Overview sulla nuove terapie dellepatite HCV correlata Raffaele
































- Slides: 82

Overview sulla nuove terapie dell'epatite HCV correlata Raffaele Bruno Division of Infectious and Tropical Diseases Hepatology Outpatient University of Pavia-IRCCS San Matteo

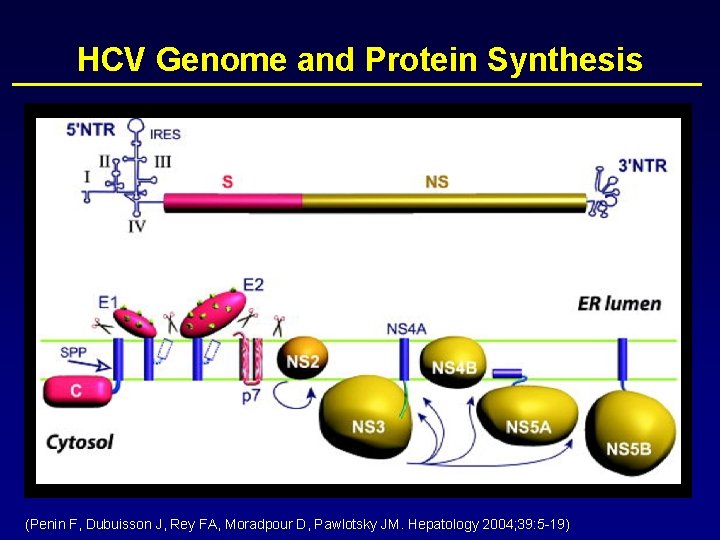
HCV Genome and Protein Synthesis (Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Hepatology 2004; 39: 5 -19)

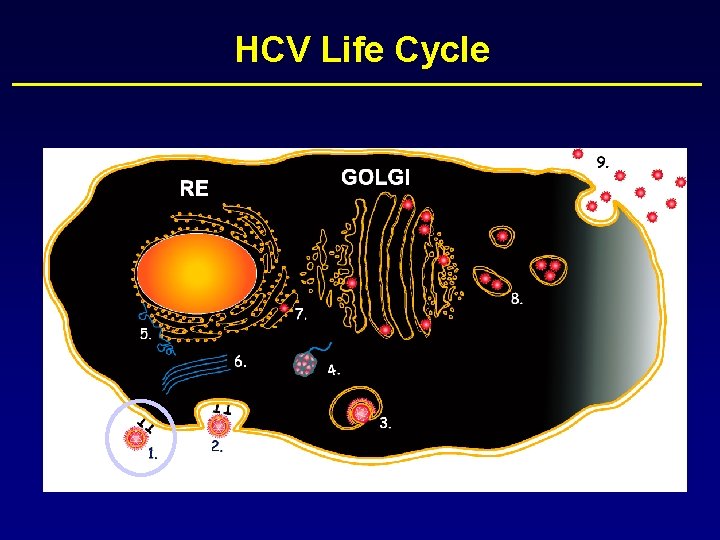
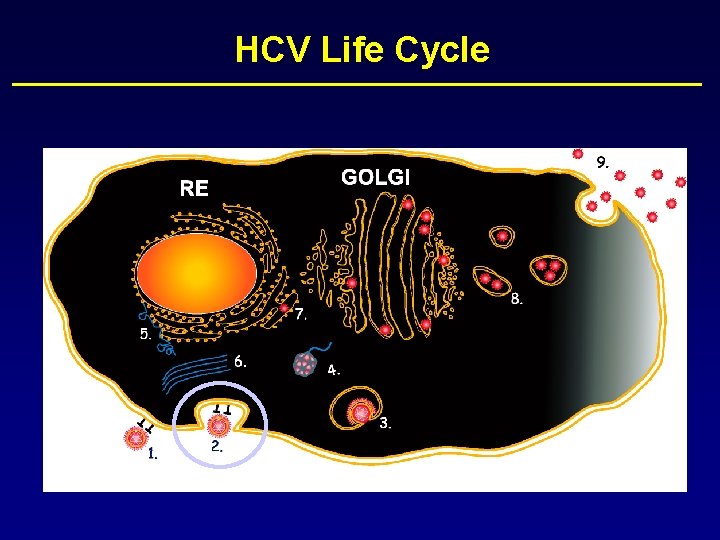
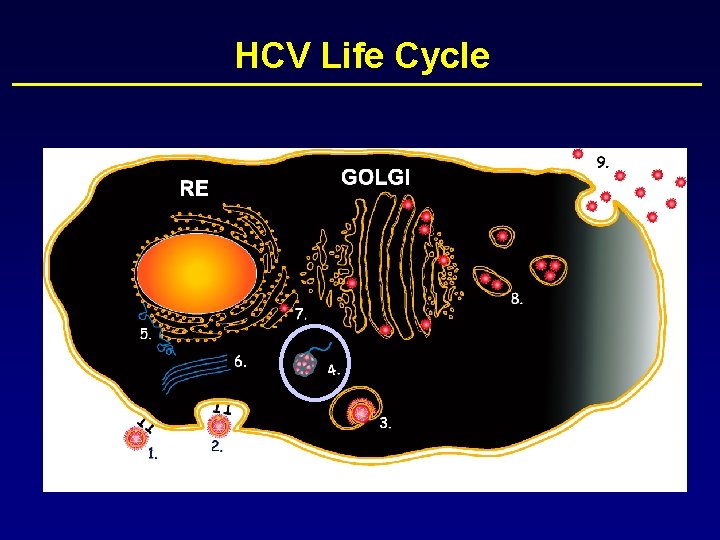
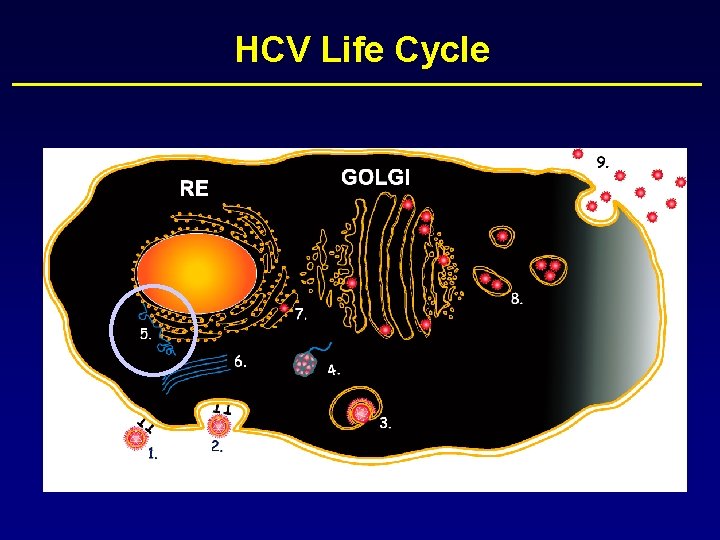
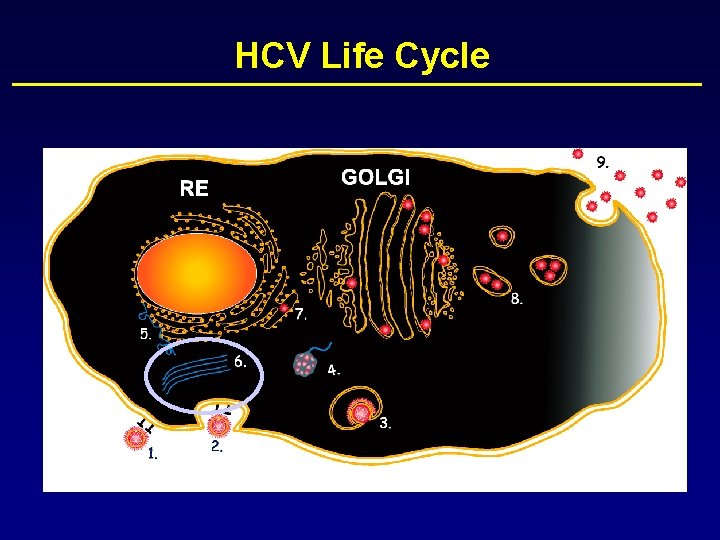
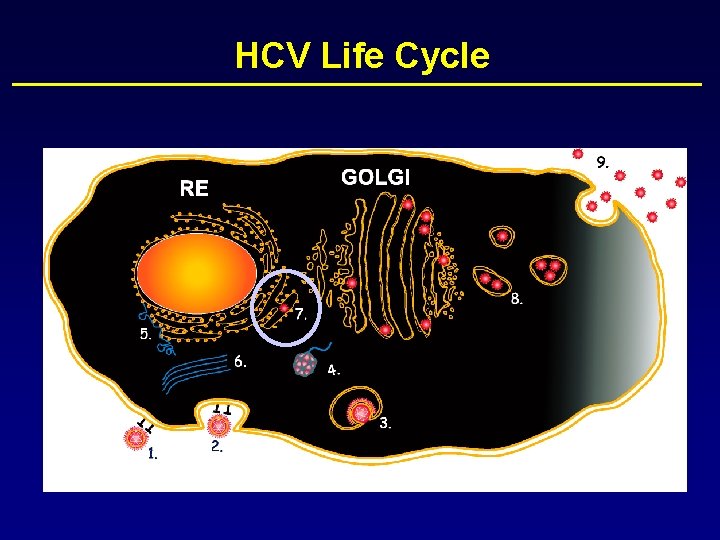
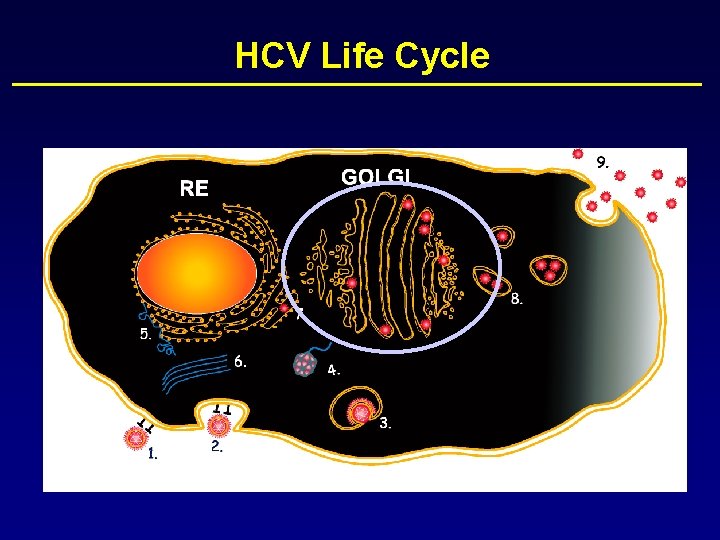
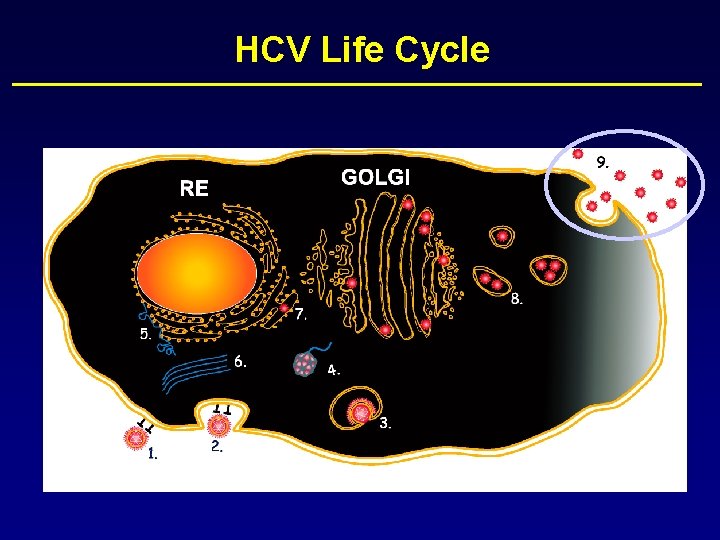
HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

HCV Life Cycle

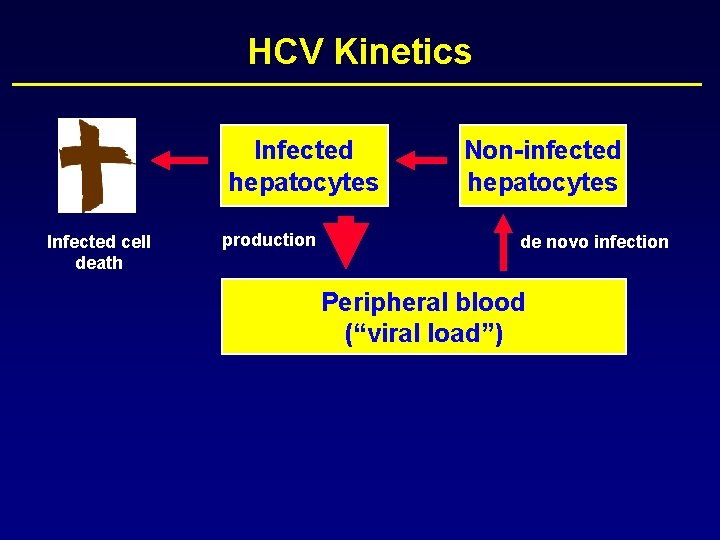
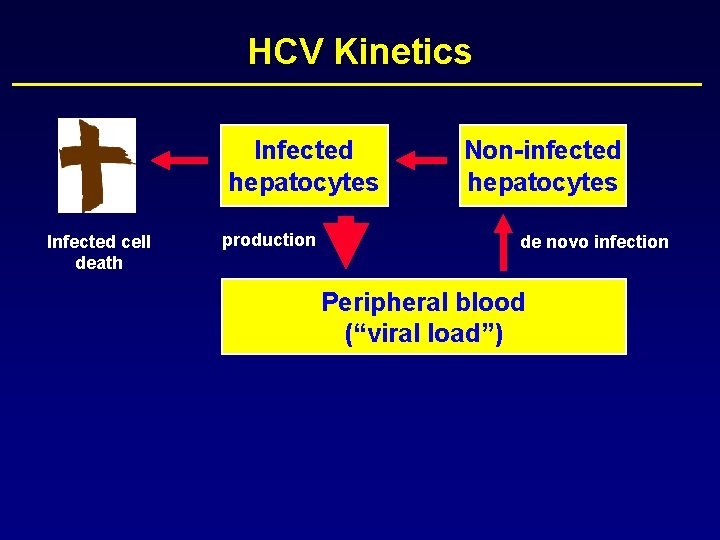
HCV Kinetics Infected hepatocytes Non-infected hepatocytes

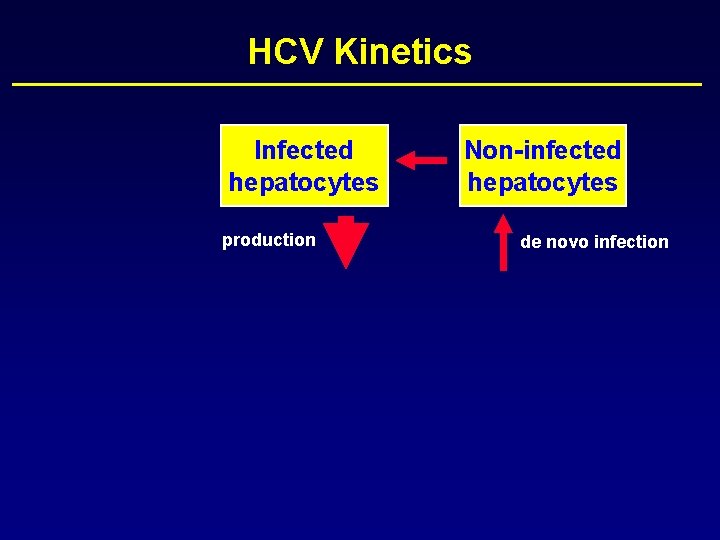
HCV Kinetics Infected hepatocytes production Non-infected hepatocytes

HCV Kinetics Infected hepatocytes production Non-infected hepatocytes de novo infection

HCV Kinetics Infected hepatocytes production Non-infected hepatocytes de novo infection

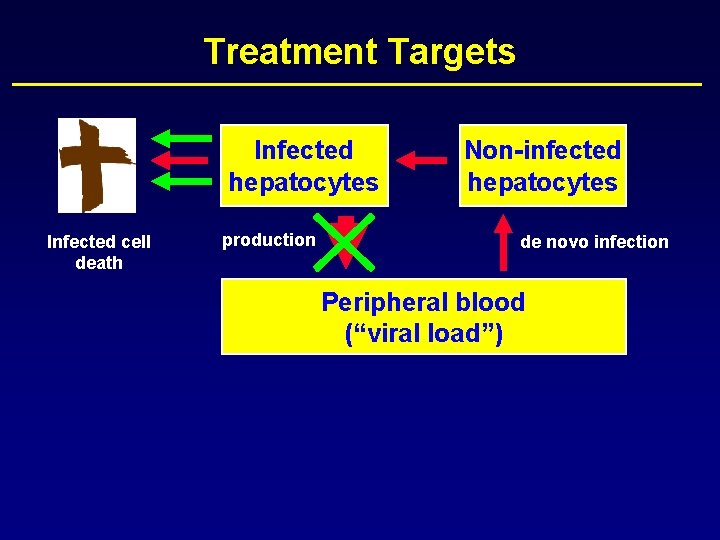
HCV Kinetics Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection

HCV Kinetics Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

HCV Kinetics Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

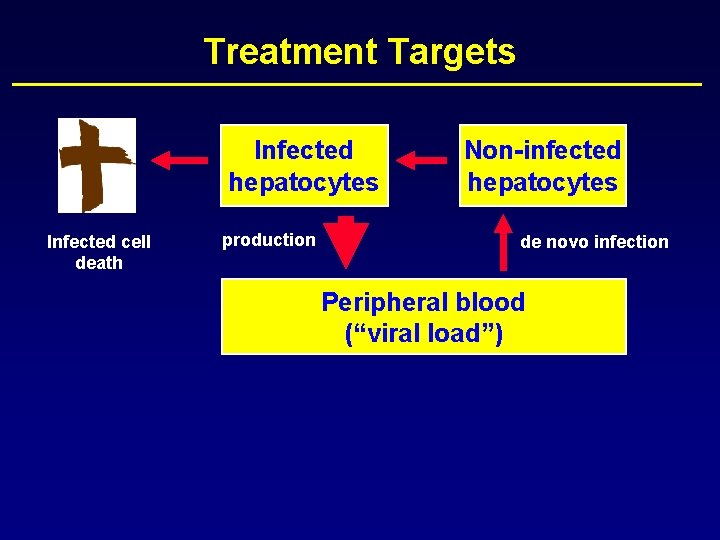
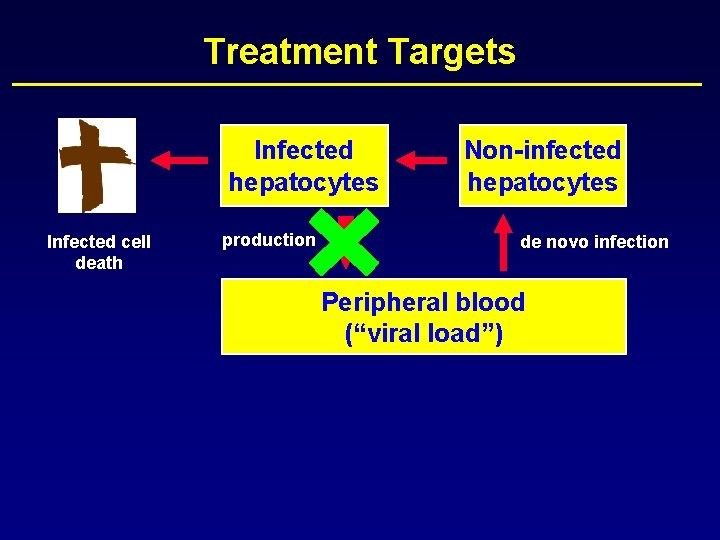
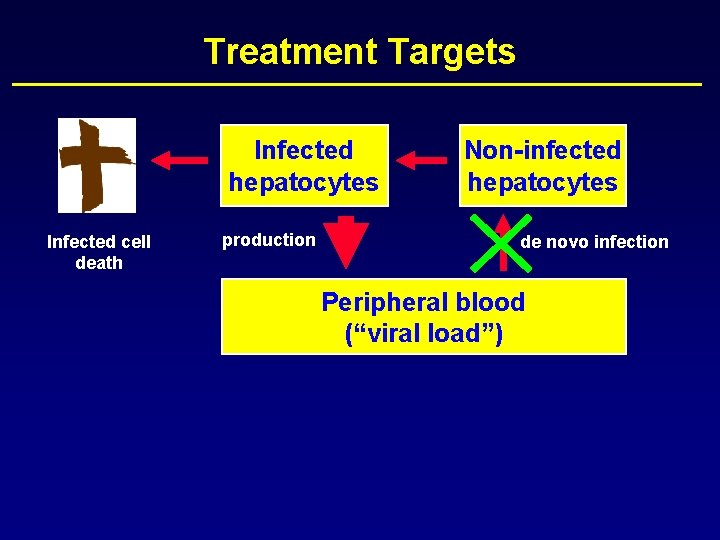
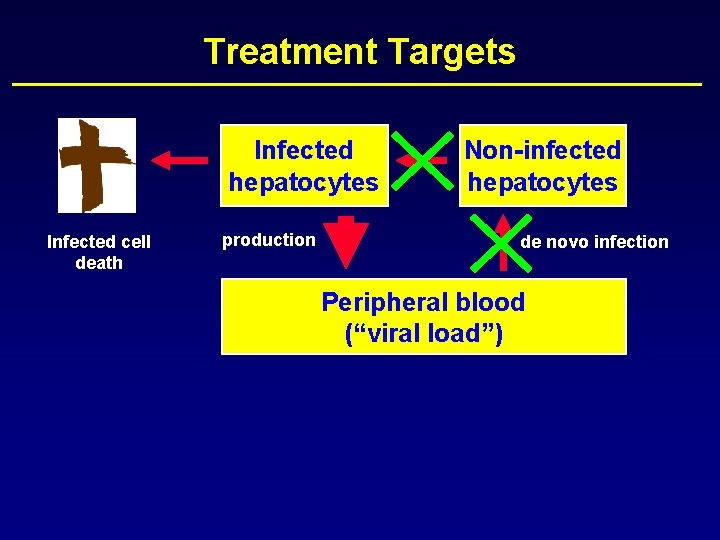
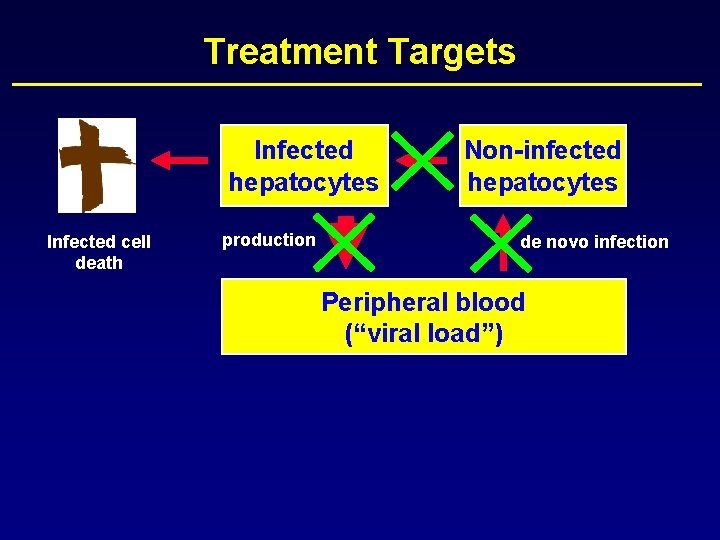
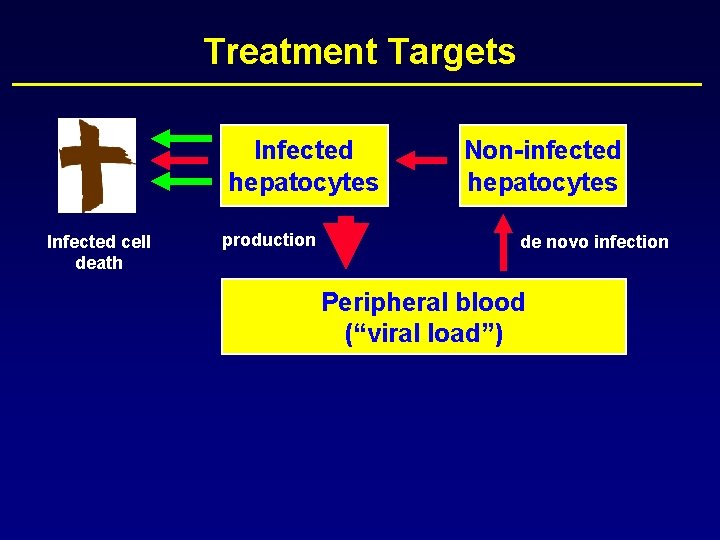
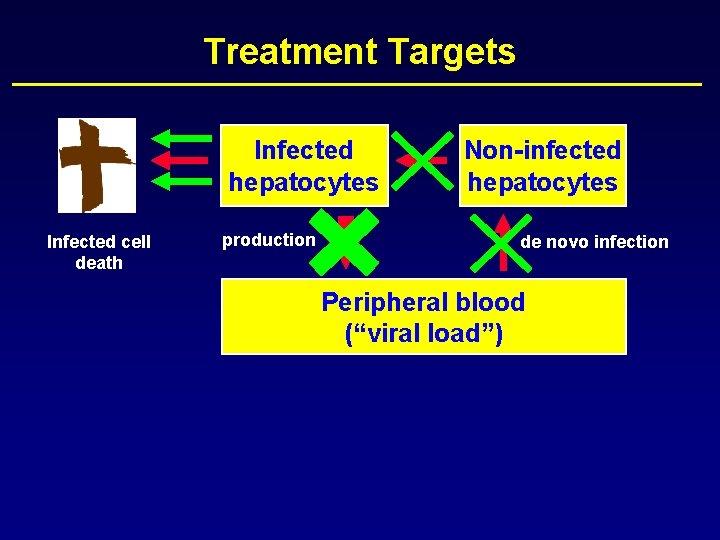
Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

Treatment Targets Infected hepatocytes Infected cell death production Non-infected hepatocytes de novo infection Peripheral blood (“viral load”)

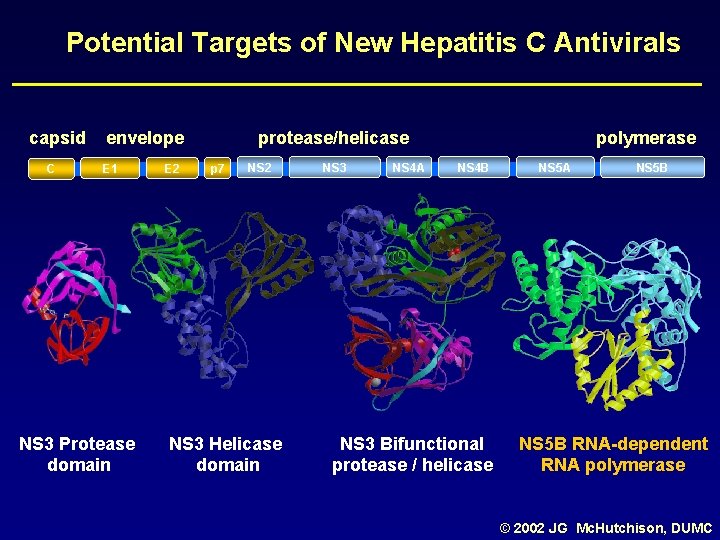
Potential Targets of New Hepatitis C Antivirals capsid C envelope E 1 NS 3 Protease domain E 2 protease/helicase p 7 NS 2 NS 3 Helicase domain NS 3 NS 4 A polymerase NS 4 B NS 3 Bifunctional protease / helicase NS 5 A NS 5 B RNA-dependent RNA polymerase © 2002 JG Mc. Hutchison, DUMC

New Compounds - Agenda • • • NM 283 Valopicitabine (Idenix) SCH 503034 (Schering Plough) VX-950 (Vertex) Albuferon (HGS) Viramidine (Valeant) CPG 10101

Early Clearance of HCV RNA with Valopicitabine (NM 283) plus Peg-Interferon in Treatment-Naïve Patients with HCV 1 infection: First Results from a Phase IIb Trial D Dieterich, E Lawitz, T Nguyen, Z Younes, J Santoro, N Gitlin, D Mc. Eniry, R Chasen, J Goff, S Knox, K Kleber, B Belanger K, N Brown and the Valopicitabine 006 Study Group EASL Annual Meeting April 29, 2006 Vienna, Austria

Valopicitabine (NM 283) First Nucleoside-Type HCV Pol Inhibitor • NS 5 b polymerase inhibitor – Ribonucleoside; cytidine analogue – NM 107 -triphosphate inhibits viral polymerase by viral RNA chain termination • Oral agent – Valyl ester pro-drug provides high oral bioavailability – Plasma half life (4 -6 hrs) & intracellular half life (15 hrs) support once daily dosing NH 2 NH O N HO O CH 3 Val-O OH Valopicitabine (NM 283) 2’-C-methylcytidine-3’-O-L-valine ester

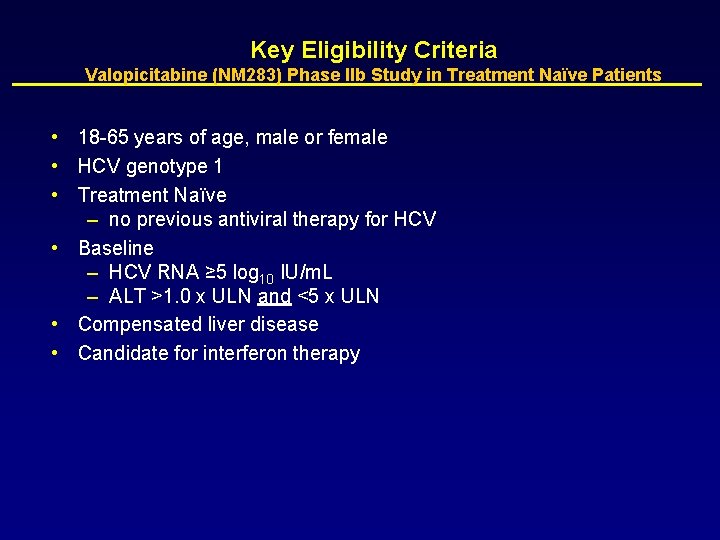
Key Eligibility Criteria Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients • 18 -65 years of age, male or female • HCV genotype 1 • Treatment Naïve – no previous antiviral therapy for HCV • Baseline – HCV RNA ≥ 5 log 10 IU/m. L – ALT >1. 0 x ULN and <5 x ULN • Compensated liver disease • Candidate for interferon therapy

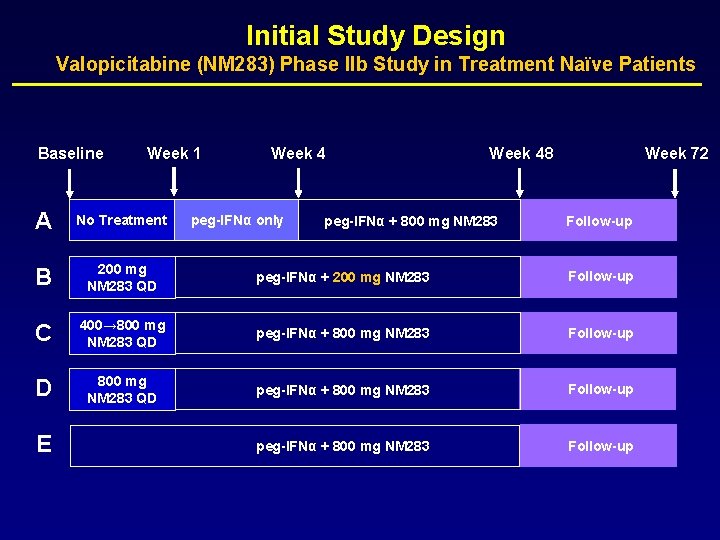
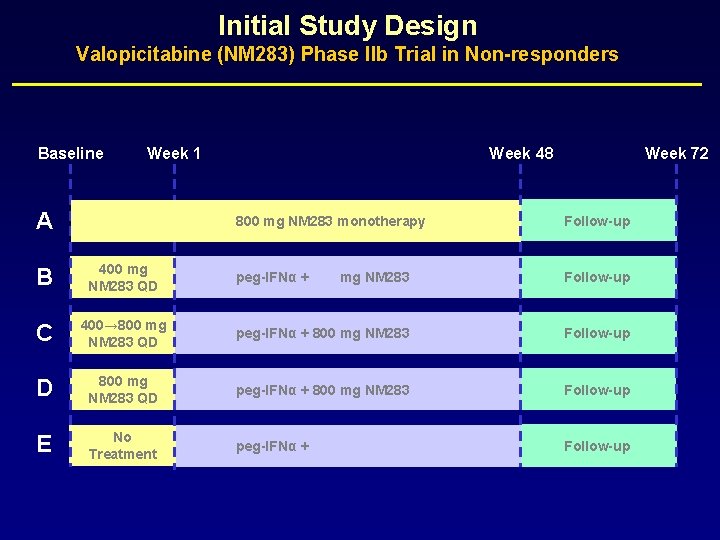
Initial Study Design Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients Baseline Week 1 Week 48 Week 72 A No Treatment B 200 mg NM 283 QD peg-IFNα + 200 mg NM 283 Follow-up C 400→ 800 mg NM 283 QD peg-IFNα + 800 mg NM 283 Follow-up D 800 mg NM 283 QD peg-IFNα + 800 mg NM 283 Follow-up E peg-IFNα only peg-IFNα + 800 mg NM 283 Follow-up

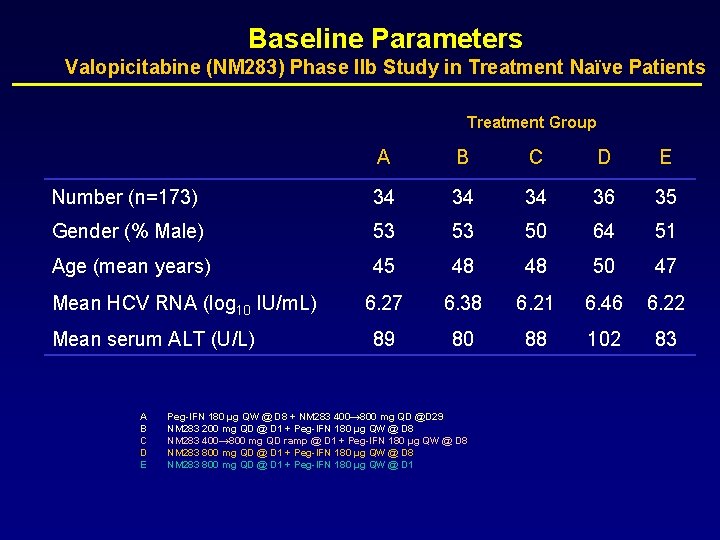
Baseline Parameters Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients Treatment Group A B C D E Number (n=173) 34 34 34 36 35 Gender (% Male) 53 53 50 64 51 Age (mean years) 45 48 48 50 47 6. 27 6. 38 6. 21 6. 46 6. 22 89 80 88 102 83 Mean HCV RNA (log 10 IU/m. L) Mean serum ALT (U/L) A B C D E Peg-IFN 180 µg QW @ D 8 + NM 283 400→ 800 mg QD @D 29 NM 283 200 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 400→ 800 mg QD ramp @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 800 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 1

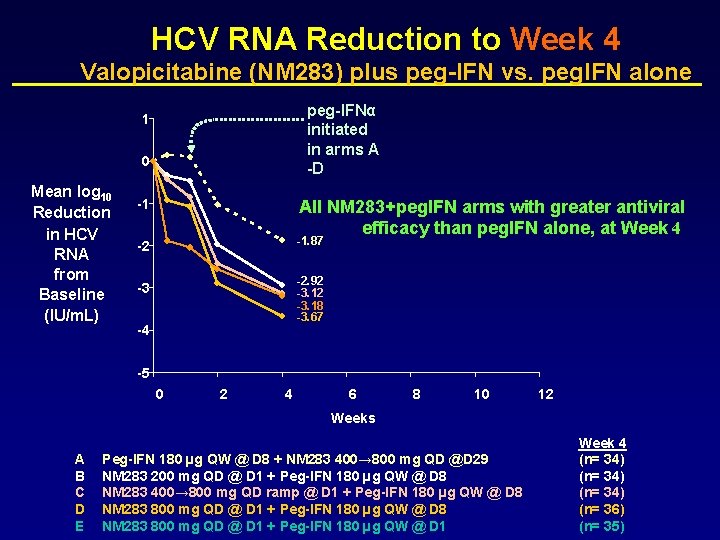
HCV RNA Reduction to Week 4 Valopicitabine (NM 283) plus peg-IFN vs. peg. IFN alone peg-IFNα initiated in arms A -D 1 0 Mean log 10 Reduction in HCV RNA from Baseline (IU/m. L) -1 All NM 283+peg. IFN arms with greater antiviral efficacy than peg. IFN alone, at Week 4 -2 -1. 87 -3 -2. 92 -3. 18 -3. 67 -4 -5 0 2 4 6 8 10 12 Weeks A B C D E Peg-IFN 180 µg QW @ D 8 + NM 283 400→ 800 mg QD @D 29 NM 283 200 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 400→ 800 mg QD ramp @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 800 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 1 Week 4 (n= 34) (n= 36) (n= 35)

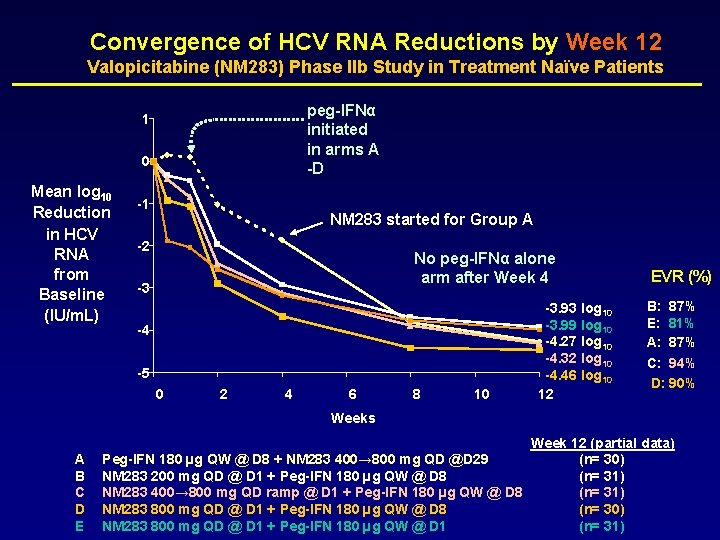
Convergence of HCV RNA Reductions by Week 12 Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients peg-IFNα initiated in arms A -D 1 0 Mean log 10 Reduction in HCV RNA from Baseline (IU/m. L) -1 NM 283 started for Group A -2 No peg-IFNα alone arm after Week 4 -3 -4 -5 0 2 4 6 8 10 -3. 93 log 10 -3. 99 log 10 -4. 27 log 10 -4. 32 log 10 -4. 46 log 10 12 EVR (%) B: 87% E: 81% A: 87% C: 94% D: 90% Weeks A B C D E Peg-IFN 180 µg QW @ D 8 + NM 283 400→ 800 mg QD @D 29 NM 283 200 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 400→ 800 mg QD ramp @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 800 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 1 Week 12 (partial data) (n= 30) (n= 31)

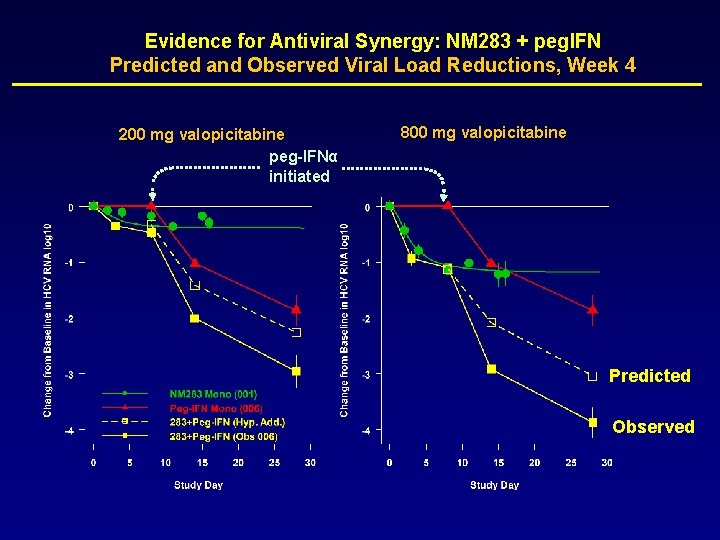
Evidence for Antiviral Synergy: NM 283 + peg. IFN Predicted and Observed Viral Load Reductions, Week 4 200 mg valopicitabine peg-IFNα initiated 800 mg valopicitabine Predicted Observed

Percent of Patients with HCV RNA PCR Negative Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients Solid Bars: < 600 IU/m. L (Amplicor detection limit) Stippled Bars: < 20 IU/m. L (Taqman detection limit) 70 71 77 83 77 73 76 80 67 65 Percent Of Patients Week 12 A B C D E Week 16 Peg-IFN 180 µg QW @ D 8 + NM 283 400→ 800 mg QD @D 29 NM 283 200 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 400→ 800 mg QD ramp @ D 1 + Peg-IFN 180 µg QW @ D 8 NM 283 800 mg QD @ D 1 + Peg-IFN 180 µg QW @ D 1

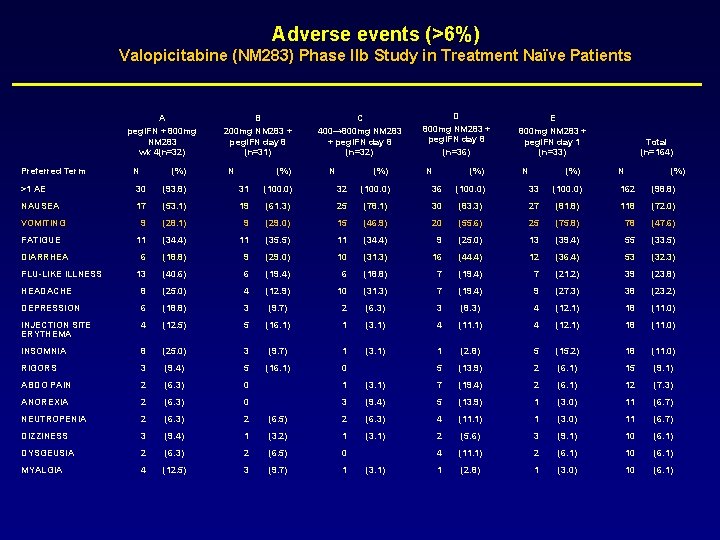
Adverse events (>6%) Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients A peg. IFN + 800 mg NM 283 wk 4(n=32) Preferred Term N (%) B 200 mg NM 283 + peg. IFN day 8 (n=31) N (%) C 400→ 800 mg NM 283 + peg. IFN day 8 (n=32) N (%) D 800 mg NM 283 + peg. IFN day 8 (n=36) N (%) E 800 mg NM 283 + peg. IFN day 1 (n=33) N (%) Total (n=164) N (%) >1 AE 30 (93. 8) 31 (100. 0) 32 (100. 0) 36 (100. 0) 33 (100. 0) 162 (98. 8) NAUSEA 17 (53. 1) 19 (61. 3) 25 (78. 1) 30 (83. 3) 27 (81. 8) 118 (72. 0) VOMITING 9 (28. 1) 9 (29. 0) 15 (46. 9) 20 (55. 6) 25 (75. 8) 78 (47. 6) FATIGUE 11 (34. 4) 11 (35. 5) 11 (34. 4) 9 (25. 0) 13 (39. 4) 55 (33. 5) 6 (18. 8) 9 (29. 0) 10 (31. 3) 16 (44. 4) 12 (36. 4) 53 (32. 3) 13 (40. 6) 6 (19. 4) 6 (18. 8) 7 (19. 4) 7 (21. 2) 39 (23. 8) HEADACHE 8 (25. 0) 4 (12. 9) 10 (31. 3) 7 (19. 4) 9 (27. 3) 38 (23. 2) DEPRESSION 6 (18. 8) 3 (9. 7) 2 (6. 3) 3 (8. 3) 4 (12. 1) 18 (11. 0) INJECTION SITE ERYTHEMA 4 (12. 5) 5 (16. 1) 1 (3. 1) 4 (11. 1) 4 (12. 1) 18 (11. 0) INSOMNIA 8 (25. 0) 3 (9. 7) 1 (3. 1) 1 (2. 8) 5 (15. 2) 18 (11. 0) RIGORS 3 (9. 4) 5 (16. 1) 0 5 (13. 9) 2 (6. 1) 15 (9. 1) ABDO PAIN 2 (6. 3) 0 1 (3. 1) 7 (19. 4) 2 (6. 1) 12 (7. 3) ANOREXIA 2 (6. 3) 0 3 (9. 4) 5 (13. 9) 1 (3. 0) 11 (6. 7) NEUTROPENIA 2 (6. 3) 2 (6. 5) 2 (6. 3) 4 (11. 1) 1 (3. 0) 11 (6. 7) DIZZINESS 3 (9. 4) 1 (3. 2) 1 (3. 1) 2 (5. 6) 3 (9. 1) 10 (6. 1) DYSGEUSIA 2 (6. 3) 2 (6. 5) 0 4 (11. 1) 2 (6. 1) 10 (6. 1) MYALGIA 4 (12. 5) 3 (9. 7) 1 1 (2. 8) 1 (3. 0) 10 (6. 1) DIARRHEA FLU-LIKE ILLNESS (3. 1)

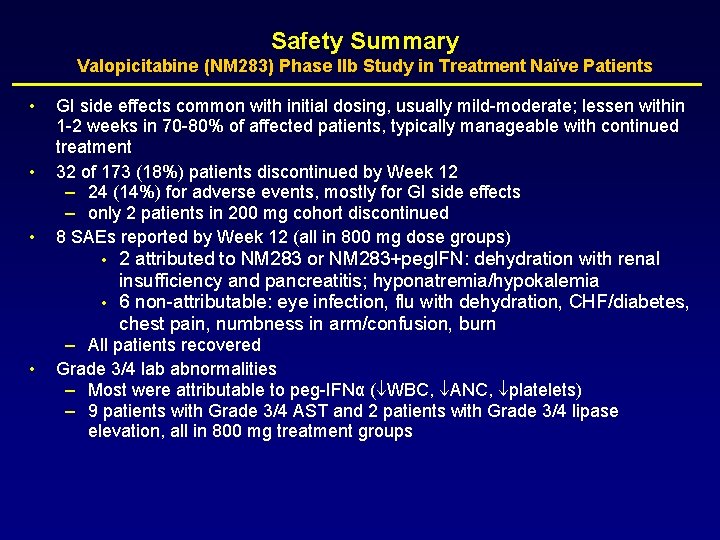
Safety Summary Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients • • • GI side effects common with initial dosing, usually mild-moderate; lessen within 1 -2 weeks in 70 -80% of affected patients, typically manageable with continued treatment 32 of 173 (18%) patients discontinued by Week 12 – 24 (14%) for adverse events, mostly for GI side effects – only 2 patients in 200 mg cohort discontinued 8 SAEs reported by Week 12 (all in 800 mg dose groups) • • • 2 attributed to NM 283 or NM 283+peg. IFN: dehydration with renal insufficiency and pancreatitis; hyponatremia/hypokalemia 6 non-attributable: eye infection, flu with dehydration, CHF/diabetes, chest pain, numbness in arm/confusion, burn – All patients recovered Grade 3/4 lab abnormalities – Most were attributable to peg-IFNα ( WBC, ANC, platelets) – 9 patients with Grade 3/4 AST and 2 patients with Grade 3/4 lipase elevation, all in 800 mg treatment groups

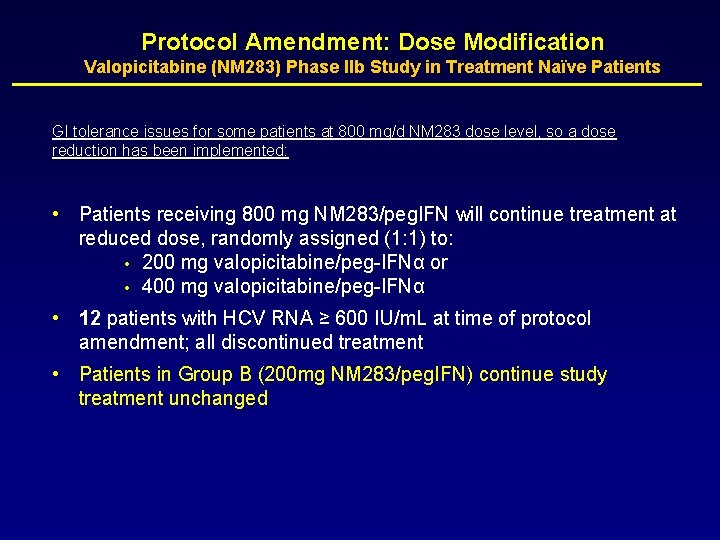
Protocol Amendment: Dose Modification Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients GI tolerance issues for some patients at 800 mg/d NM 283 dose level, so a dose reduction has been implemented: • Patients receiving 800 mg NM 283/peg. IFN will continue treatment at reduced dose, randomly assigned (1: 1) to: • 200 mg valopicitabine/peg-IFNα or • 400 mg valopicitabine/peg-IFNα • 12 patients with HCV RNA ≥ 600 IU/m. L at time of protocol amendment; all discontinued treatment • Patients in Group B (200 mg NM 283/peg. IFN) continue study treatment unchanged

Valopicitabine Development: Next Steps First nucleoside-type HCV polymerase inhibitor • 200 -400 mg valopicitabine doses chosen for further study in treatment-naïve patients – Good antiviral efficacy with good safety/tolerance – Antiviral efficacy at Week 12 comparable to higher (800 mg/d) dosing regimens • Ribavirin / NM 283 interaction study – starts 2 Q 2006 • Potential investigation of double and triple regimens (NM 283 + peg. IFN + ribavirin) in phase III clinical trials • Global Phase III program as collaboration between Idenix and Novartis

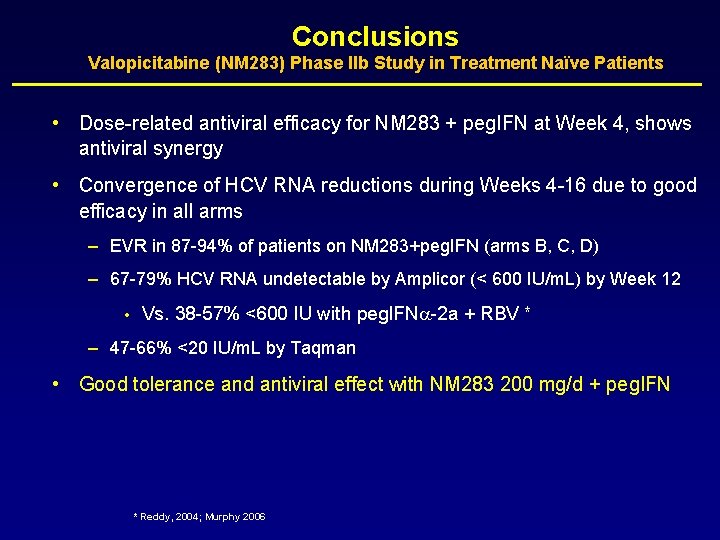
Conclusions Valopicitabine (NM 283) Phase IIb Study in Treatment Naïve Patients • Dose-related antiviral efficacy for NM 283 + peg. IFN at Week 4, shows antiviral synergy • Convergence of HCV RNA reductions during Weeks 4 -16 due to good efficacy in all arms – EVR in 87 -94% of patients on NM 283+peg. IFN (arms B, C, D) – 67 -79% HCV RNA undetectable by Amplicor (< 600 IU/m. L) by Week 12 • Vs. 38 -57% <600 IU with peg. IFN -2 a + RBV * – 47 -66% <20 IU/m. L by Taqman • Good tolerance and antiviral effect with NM 283 200 mg/d + peg. IFN * Reddy, 2004; Murphy 2006

Randomized Trial of Valopicitabine (NM 283) Alone and in Combination with Peg-Interferon vs. Retreatment with Peg-Interferon plus Ribavirin (Peg. IFN/RBV) in Hepatitis C Patients with Previous Non-Response to Peg. IFN/RBV: Second Interim Results N. Afdhal, C. O’Brien, E. Godofsky, M. Rodriguez-Torres, S. Pappas, P. Pockros, E. Lawitz, N. Bzowej, V. Rustgi, M. Sulkowski, K. Sherman, I. Jacobson, G. Chao, S. Knox, K. Pietropaolo, and N. Brown EASL Annual Meeting April 28, 2006 Vienna, Austria

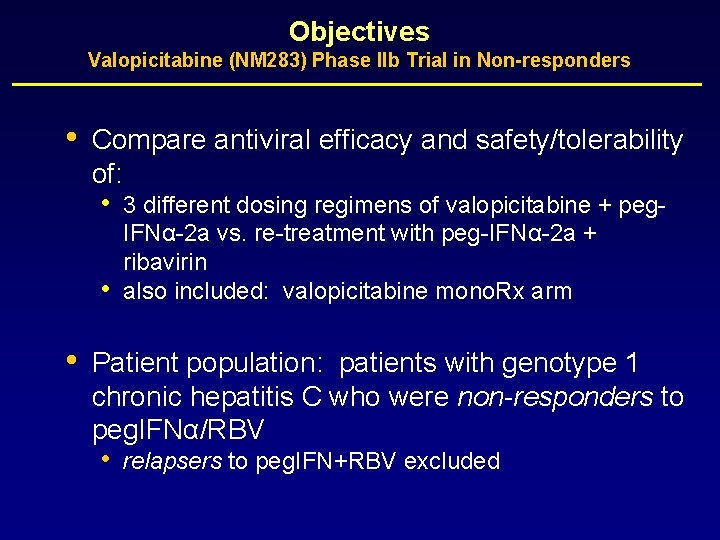
Objectives Valopicitabine (NM 283) Phase IIb Trial in Non-responders • Compare antiviral efficacy and safety/tolerability of: • • • 3 different dosing regimens of valopicitabine + peg. IFNα-2 a vs. re-treatment with peg-IFNα-2 a + ribavirin also included: valopicitabine mono. Rx arm Patient population: patients with genotype 1 chronic hepatitis C who were non-responders to peg. IFNα/RBV • relapsers to peg. IFN+RBV excluded

Key Eligibility Criteria Valopicitabine (NM 283) Phase IIb Trial in Non-responders • • • 18 -65 years of age, male or female HCV genotype 1 Non-responders to previous adequate treatment course • At least 12 weeks of peg. IFN + RBV • At least 75% of the prescribed doses peg. IFN/RBV • Failed to clear HCV RNA to non-detectable levels • Patients must have previously failed for efficacy, not safety Screen/Baseline • HCV RNA ≥ 105 IU/m. L • ALT < 5 x ULN Compensated liver disease

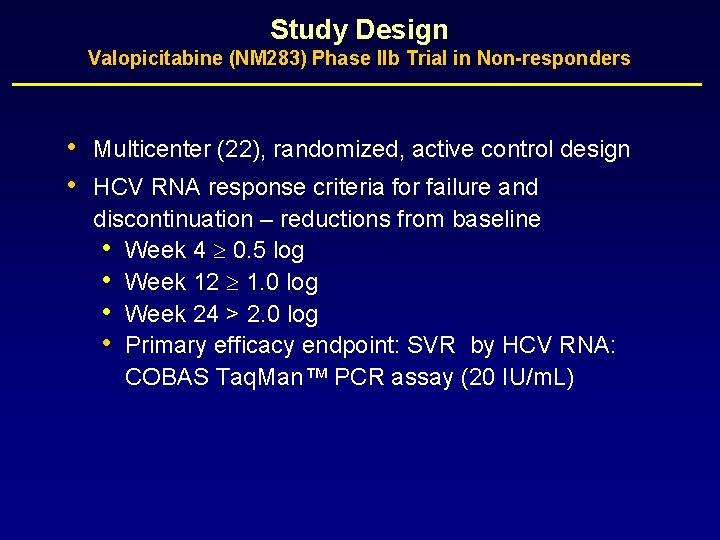
Study Design Valopicitabine (NM 283) Phase IIb Trial in Non-responders • • Multicenter (22), randomized, active control design HCV RNA response criteria for failure and discontinuation – reductions from baseline • Week 4 0. 5 log • Week 12 1. 0 log • Week 24 > 2. 0 log • Primary efficacy endpoint: SVR by HCV RNA: COBAS Taq. Man™ PCR assay (20 IU/m. L)

Initial Study Design Valopicitabine (NM 283) Phase IIb Trial in Non-responders Baseline Week 1 A Week 48 Week 72 800 mg NM 283 monotherapy Follow-up B 400 mg NM 283 QD peg-IFNα + 400 mg NM 283 Follow-up C 400→ 800 mg NM 283 QD peg-IFNα + 800 mg NM 283 Follow-up D 800 mg NM 283 QD peg-IFNα + 800 mg NM 283 Follow-up E No Treatment peg-IFNα + 1000 -1200 mg ribavirin Follow-up

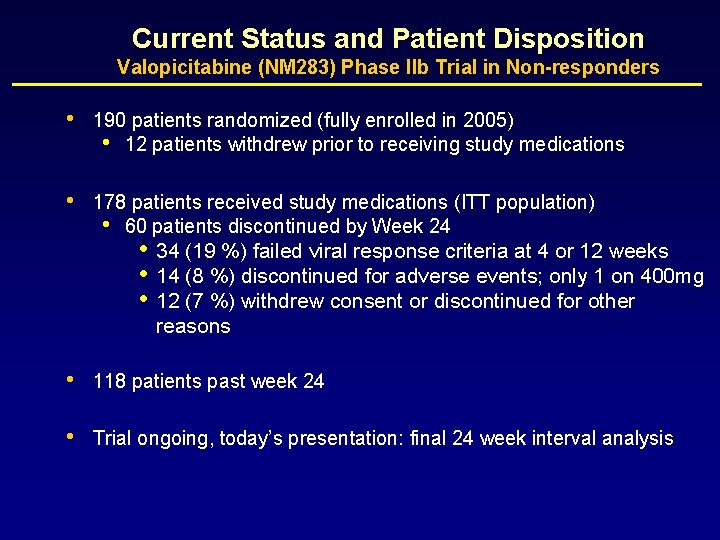
Current Status and Patient Disposition Valopicitabine (NM 283) Phase IIb Trial in Non-responders • 190 patients randomized (fully enrolled in 2005) • 12 patients withdrew prior to receiving study medications • 178 patients received study medications (ITT population) • 60 patients discontinued by Week 24 • • • 34 (19 %) failed viral response criteria at 4 or 12 weeks 14 (8 %) discontinued for adverse events; only 1 on 400 mg 12 (7 %) withdrew consent or discontinued for other reasons • 118 patients past week 24 • Trial ongoing, today’s presentation: final 24 week interval analysis

Mean Reduction HCV RNA to Week 24 Valopicitabine (NM 283) Phase IIb Trial in Non-responders 0 Wks 12 -24 n=7 -0. 5 Serum HCV RNA (Mean Log 10 Change From Baseline) A 0. 46 log -1 -1. 5 -2 -2. 5 E 2. 27 log B 2. 45 log -3 C 2. 99 log D 3. 29 log -3. 5 0 6 12 18 24 30 Study Week A B C D E NM 283 800 mg QD NM 283 400 mg QD + peg-IFN 180 µg QW NM 283 400→ 800 mg QD ramp (1 st week)→ 800 mg QD +peg-IFN 180 µg QW NM 283 800 mg QD + peg-IFN 180 µg QW Ribavirin + peg-IFN 180 µg QW @ D 8 (n=21) (n=41) (n=34) n = ITT Population

Antiviral Efficacy at Week 24 Valopicitabine (NM 283) Phase IIb Trial in Non-responders >2 log Reduction N Mean HCV RNA (log 10 IU/m. L) PCR –ve Number (%) A NM 283 mono. Rx 21 0. 46 0 0 (0%) B NM 283 400 + peg. IFN 41 2. 45 23 (56%) 7 (17%) C NM 283 400 -800 + Peg. IFN 41 2. 99 * 30 (73%) * 5 (12%) D NM 283 800 + peg. IFN 41 3. 29 * 29 (71%) * 10 (24%) E peg. IFN + RBV retreatment 34 2. 27 16 (47%) 6 (18%)# Treatment Group * p < 0. 02 vs peg. IFN + RBV (comparisons of C and D to E) #Residual viral load (median HCV RNA level) is ca. 1. 8 log 10 higher in control arm E vs. high-dose NM 283 arms (C and D) at W 24. Favors increasing difference in HCV RNA clearance to non-detectable over time.

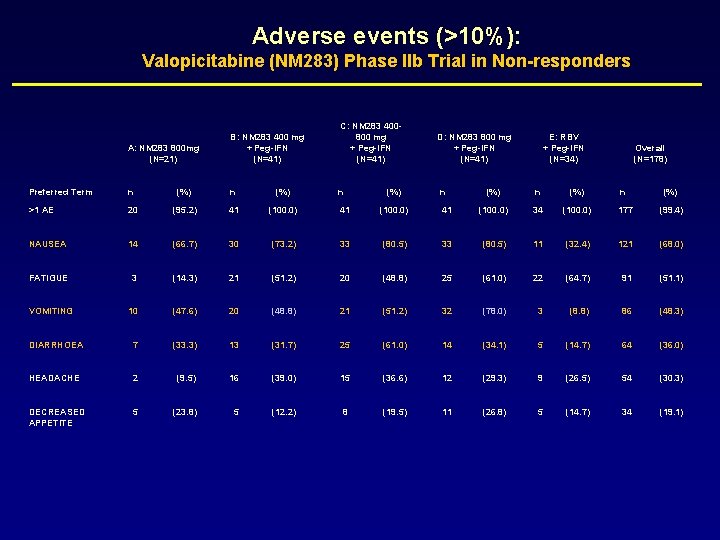
Adverse events (>10%): Valopicitabine (NM 283) Phase IIb Trial in Non-responders A: NM 283 800 mg (N=21) B: NM 283 400 mg + Peg-IFN (N=41) C: NM 283 400800 mg + Peg-IFN (N=41) Preferred Term n (%) n >1 AE 20 (95. 2) 41 (100. 0) NAUSEA 14 (66. 7) 30 (73. 2) 33 FATIGUE 3 (14. 3) 21 (51. 2) VOMITING 10 (47. 6) 20 DIARRHOEA 7 (33. 3) HEADACHE 2 DECREASED APPETITE 5 (%) D: NM 283 800 mg + Peg-IFN (N=41) n E: RBV + Peg-IFN (N=34) Overall (N=178) (%) n (%) 41 (100. 0) 34 (100. 0) 177 (99. 4) (80. 5) 33 (80. 5) 11 (32. 4) 121 (68. 0) 20 (48. 8) 25 (61. 0) 22 (64. 7) 91 (51. 1) (48. 8) 21 (51. 2) 32 (78. 0) 3 (8. 8) 86 (48. 3) 13 (31. 7) 25 (61. 0) 14 (34. 1) 5 (14. 7) 64 (36. 0) (9. 5) 16 (39. 0) 15 (36. 6) 12 (29. 3) 9 (26. 5) 54 (30. 3) (23. 8) 5 (12. 2) 8 (19. 5) 11 (26. 8) 5 (14. 7) 34 (19. 1)

Safety Summary to Week 24 Valopicitabine (NM 283) Phase IIb Trial in Non-responders • Nausea (with/without vomiting) common with initiation of NM 283 + peg. IFN treatment; diarrhea is less common; usually transient or intermittent - 4 patients (3%) discontinued for GI side effects • 24 serious adverse events (SAEs) through Week 24 • 6 reported possibly attributed to NM 283 (anemia, colitis, dehydration, fatigue, pancreatitis, gram-negative bacteremia 2° to UTI) • Sporadic elevations of amylase, lipase, AST, ALT • rarely treatment-limiting

SCH 503034 A HCV Protease Inhibitor

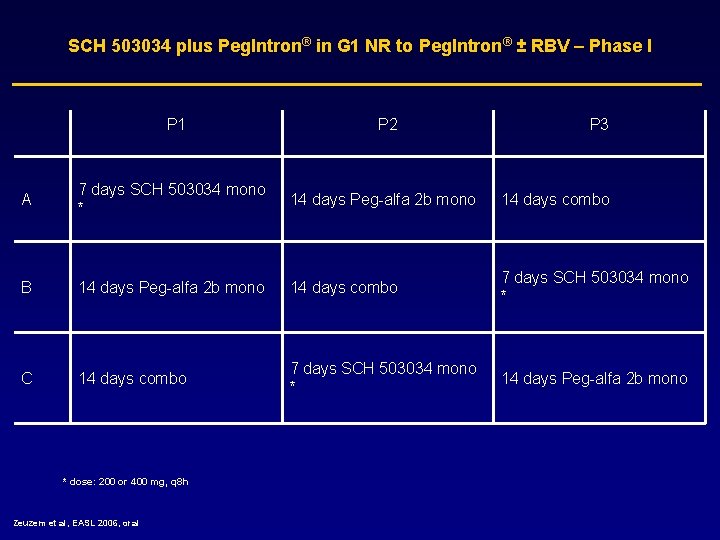
SCH 503034 plus Peg. Intron® in G 1 NR to Peg. Intron® ± RBV – Phase I P 1 P 2 P 3 A 7 days SCH 503034 mono * 14 days Peg-alfa 2 b mono 14 days combo B 14 days Peg-alfa 2 b mono 14 days combo 7 days SCH 503034 mono * C 14 days combo 7 days SCH 503034 mono * 14 days Peg-alfa 2 b mono * dose: 200 or 400 mg, q 8 h Zeuzem et al, EASL 2006, oral

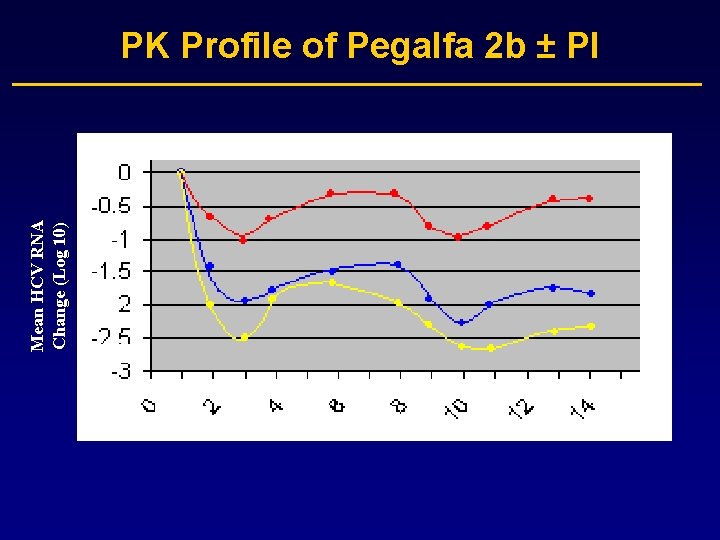
Mean HCV RNA Change (Log 10) PK Profile of Pegalfa 2 b ± PI

SCH 503034 plus Peg-alfa 2 b in G 1 NR to Pegalfa 2 b ± RBV – Phase I • Results: – Mean max log 10 viral load drop 2. 4 and 2. 9 log for 200 and 400 mg combined with Peg-IFN alfa 2 b • 4/10 became HCV RNA negative on combination therapy with 400 mg SCH 503034 • HCV RNA reduction disappointing for a PI (mean 1. 5 log highest monotherapy dose) • No resistance reported • q 8 h dosing is a challenge for patients Zeuzem et al, EASL 2006, oral

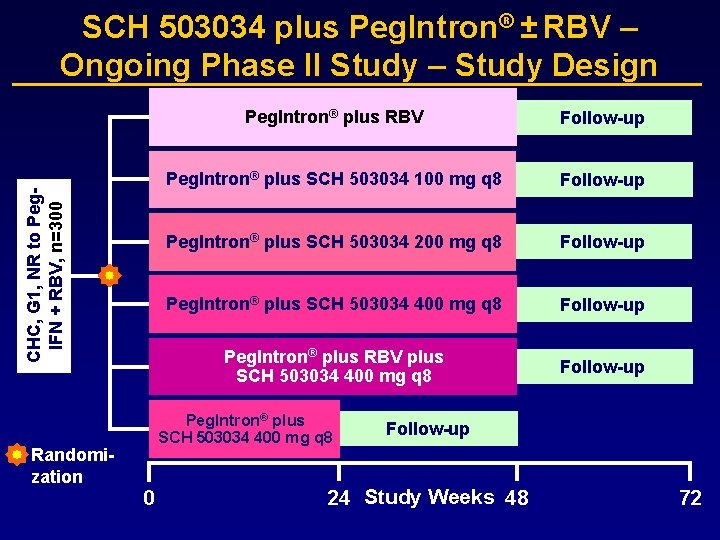
CHC, G 1, NR to Peg. IFN + RBV, n=300 SCH 503034 plus Peg. Intron® ± RBV – Ongoing Phase II Study – Study Design Randomization Peg. Intron® plus RBV Follow-up Peg. Intron® plus SCH 503034 100 mg q 8 Follow-up Peg. Intron® plus SCH 503034 200 mg q 8 Follow-up Peg. Intron® plus SCH 503034 400 mg q 8 Follow-up Peg. Intron® plus RBV plus SCH 503034 400 mg q 8 Follow-up Peg. Intron® plus SCH 503034 400 mg q 8 0 Follow-up 24 Study Weeks 48 72

VX-950 A HCV Protease Inhibitor

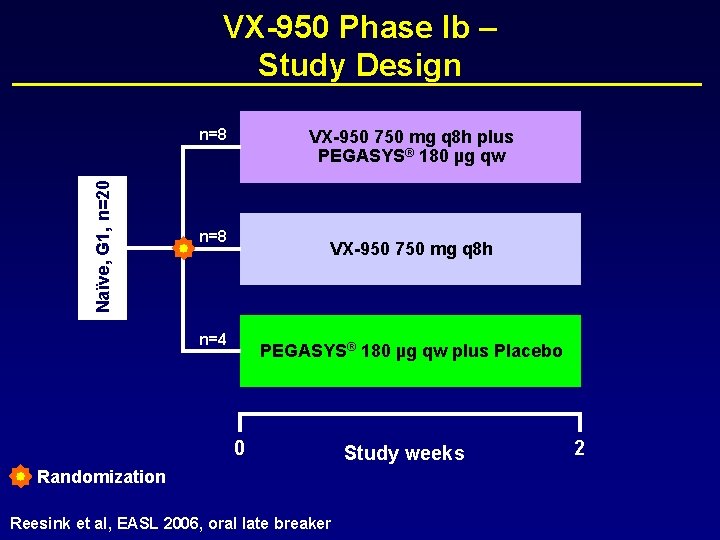
VX-950 Phase Ib – Study Design Naïve, G 1, n=20 n=8 VX-950 750 mg q 8 h plus PEGASYS® 180 µg qw n=8 VX-950 750 mg q 8 h n=4 PEGASYS® 180 µg qw plus Placebo 0 Randomization Reesink et al, EASL 2006, oral late breaker Study weeks 2

VX-950 – Results Reesink et al, EASL 2006, oral late breaker

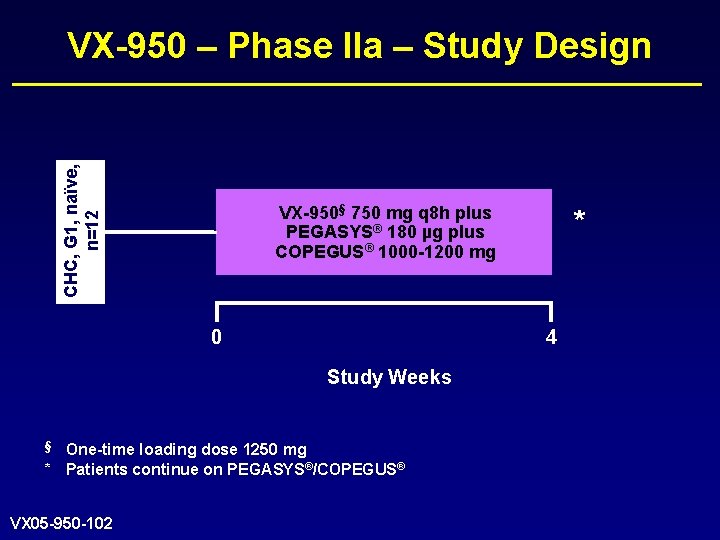
CHC, G 1, naïve, n=12 VX-950 – Phase IIa – Study Design VX-950§ 750 mg q 8 h plus PEGASYS® 180 µg plus COPEGUS® 1000 -1200 mg 0 4 Study Weeks § One-time loading dose 1250 mg * Patients continue on PEGASYS®/COPEGUS® VX 05 -950 -102 *

VX-950 – Results End of VX 05 -950 -102

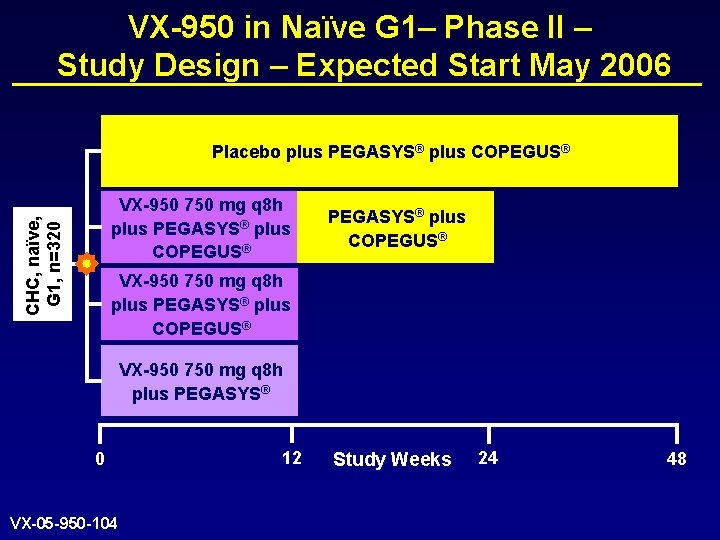
VX-950 in Naïve G 1– Phase II – Study Design – Expected Start May 2006 Placebo plus PEGASYS® plus COPEGUS® CHC, naïve, G 1, n=320 VX-950 750 mg q 8 h plus PEGASYS® plus COPEGUS® VX-950 750 mg q 8 h plus PEGASYS® 0 VX-05 -950 -104 12 Study Weeks 24 48

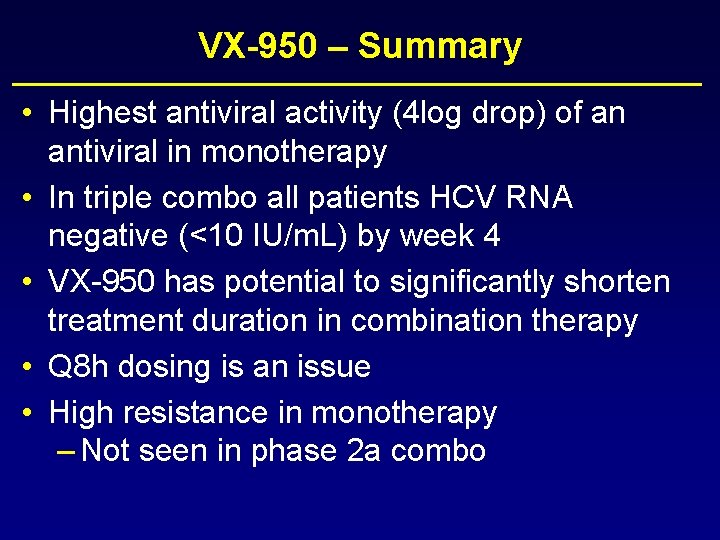
VX-950 – Summary • Highest antiviral activity (4 log drop) of an antiviral in monotherapy • In triple combo all patients HCV RNA negative (<10 IU/m. L) by week 4 • VX-950 has potential to significantly shorten treatment duration in combination therapy • Q 8 h dosing is an issue • High resistance in monotherapy – Not seen in phase 2 a combo

New Long Acting IFNs Albuferon

Albuferon Characteristics • IFN alfa-2 fused with human serum albumin • higher efficacy not expected • better convenience because of less frequent dosing • currently in phase II • possible launch 2010

CHC, naïve, G 1, n=458 Albuferon in Naïve G 1 – Phase IIb – Study Design 0 PEGASYS® 180 µg qw plus COPEGUS® 1000/1200 mg/d Follow-up Albuferon 900 µg q 2 w plus RBV 1000/1200 mg/d Follow-up Albuferon 1200 µg q 4 w plus RBV 1000/1200 mg/d Follow-up Study Weeks Zeuzem et al, EASL 2006, oral late breaker 48 72

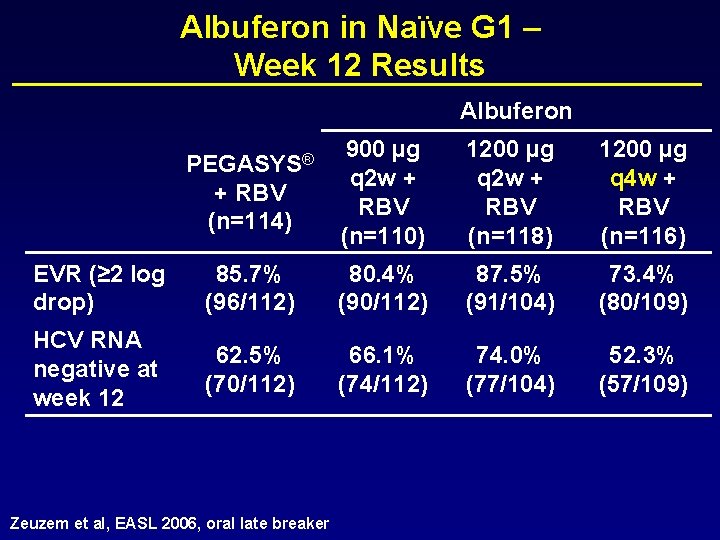
Albuferon in Naïve G 1 – Week 12 Results Albuferon PEGASYS® + RBV (n=114) 900 µg q 2 w + RBV (n=110) 1200 µg q 2 w + RBV (n=118) 1200 µg q 4 w + RBV (n=116) EVR (≥ 2 log drop) 85. 7% (96/112) 80. 4% (90/112) 87. 5% (91/104) 73. 4% (80/109) HCV RNA negative at week 12 62. 5% (70/112) 66. 1% (74/112) 74. 0% (77/104) 52. 3% (57/109) Zeuzem et al, EASL 2006, oral late breaker

Viramidine A Ribavirin Prodrug

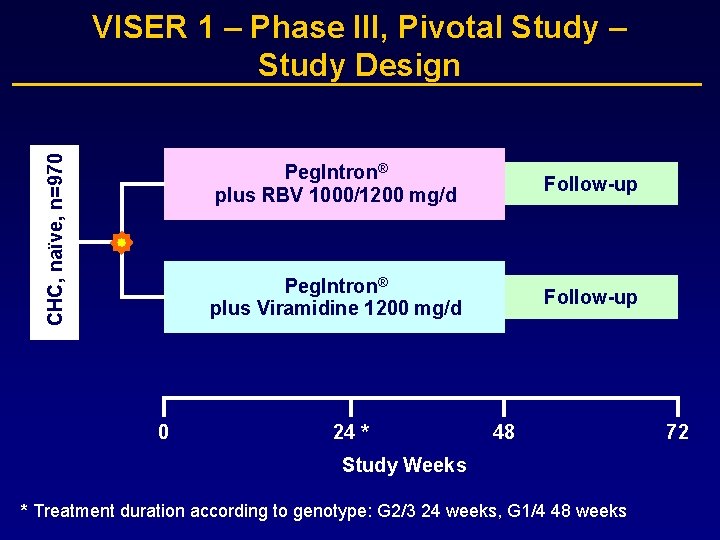
CHC, naïve, n=970 VISER 1 – Phase III, Pivotal Study – Study Design 0 Peg. Intron® plus RBV 1000/1200 mg/d Follow-up Peg. Intron® plus Viramidine 1200 mg/d Follow-up 24 * 48 Study Weeks * Treatment duration according to genotype: G 2/3 24 weeks, G 1/4 48 weeks 72

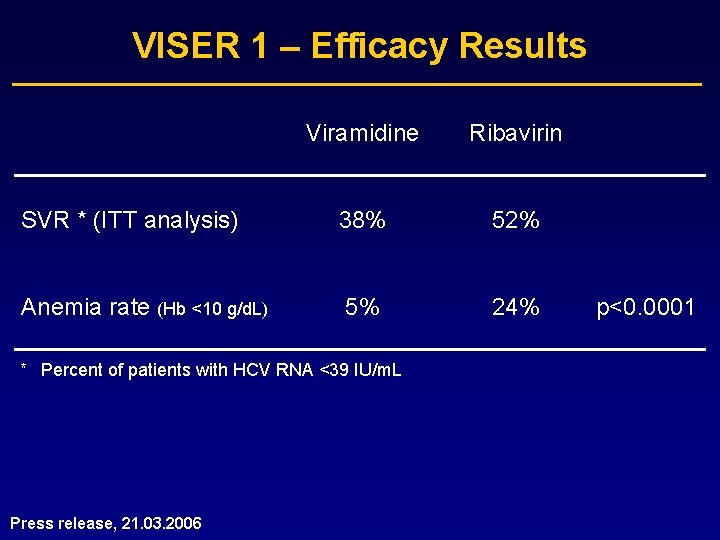
VISER 1 – Efficacy Results Viramidine Ribavirin SVR * (ITT analysis) 38% 52% Anemia rate (Hb <10 g/d. L) 5% 24% * Percent of patients with HCV RNA <39 IU/m. L Press release, 21. 03. 2006 p<0. 0001

VISER 1 – Viramidine * Weight-Based Analysis Viramidine dose n SVR § Anemia ‡ ≤ 18 mg/kg 323 47% 4. 3% 19 -22 mg/kg 82 66% 2. 4% ≥ 23 mg/kg 16 81% 12. 5% * Fixed dose of Viramidine averaged ~15 mg/kg, based on mean weight for the study population § HCV RNA <39 IU/m. L, per protocol analysis ‡ Hb <10 g/d. L Press release, 21. 03. 2006

VISER 2 • Identical study design to VISER 1 but PEGASYS® instead of Peg. Intron® • Results available in Q 3/2006

CPG 10101

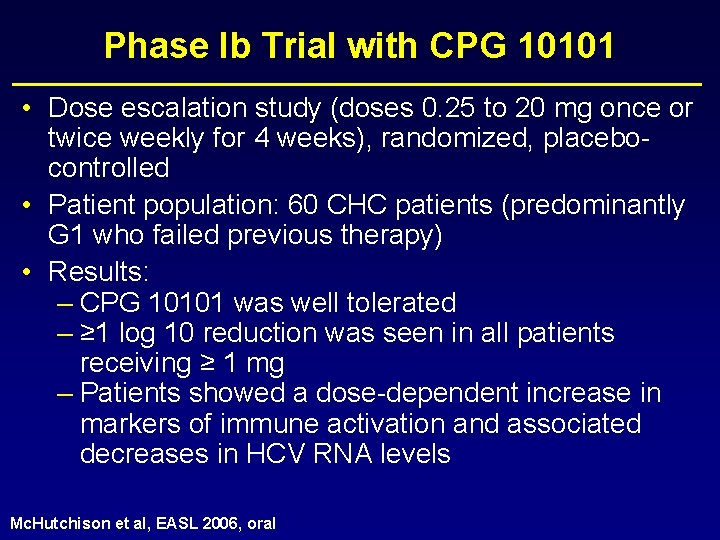
Phase Ib Trial with CPG 10101 • CPG 10101 is a synthetic oligonucleotide and selective Toll. Like. Receptor 9 agonist which enhances the ability of dendritic cells to activate killer T cells against invaders. Actilon appears to stimulate TLR 9 in a different way resulting in significantly stronger activation of interferon-α production by the plasmacytoid dendritic cells.

Phase Ib Trial with CPG 10101 • Dose escalation study (doses 0. 25 to 20 mg once or twice weekly for 4 weeks), randomized, placebocontrolled • Patient population: 60 CHC patients (predominantly G 1 who failed previous therapy) • Results: – CPG 10101 was well tolerated – ≥ 1 log 10 reduction was seen in all patients receiving ≥ 1 mg – Patients showed a dose-dependent increase in markers of immune activation and associated decreases in HCV RNA levels Mc. Hutchison et al, EASL 2006, oral

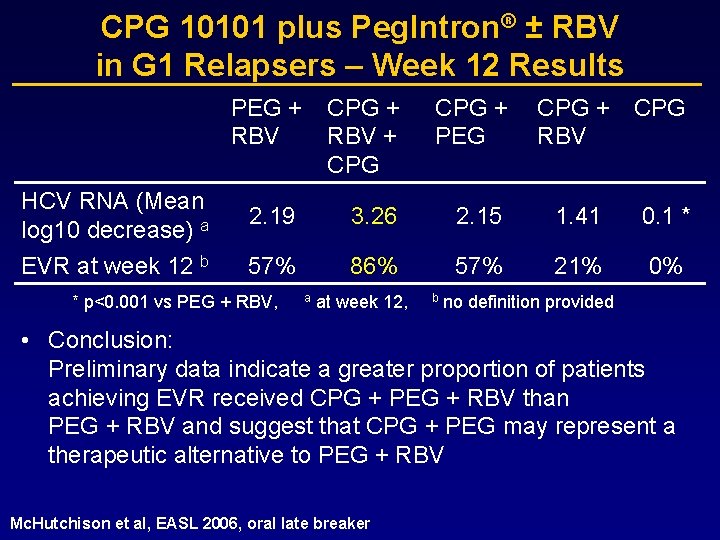
CHC, G 1, relapsers to PEG-IFN + RBV, n=74 CPG 10101 plus Peg. Intron® ± RBV in G 1 Relapsers – Study Design Peg. Intron® 1. 5 µg/kg/wk plus RBV 800 -1400 mg/d Follow-up Peg. Intron® 1. 5 µg/kg/wk plus RBV 800 -1400 mg/d plus CPG 0. 2 mg/kg/wk Follow-up Peg. Intron® 1. 5 µg/kg/wk plus CPG 0. 2 mg/kg/wk Follow-up CPG 0. 2 mg/kg/wk plus RBV 800 -1400 mg/d Follow-up CPG 0. 2 mg/kg/wk Follow-up 0 Randomization Study Weeks 48 72

CPG 10101 plus Peg. Intron® ± RBV in G 1 Relapsers – Week 12 Results PEG + RBV CPG + RBV + CPG + PEG HCV RNA (Mean log 10 decrease) a 2. 19 3. 26 2. 15 1. 41 0. 1 * EVR at week 12 b 57% 86% 57% 21% 0% * p<0. 001 vs PEG + RBV, a at week 12, b CPG + CPG RBV no definition provided • Conclusion: Preliminary data indicate a greater proportion of patients achieving EVR received CPG + PEG + RBV than PEG + RBV and suggest that CPG + PEG may represent a therapeutic alternative to PEG + RBV Mc. Hutchison et al, EASL 2006, oral late breaker

Summary • Antiviral compounds are expected to change HCV treatment significantly by – increasing SVR rates – shortening treatment duration – Until now Peg seems to be the backbone of HCV therapy in the future.
 Uda nuove risorse digitali e loro impatto sulla didattica
Uda nuove risorse digitali e loro impatto sulla didattica Nuove risorse digitali e loro impatto sulla didattica
Nuove risorse digitali e loro impatto sulla didattica Exercitii dislalie
Exercitii dislalie Elektrokonvulzivní terapie zkušenosti
Elektrokonvulzivní terapie zkušenosti Hcv treatment
Hcv treatment Hcv symptoms female
Hcv symptoms female Ultimate analysis formula
Ultimate analysis formula Hcv
Hcv Hcv treatment
Hcv treatment Hiv test window period
Hiv test window period Moral anomalies by raffaele garofalo
Moral anomalies by raffaele garofalo Raffaele flaminio
Raffaele flaminio Dimer san raffaele milano
Dimer san raffaele milano Azzolini gastroenterologo
Azzolini gastroenterologo Kristina gregorc
Kristina gregorc Gigino milia
Gigino milia Raffaele colaizzo
Raffaele colaizzo Raffaele pozzi
Raffaele pozzi Raffaele gratton
Raffaele gratton Raffaele flaminio
Raffaele flaminio Raffaele flaminio
Raffaele flaminio Ente per le nuove tecnologie
Ente per le nuove tecnologie Nuove tecnologie fotovoltaico
Nuove tecnologie fotovoltaico Vecchie e nuove dipendenze
Vecchie e nuove dipendenze Tesina sulle nuove tecnologie
Tesina sulle nuove tecnologie Le nuove teorie di legame zanichelli
Le nuove teorie di legame zanichelli Nuove tendenze del turismo
Nuove tendenze del turismo I limiti della teoria di lewis
I limiti della teoria di lewis Nuove linee guida blsd
Nuove linee guida blsd Ente per le nuove tecnologie
Ente per le nuove tecnologie Eugenio montale satura
Eugenio montale satura Aumento sigarette camel
Aumento sigarette camel Camminiamo sulla strada testo
Camminiamo sulla strada testo Due cuori a distanza frasi
Due cuori a distanza frasi Annotazioni sulla verifica effettuata peer to peer
Annotazioni sulla verifica effettuata peer to peer I pesci come sono fatti
I pesci come sono fatti Madre teresa frasi
Madre teresa frasi La netiquette il galateo di internet
La netiquette il galateo di internet Conversazione clinica esempio
Conversazione clinica esempio Padrino cresima mano sulla spalla
Padrino cresima mano sulla spalla Mappa concettuale sulla libertà
Mappa concettuale sulla libertà Esercizi piano cartesiano e retta
Esercizi piano cartesiano e retta Unità didattica sulla favola scuola primaria
Unità didattica sulla favola scuola primaria Sulla inimicos suos
Sulla inimicos suos Similitudini sulla luna esempi
Similitudini sulla luna esempi I lipidi lasciano una macchia traslucida sulla carta
I lipidi lasciano una macchia traslucida sulla carta Prove della sfericità della terra zanichelli
Prove della sfericità della terra zanichelli Fonti produzione
Fonti produzione Carme 101 catullo analisi
Carme 101 catullo analisi Video povertà nel mondo
Video povertà nel mondo Rap della costituzione testo
Rap della costituzione testo U.d.a sulla gestione dei conflitti
U.d.a sulla gestione dei conflitti Frase negativa e interrogativa in francese
Frase negativa e interrogativa in francese Piove di d'annunzio
Piove di d'annunzio Slide sulla ricerca attiva del lavoro
Slide sulla ricerca attiva del lavoro Attributi essere parmenide
Attributi essere parmenide Mappa concettuale fegato
Mappa concettuale fegato Volume acqua sulla terra
Volume acqua sulla terra Racconti sulla pioggia scuola infanzia
Racconti sulla pioggia scuola infanzia Altimetro basato sulla posizione
Altimetro basato sulla posizione Parole gentili
Parole gentili Protuberanze sulla lingua
Protuberanze sulla lingua Frasi sulla testa dura
Frasi sulla testa dura Setacciatura metodo di separazione
Setacciatura metodo di separazione Casa sulla michaelerplatz
Casa sulla michaelerplatz La bella zoppa
La bella zoppa Stelle sulla terra trama
Stelle sulla terra trama Astolfo sulla luna riassunto
Astolfo sulla luna riassunto Cinque bagnanti cezanne
Cinque bagnanti cezanne Ricerca
Ricerca Modello delle credenze sulla salute
Modello delle credenze sulla salute Insieme delle acque presenti sulla terra
Insieme delle acque presenti sulla terra Uda legalità scuola primaria
Uda legalità scuola primaria Differenza tra nota integrativa e relazione sulla gestione
Differenza tra nota integrativa e relazione sulla gestione Vieni santo spirito come vento soffia sulla chiesa
Vieni santo spirito come vento soffia sulla chiesa Istituto omnicomprensivo montesano sulla marcellana
Istituto omnicomprensivo montesano sulla marcellana Resta accanto a me testo
Resta accanto a me testo Racconti sulla legalità scuola media
Racconti sulla legalità scuola media Ricerca sulla finlandia
Ricerca sulla finlandia Monaco sulla spiaggia friedrich
Monaco sulla spiaggia friedrich A livella poesia testo
A livella poesia testo Mappa testo espositivo
Mappa testo espositivo Le grazie naturali magritte
Le grazie naturali magritte