Phase Field Modeling of Electrochemistry J E Guyer




- Slides: 41

Phase Field Modeling of Electrochemistry J. E. Guyer, W. J. Boettinger, J. A. Warren & G. B. Mc. Fadden • Features common to electrochemistry & melt growth • Review of – Sharp interface model of electroplating – Interface structure • Phase field model – 1 -D Equilibrium Results Bob Sekerka NASA

• Mullins-Sekerka widely quoted and adapted to predict roughening of plated surface; e. g. Aogaki et al. (1980 -1995) • “A theory of dendritic growth in electrolytes”, D. R Hamilton, Electrochimica Acta 8 (1963) 731. Ivantsov / maximum growth rate hypothesis to fit velocity vs. overpotential data of Barton & Bockris (Ag dendrites growing from Ag. NO 3 in Na. NO 3 -KNO 3 molten salt eutectic). current~velocity; undercooling~overpotential Cu from aqueous solution: Barkey, Oberholtzer & Wu, PRL 75(1995) 2980

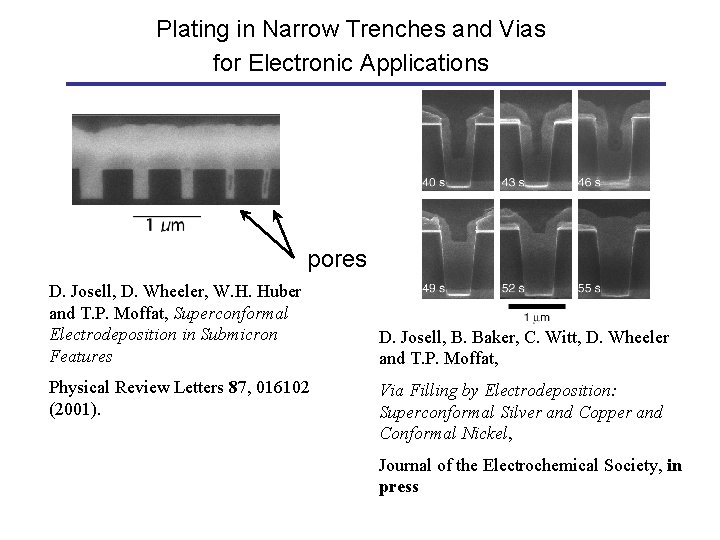
Plating in Narrow Trenches and Vias for Electronic Applications pores D. Josell, D. Wheeler, W. H. Huber and T. P. Moffat, Superconformal Electrodeposition in Submicron Features Physical Review Letters 87, 016102 (2001). D. Josell, B. Baker, C. Witt, D. Wheeler and T. P. Moffat, Via Filling by Electrodeposition: Superconformal Silver and Copper and Conformal Nickel, Journal of the Electrochemical Society, in press

Some possible uses of phase-field modeling in electrochemistry • Plating in submicron features – High curvatures, high electric field gradients – Avoid approximations • Pulse plating • Alloy Plating • Alloy Dissolution • Reveal new insight into relationship between interface charge distribution / adsorption and kinetics

Electrochemistry

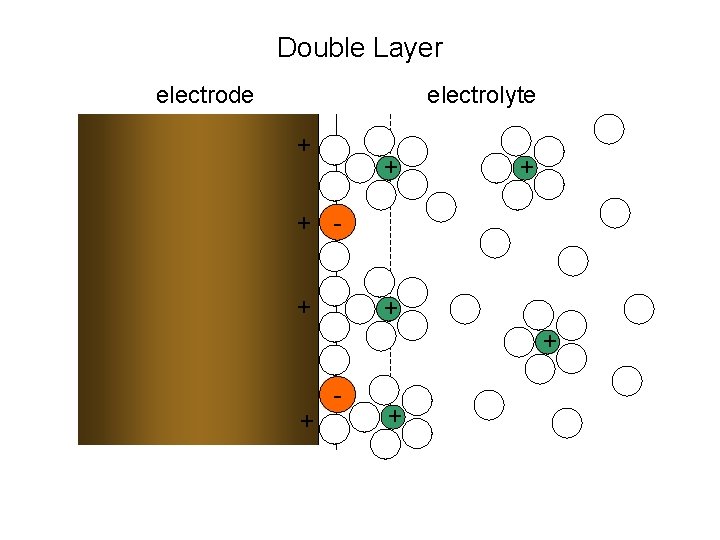
Double Layer electrode electrolyte + + + + - +

Length Scales in Electrochemistry a) Thickness of Electrode-Electrolyte interface b) Charge separation distance (Debye layer); related to concentrations and dielectric constant c) Long range concentration decay length due to diffusion/convection in electrolyte Helmholtz model Gouy-Chapman-Stern model

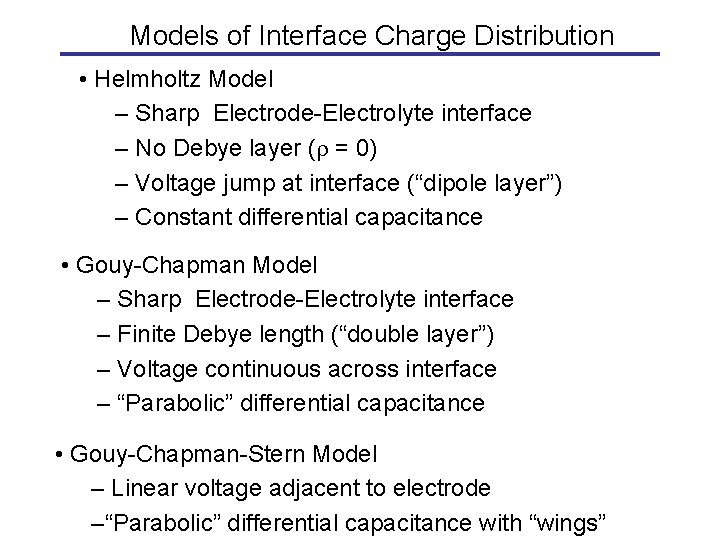
Models of Interface Charge Distribution • Helmholtz Model – Sharp Electrode-Electrolyte interface – No Debye layer ( = 0) – Voltage jump at interface (“dipole layer”) – Constant differential capacitance • Gouy-Chapman Model – Sharp Electrode-Electrolyte interface – Finite Debye length (“double layer”) – Voltage continuous across interface – “Parabolic” differential capacitance • Gouy-Chapman-Stern Model – Linear voltage adjacent to electrode –“Parabolic” differential capacitance with “wings”

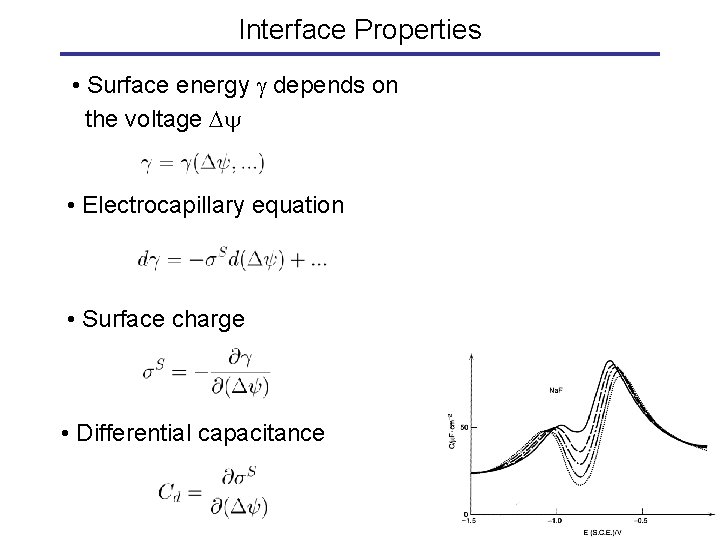
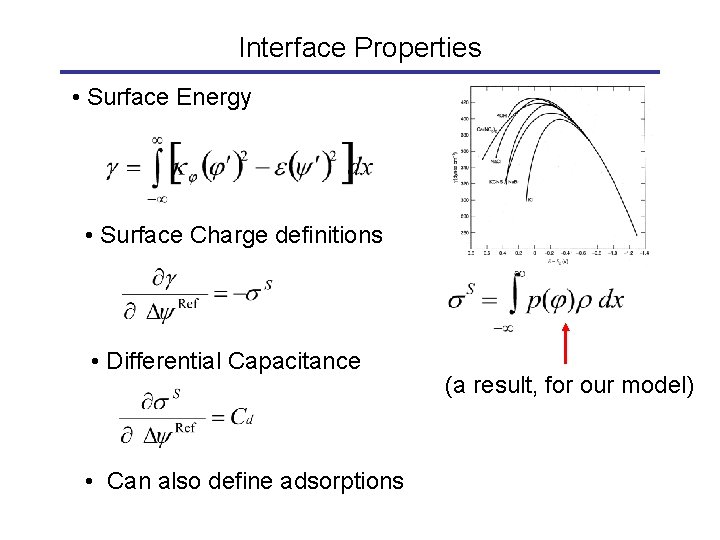
Interface Properties • Surface energy depends on the voltage • Electrocapillary equation • Surface charge • Differential capacitance

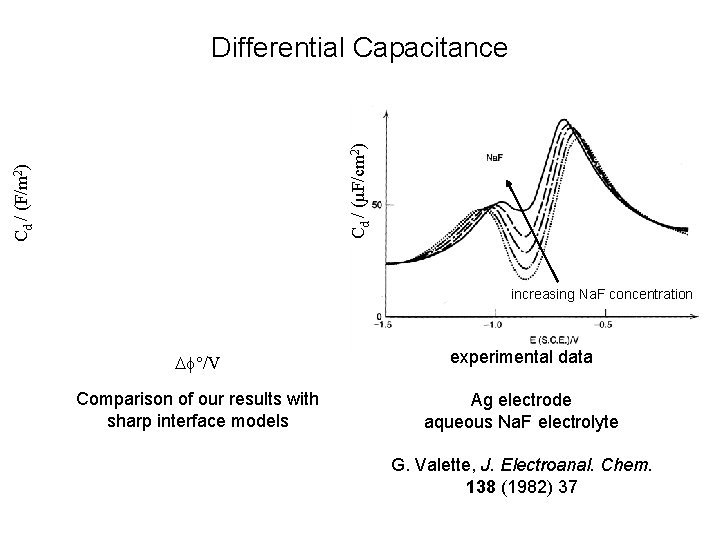
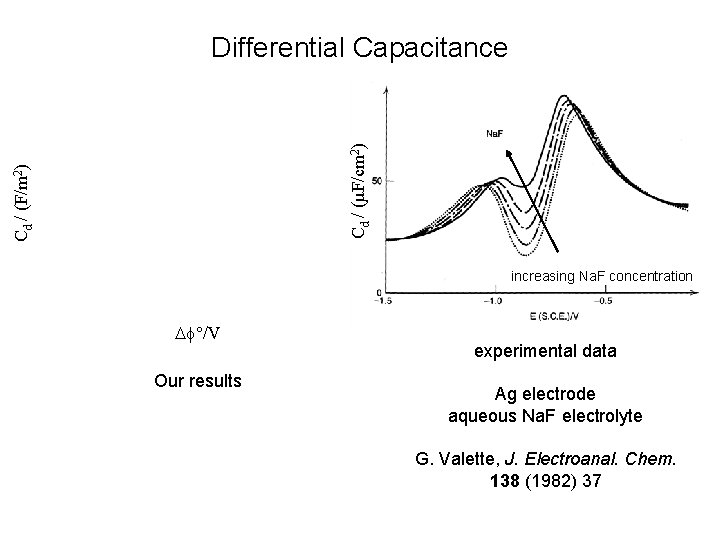
Cd / (F/m 2) Cd / (µF/cm 2) Differential Capacitance increasing Na. F concentration f°/V experimental data Comparison of our results with sharp interface models Ag electrode aqueous Na. F electrolyte G. Valette, J. Electroanal. Chem. 138 (1982) 37

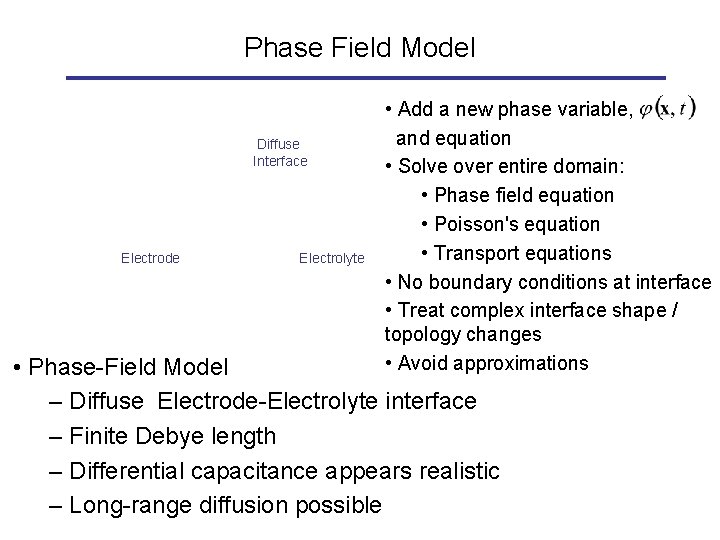
Phase Field Model Diffuse Interface Electrode Electrolyte • Add a new phase variable, and equation • Solve over entire domain: • Phase field equation • Poisson's equation • Transport equations • No boundary conditions at interface • Treat complex interface shape / topology changes • Avoid approximations • Phase-Field Model – Diffuse Electrode-Electrolyte interface – Finite Debye length – Differential capacitance appears realistic – Long-range diffusion possible

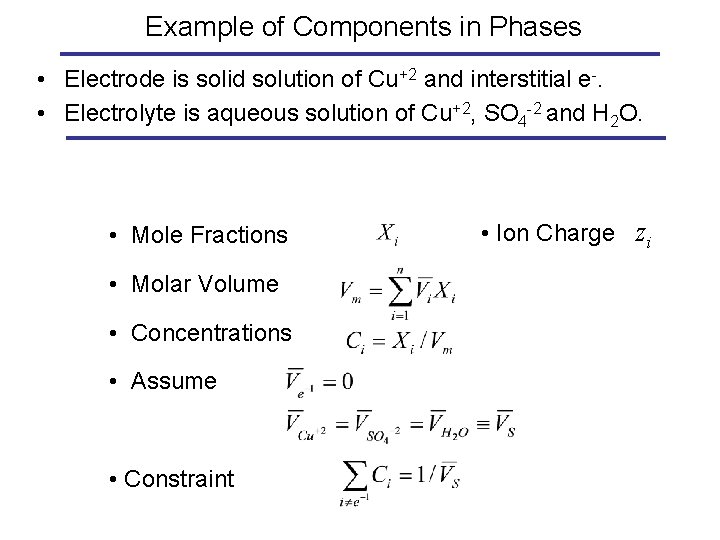
Example of Components in Phases • Electrode is solid solution of Cu+2 and interstitial e-. • Electrolyte is aqueous solution of Cu+2, SO 4 -2 and H 2 O. • Mole Fractions • Molar Volume • Concentrations • Assume • Constraint • Ion Charge zi

Free Energy

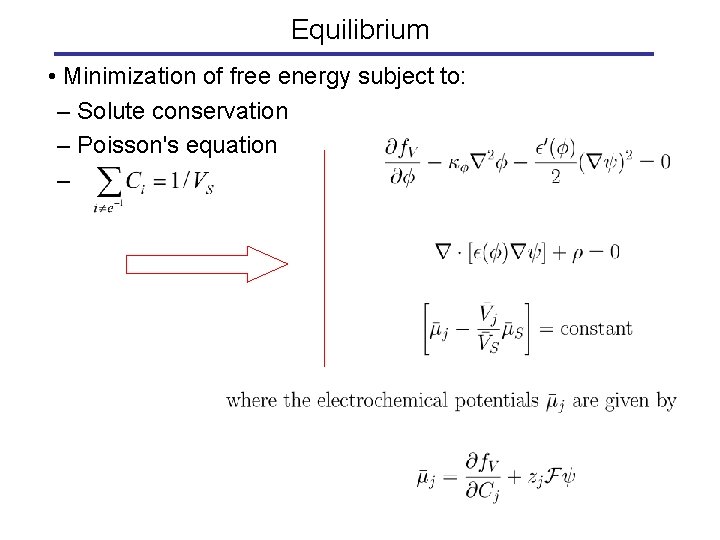
Equilibrium • Minimization of free energy subject to: – Solute conservation – Poisson's equation –

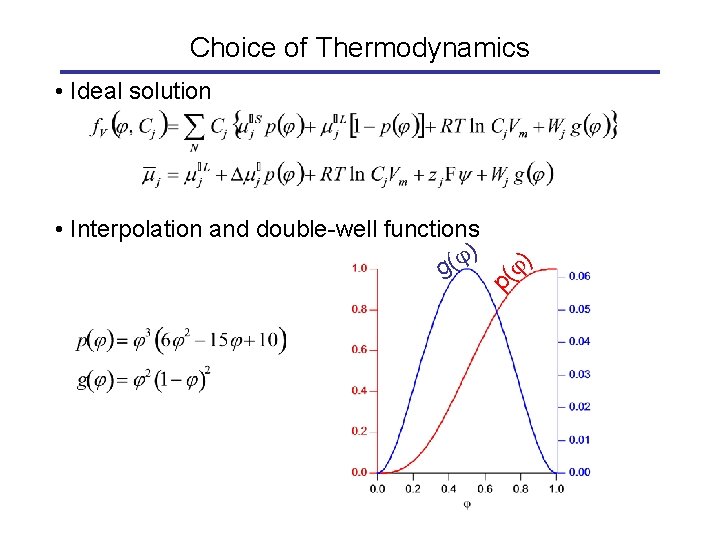
Choice of Thermodynamics p( • Interpolation and double-well functions ) ( g ) • Ideal solution

Phase Equilibria: • Xi. L, Ref, Xi. S, Ref chosen to obtain equilibrium between a liquid solution of Cu. SO 4 in H 2 O & metal (Cu+2 + 2 e-1) zero charge plane • Dy. Ref chosen to set interface charge distribution, for Ref X’s

Interface Properties • Surface Energy • Surface Charge definitions • Differential Capacitance • Can also define adsorptions (a result, for our model)

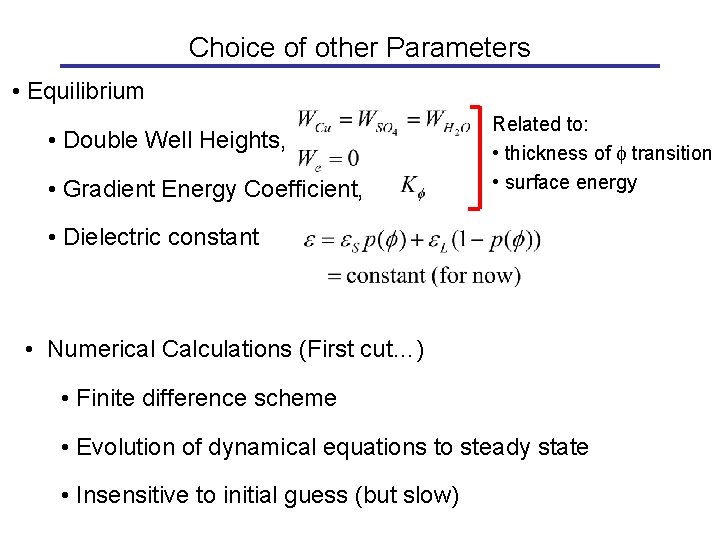
Choice of other Parameters • Equilibrium • Double Well Heights, • Gradient Energy Coefficient, Related to: • thickness of f transition • surface energy • Dielectric constant • Numerical Calculations (First cut…) • Finite difference scheme • Evolution of dynamical equations to steady state • Insensitive to initial guess (but slow)

Equilibrium Profiles

Concentration Profiles

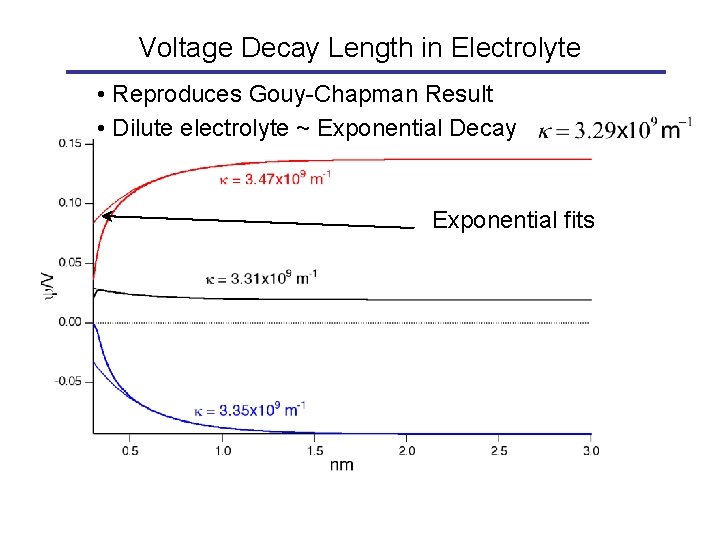
Voltage Decay Length in Electrolyte • Reproduces Gouy-Chapman Result • Dilute electrolyte ~ Exponential Decay Exponential fits

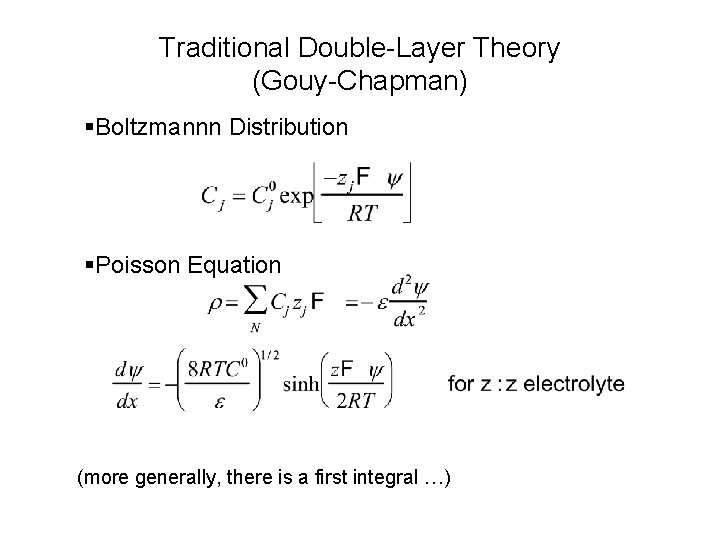
Traditional Double-Layer Theory (Gouy-Chapman) §Boltzmannn Distribution §Poisson Equation (more generally, there is a first integral …)

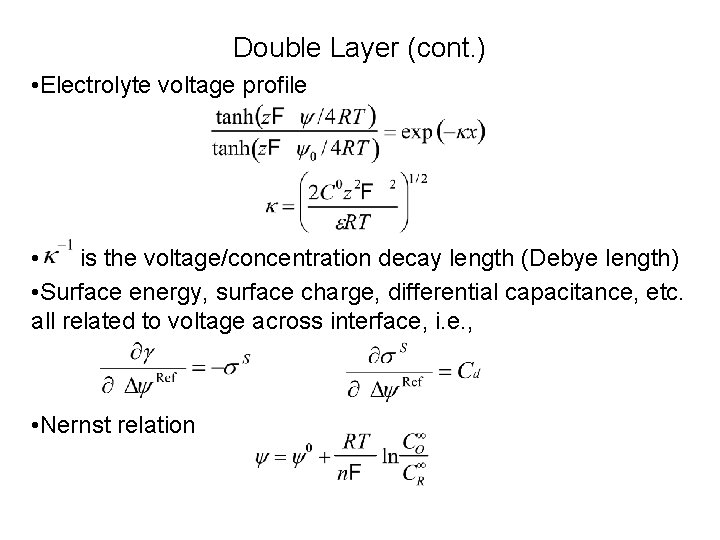
Double Layer (cont. ) • Electrolyte voltage profile • is the voltage/concentration decay length (Debye length) • Surface energy, surface charge, differential capacitance, etc. all related to voltage across interface, i. e. , • Nernst relation

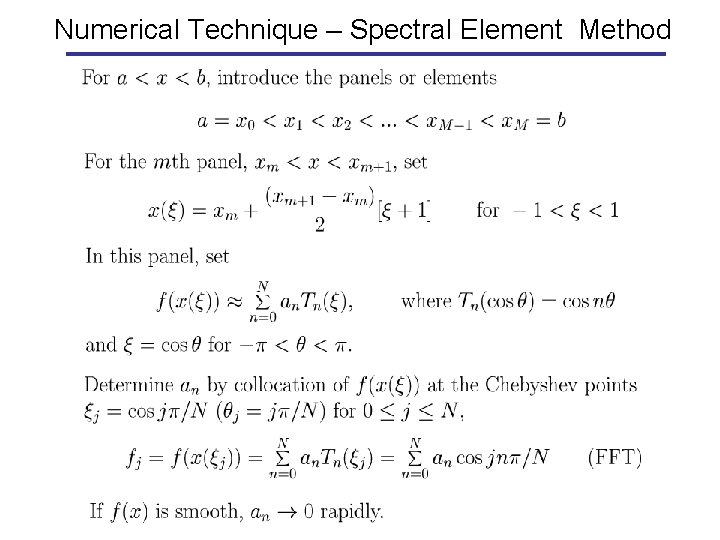
Numerical Technique – Spectral Element Method

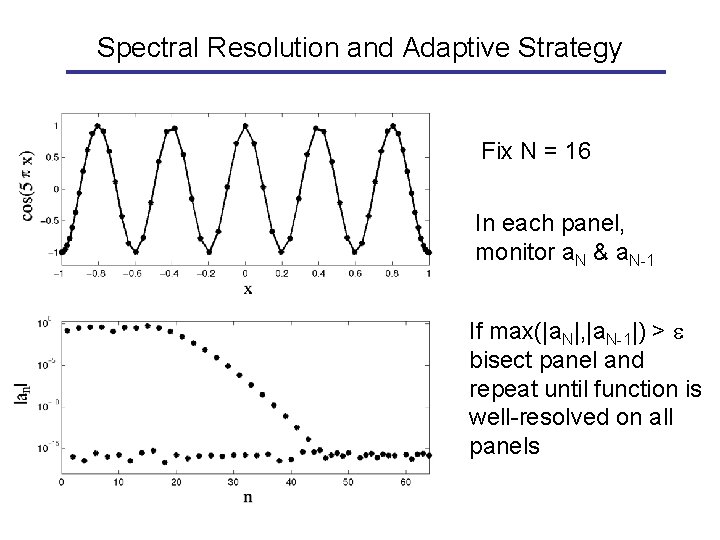
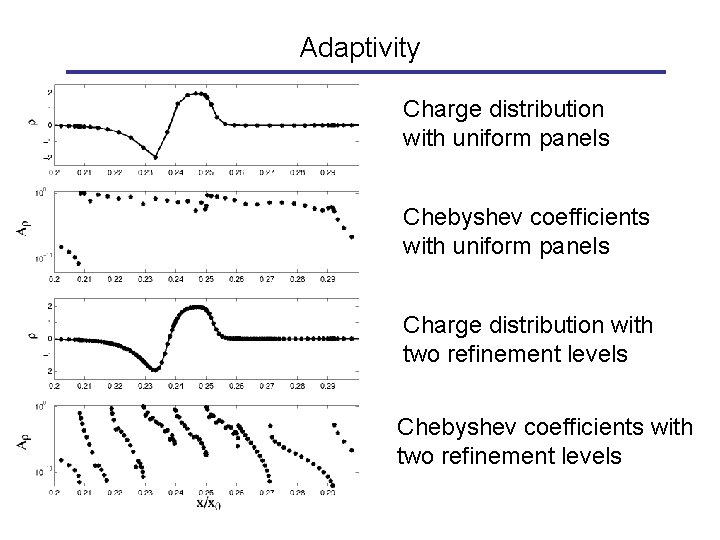
Spectral Resolution and Adaptive Strategy Fix N = 16 In each panel, monitor a. N & a. N-1 If max(|a. N|, |a. N-1|) > bisect panel and repeat until function is well-resolved on all panels

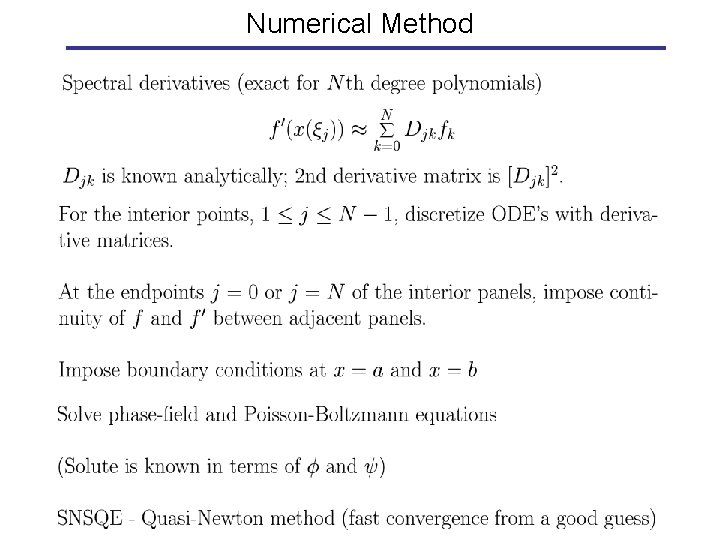
Numerical Method

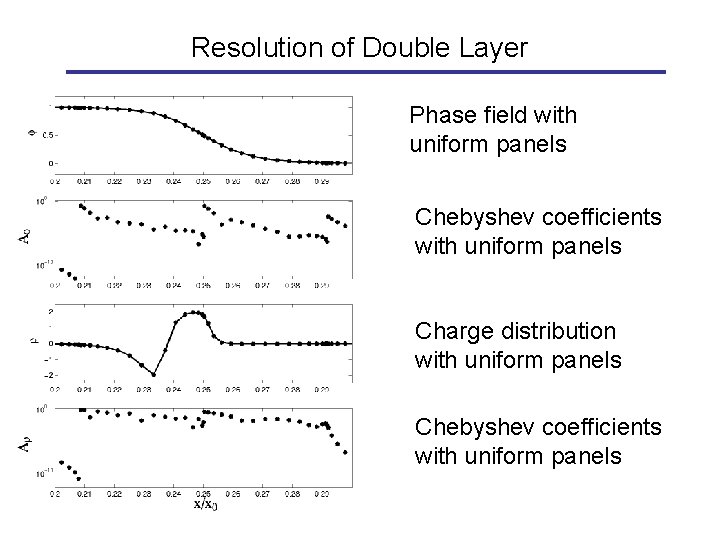
Resolution of Double Layer Phase field with uniform panels Chebyshev coefficients with uniform panels Charge distribution with uniform panels Chebyshev coefficients with uniform panels

Adaptivity Charge distribution with uniform panels Chebyshev coefficients with uniform panels Charge distribution with two refinement levels Chebyshev coefficients with two refinement levels

Spectral Computation surface energy surface charge differential capacitance

Electrocapillarity Bard & Faulkner, Electrochemical Methods 2 nd Ed. , Wiley & Sons, New York (2001) after D. C. Grahame, Chem. Rev. 41 (1947) 441

Cd / (F/m 2) Cd / (µF/cm 2) Differential Capacitance increasing Na. F concentration f°/V Our results experimental data Ag electrode aqueous Na. F electrolyte G. Valette, J. Electroanal. Chem. 138 (1982) 37

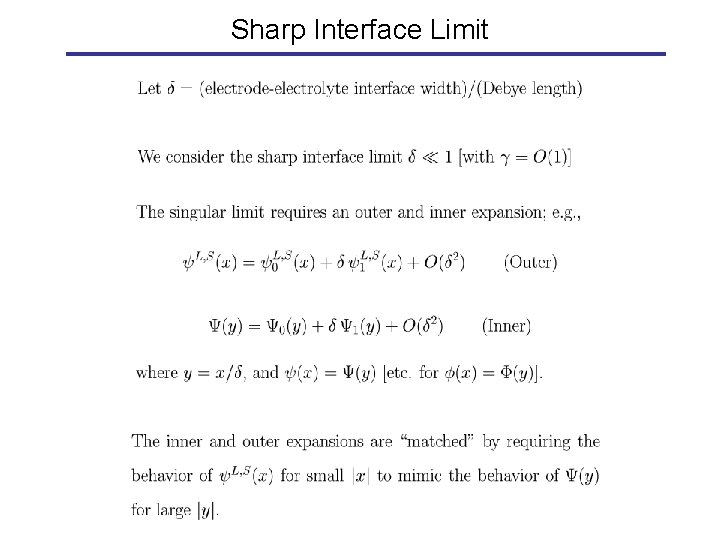
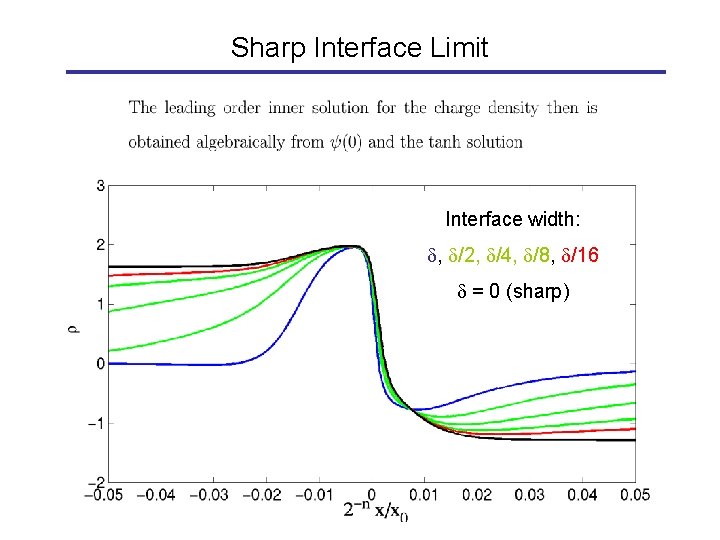
Sharp Interface Limit

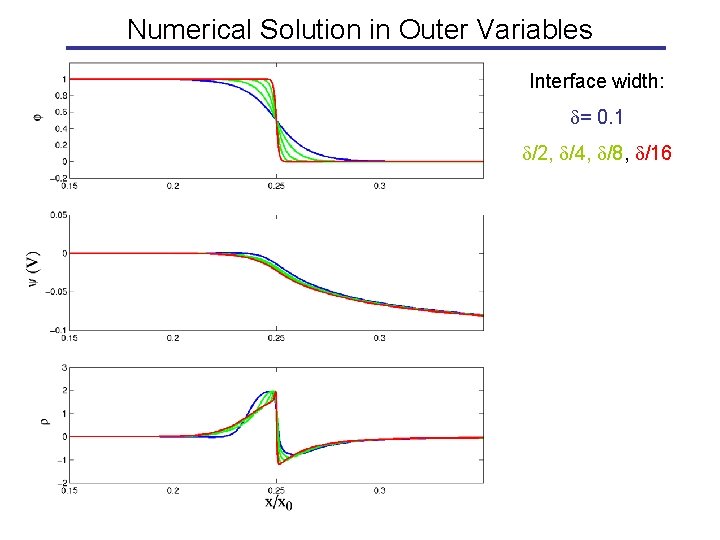
Numerical Solution in Outer Variables Interface width: = 0. 1 /2, /4, /8, /16

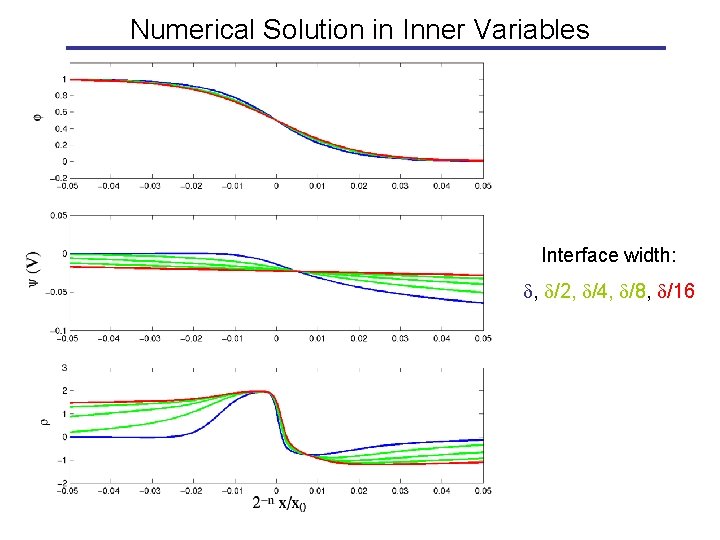
Numerical Solution in Inner Variables Interface width: , /2, /4, /8, /16

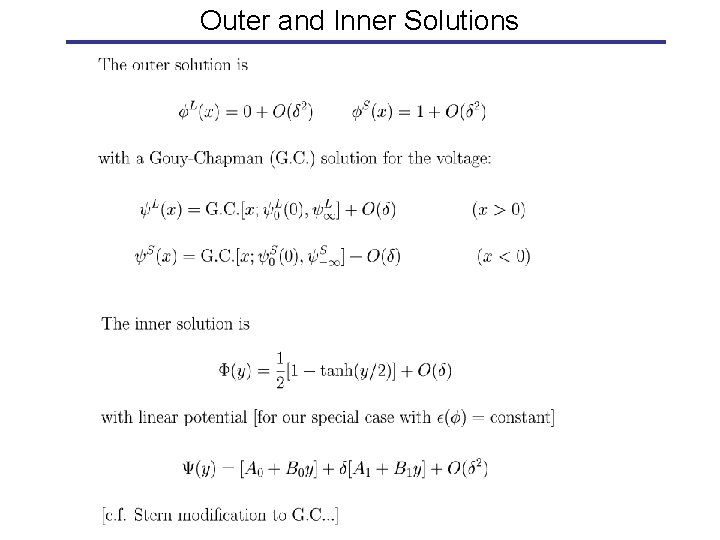
Outer and Inner Solutions

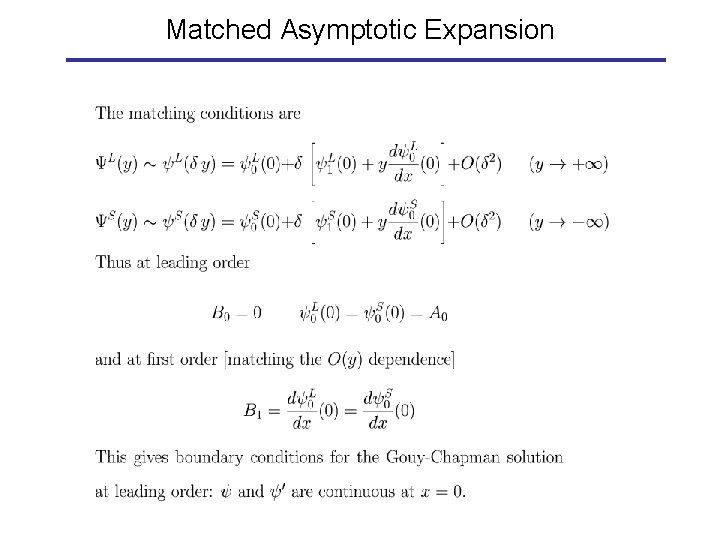
Matched Asymptotic Expansion

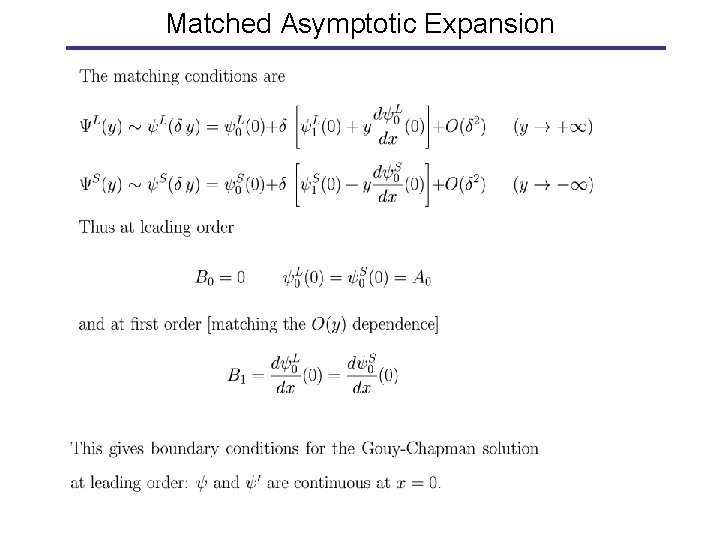
Matched Asymptotic Expansion

Surface Charge

Sharp Interface Limit Interface width: , /2, /4, /8, /16 = 0 (sharp)

Conclusions • Equilibrium 1 -D solutions of the model exhibit double layer behavior…. Consistent with Gouy-Chapman model, and incorporate – Decay length of electrostatic potential – Interface energy (“electrocapillary curves”), surface charge and differential capacitance all look reasonable

Current and Future Work • Continue study of sharp-interface limits • Kinetic studies underway • Explore behavior for We 0, non-constant ( ) • Explore effects of curvature (cylindrical electrode) • Adaptive Mesh in 2 -D • Alloy Plating/Corroding • Additives, Adsorption and inhibitors