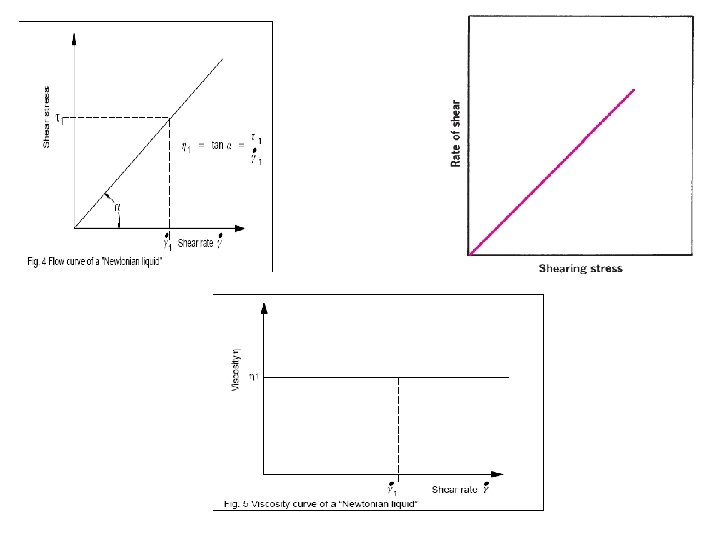
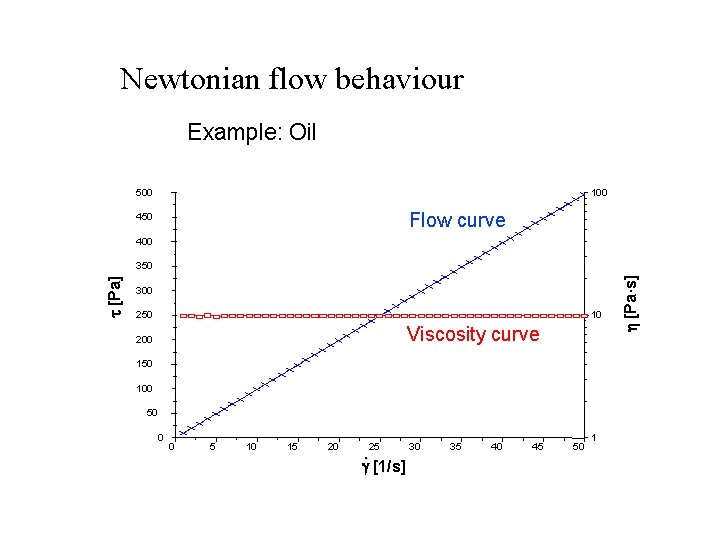
Types of flow behavior 1. Newtonian flow: when the liquid follows Newton Equation = h * The viscosity is constant and independent of shear stress or rate of shear. Example: water, mineral oils, . . Plot x-y diagram for a Newtonian fluid 2. non- Newtonian flow: when the liquid does not follow Newton Equation and the viscosity is function of the shear stress and rate of shear. Example: most of the pharmaceutical formulations.


Newtonian flow behaviour Example: Oil 500 100 Flow curve 450 400 300 250 10 Viscosity curve 200 150 100 50 0 0 5 10 15 20 25 . g [1/s] 30 35 40 45 50 1 h [Pa·s] t [Pa] 350

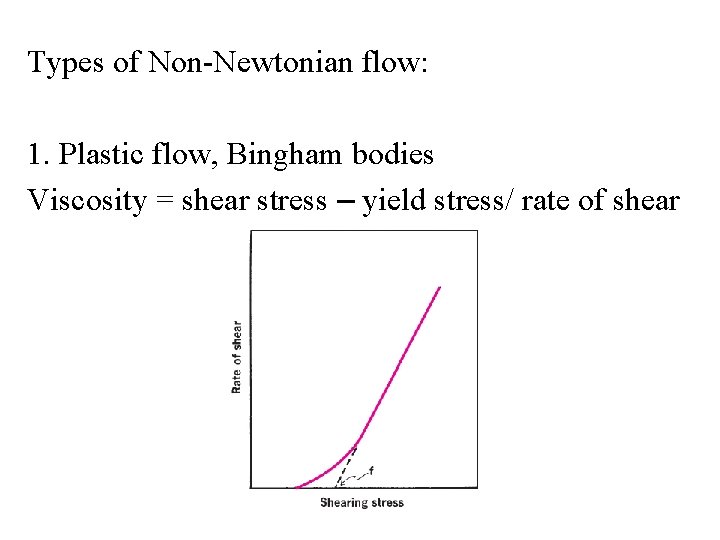
Types of Non-Newtonian flow: 1. Plastic flow, Bingham bodies Viscosity = shear stress – yield stress/ rate of shear

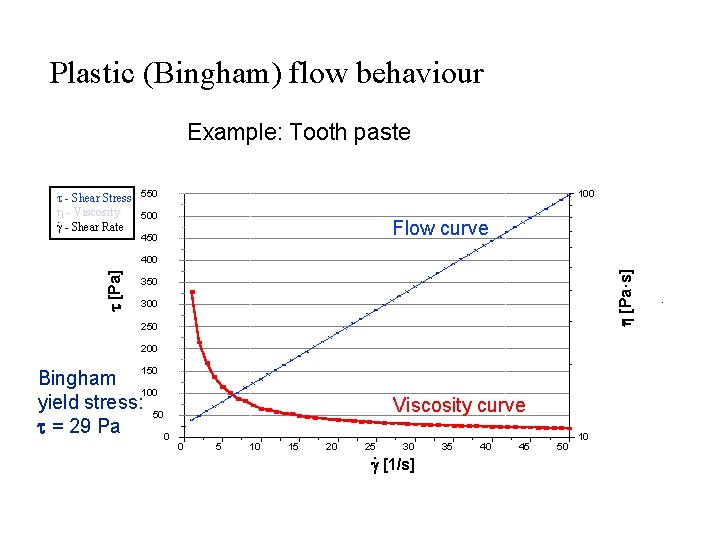
Plastic (Bingham) flow behaviour Example: Tooth paste - Shear Stress h - Viscosity. - Shear Rate 100 550 500 Flow curve 450 h [Pa·s] t [Pa] 400 350 300 250 200 150 Bingham 100 yield stress: 50 t = 29 Pa 0 Viscosity curve 0 5 10 15 20 25 30 . g [1/s] 35 40 45 50 10 .

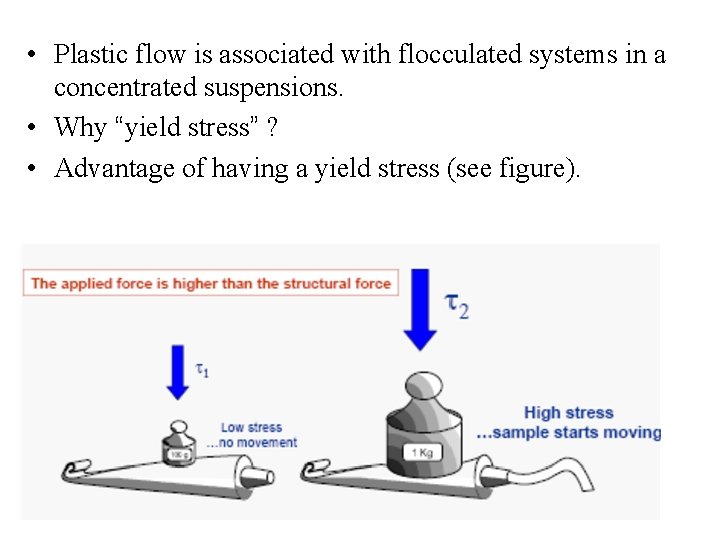
• Plastic flow is associated with flocculated systems in a concentrated suspensions. • Why “yield stress” ? • Advantage of having a yield stress (see figure).

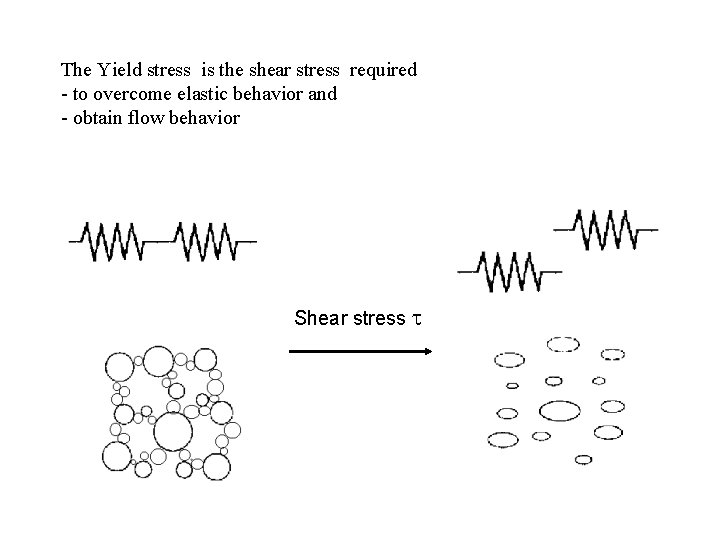
The Yield stress is the shear stress required - to overcome elastic behavior and - obtain flow behavior Shear stress

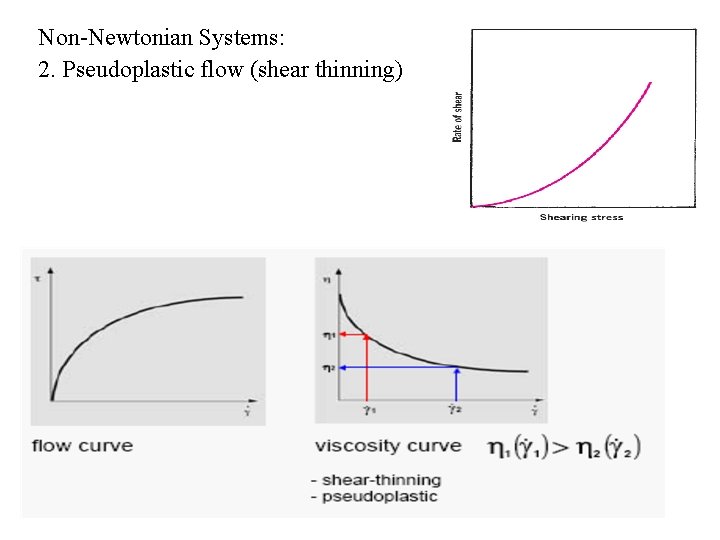
Non-Newtonian Systems: 2. Pseudoplastic flow (shear thinning)
![Pseudo plastic flow behaviour Shear thinning Example Paint 1 0 120 100 h Pas Pseudo plastic flow behaviour Shear thinning Example: Paint 1. 0 120 100 h [Pa·s]](https://slidetodoc.com/presentation_image/46ace0375c013b6a8846f2e8b2e063cd/image-19.jpg)
Pseudo plastic flow behaviour Shear thinning Example: Paint 1. 0 120 100 h [Pa·s] t [Pa] Flow Curve 80 60 40 Viscosity Curve 20 0 0 50 100 150 200 250 . g [1/s] 300 350 400 450 500 0. 1

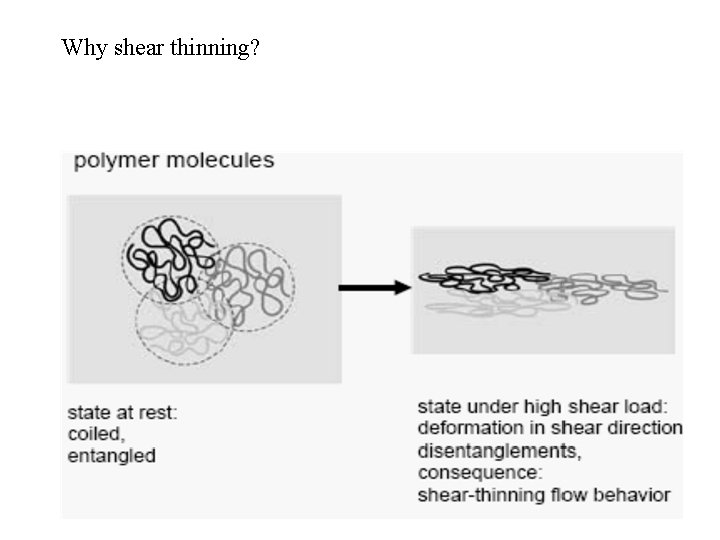
Why shear thinning?

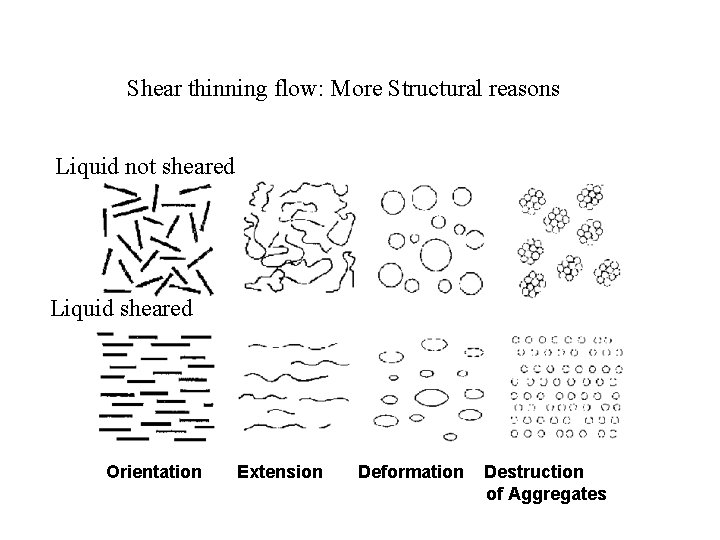
Shear thinning flow: More Structural reasons Liquid not sheared Liquid sheared Orientation Extension Deformation Destruction of Aggregates

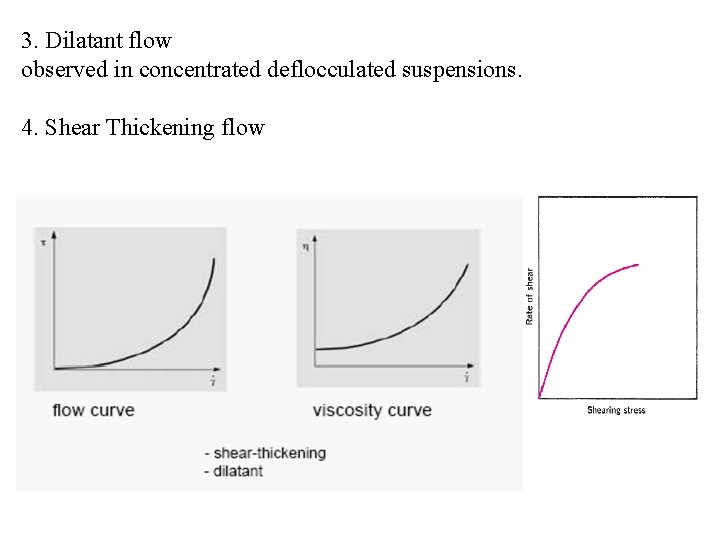
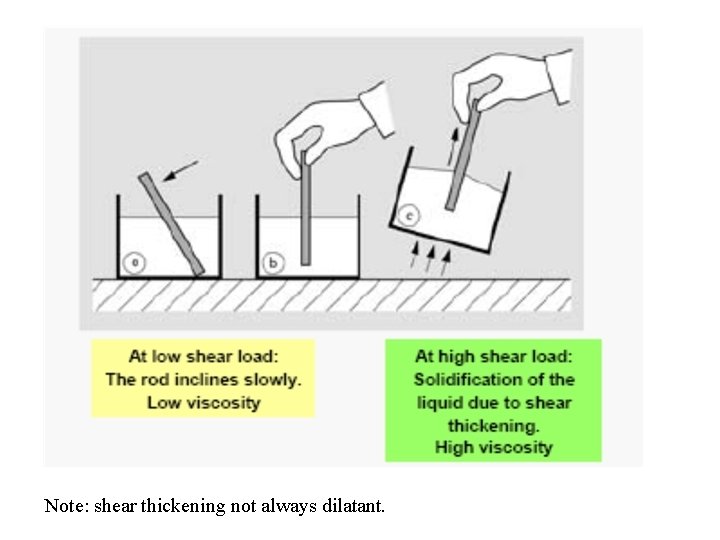
3. Dilatant flow observed in concentrated deflocculated suspensions. 4. Shear Thickening flow

Note: shear thickening not always dilatant.

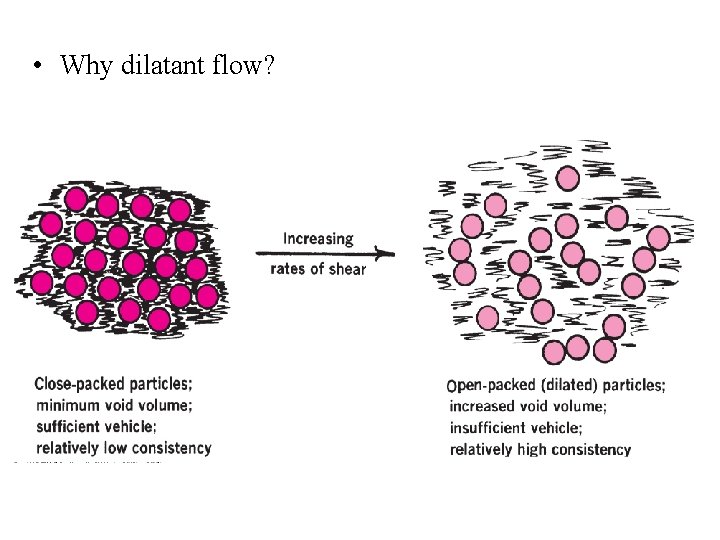
• Why dilatant flow?
![Shear thickening Example PVCPlastisol 10 3000 Viscosity Curve 2000 h Pas t Pa 2500 Shear thickening Example: PVC-Plastisol 10 3000 Viscosity Curve 2000 h [Pa·s] t [Pa] 2500](https://slidetodoc.com/presentation_image/46ace0375c013b6a8846f2e8b2e063cd/image-25.jpg)
Shear thickening Example: PVC-Plastisol 10 3000 Viscosity Curve 2000 h [Pa·s] t [Pa] 2500 1000 Flow Curve 500 0 0 50 100 150 200 . g [1/s] 250 300 350 400 1 .

Rheological properties of liquids can be described in terms of three parameters: Stress, Shear Rate and Time. Thixotropy: defined as “ an isothermal and comparatively slow recovery on standing of a material, of a consistency lost through shearing. According to this definition, thixotropy is applied only to the shear thinning systems.

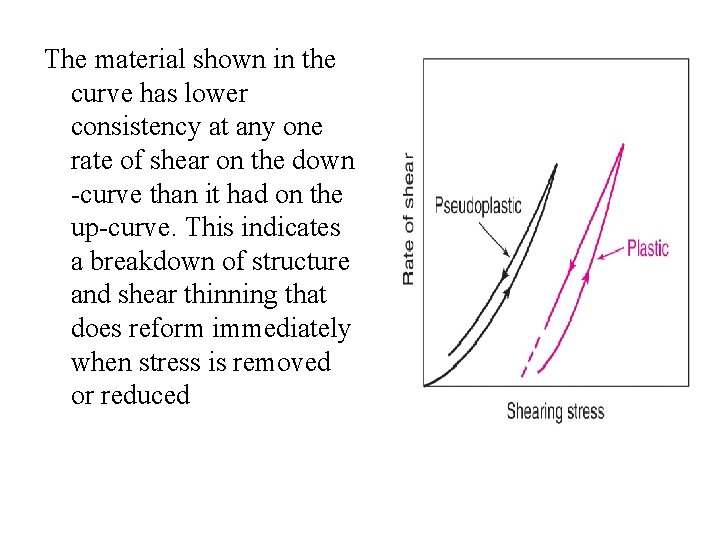
The material shown in the curve has lower consistency at any one rate of shear on the down -curve than it had on the up-curve. This indicates a breakdown of structure and shear thinning that does reform immediately when stress is removed or reduced

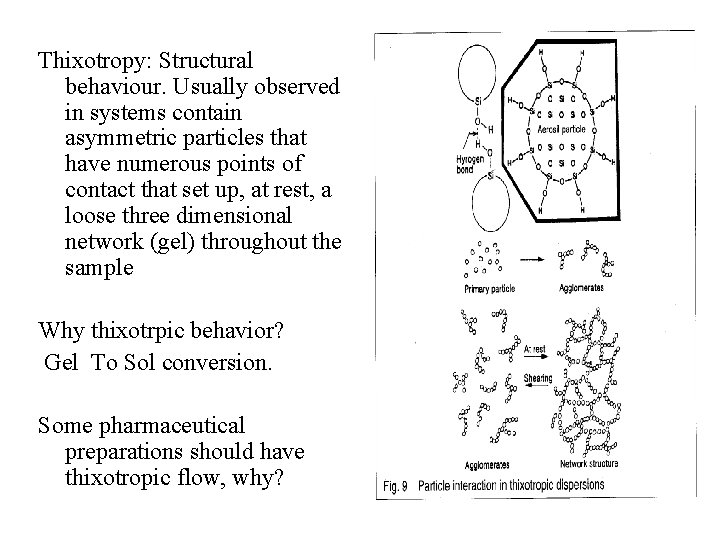
Thixotropy: Structural behaviour. Usually observed in systems contain asymmetric particles that have numerous points of contact that set up, at rest, a loose three dimensional network (gel) throughout the sample Why thixotrpic behavior? Gel To Sol conversion. Some pharmaceutical preparations should have thixotropic flow, why?
![450 400 t Pa A quantitative measurement of thixotropy is done by measuring the 450 400 t [Pa] A quantitative measurement of thixotropy is done by measuring the](https://slidetodoc.com/presentation_image/46ace0375c013b6a8846f2e8b2e063cd/image-29.jpg)
450 400 t [Pa] A quantitative measurement of thixotropy is done by measuring the thixotropic loop area 500 350 Thixotropic loop area 300 250 200 150 100 50 0 0 50 100 150 200 250 300 350 . g [1/s] 400 Thixotropy: Flow curve (thixotropic loop measurement. ) 450 500

Determination of rheological properties The Finger the cheapest viscometer • simple • cheap • quick • easy to clean

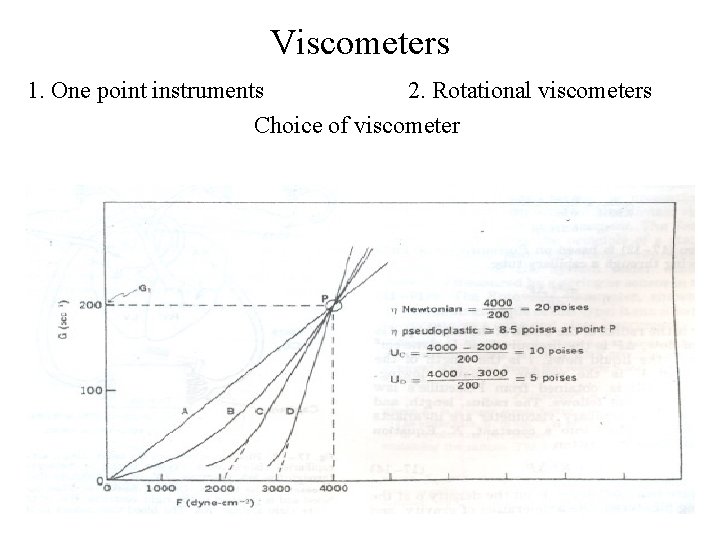
Viscometers 1. One point instruments 2. Rotational viscometers Choice of viscometer

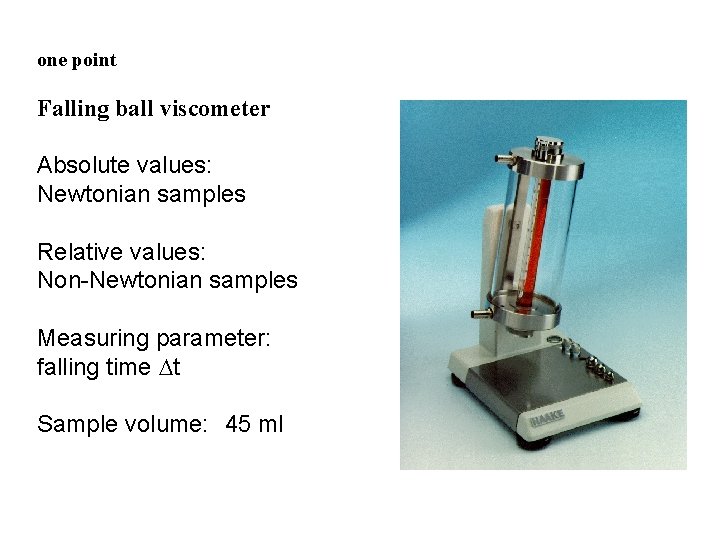
one point Falling ball viscometer Absolute values: Newtonian samples Relative values: Non-Newtonian samples Measuring parameter: falling time Dt Sample volume: 45 ml

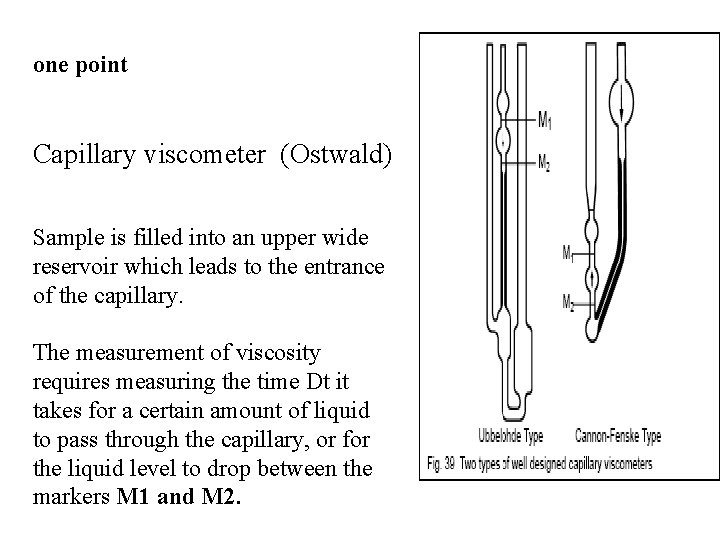
one point Capillary viscometer (Ostwald) Sample is filled into an upper wide reservoir which leads to the entrance of the capillary. The measurement of viscosity requires measuring the time Dt it takes for a certain amount of liquid to pass through the capillary, or for the liquid level to drop between the markers M 1 and M 2.

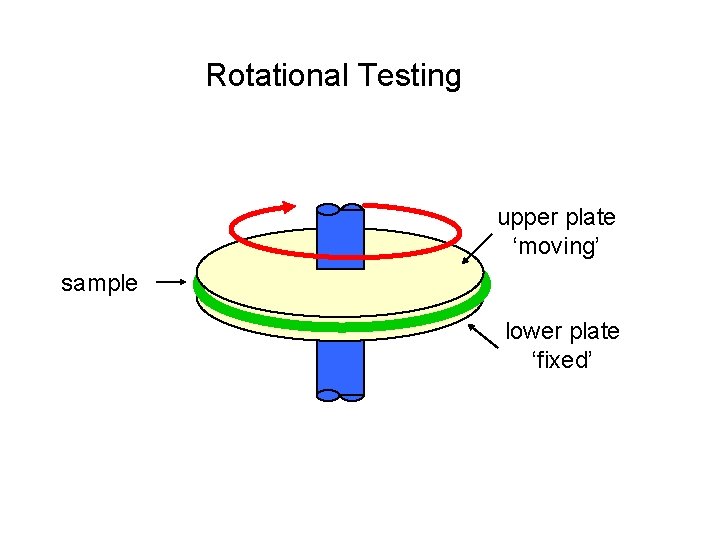
Rotational Testing upper plate ‘moving’ sample lower plate ‘fixed’

Rotational viscometers Measures absolute values for Newtonian samples And Relative values for Non-newtonian samples Measuring friction of the sample in dependence on defined rotation speeds (0. 1 < rounds/min-1 < 200) Sample volume: min. 400 ml • Time curve • Flow curve

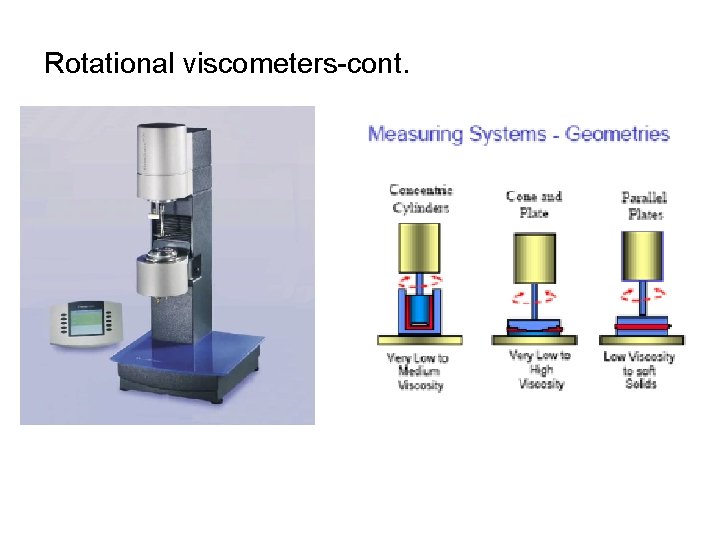
Rotational viscometers-cont.