Organic Chemistry 10 The Alkanes Leaving Certificate Chemistry








- Slides: 36

Organic Chemistry 10 – The Alkanes Leaving Certificate Chemistry

Contents The Alkanes • Chemical Structures of the Alkanes • Saturated compounds Isomers • Butane • Propane Physical Properties & Cyclohexa ne Reactions of the Alkanes • Chemical Structure of Cyclohexane • Formation of a chloroalkane

Hydrocarbons 1. The Alkanes 2. The Alkenes 3. The Alkynes (Ethyne) 4. Aromatic

Objectives Today you should learn about. . • What a homologous series is • What saturated means and how to test for it • What an isomer is and how to name isomers of alkanes

Homework • From the worksheet: Questions 114 - 121 • Learn the definitions of : • Homologous series, saturated and isomer

2006 Q. 9 (a) (5)

The Alkanes: Cn. H 2 n+2 These are the main constituents of crude oil The simplest member is methane Methane – CH 4

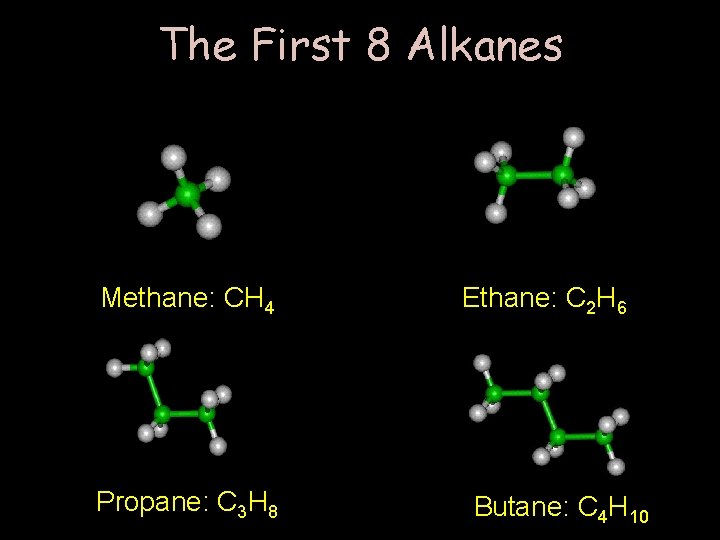
The First 8 Alkanes Methane: CH 4 Propane: C 3 H 8 Ethane: C 2 H 6 Butane: C 4 H 10

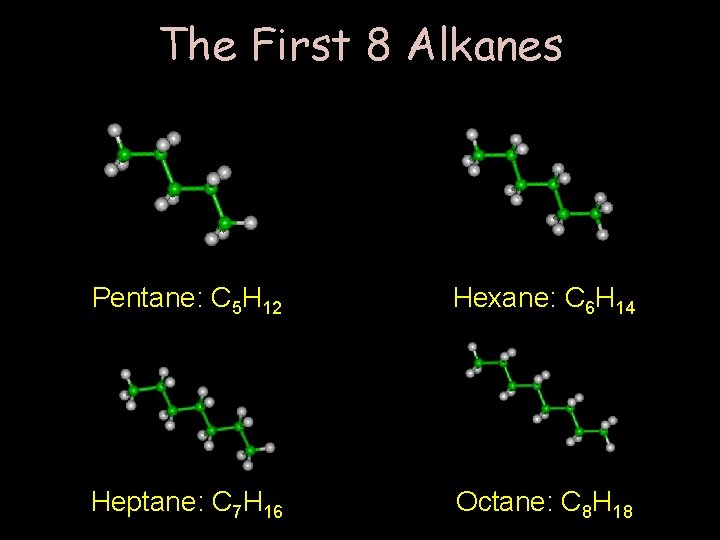
The First 8 Alkanes Pentane: C 5 H 12 Hexane: C 6 H 14 Heptane: C 7 H 16 Octane: C 8 H 18

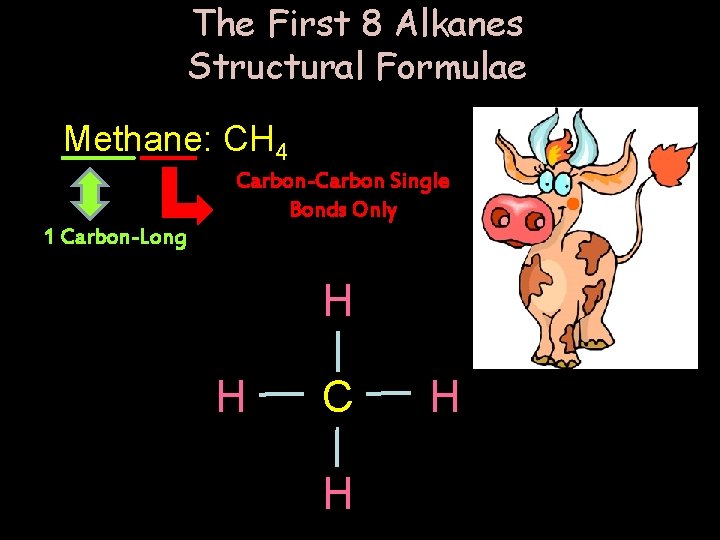
The First 8 Alkanes Structural Formulae Methane: CH 4 Carbon-Carbon Single Bonds Only 1 Carbon-Long H H C H H

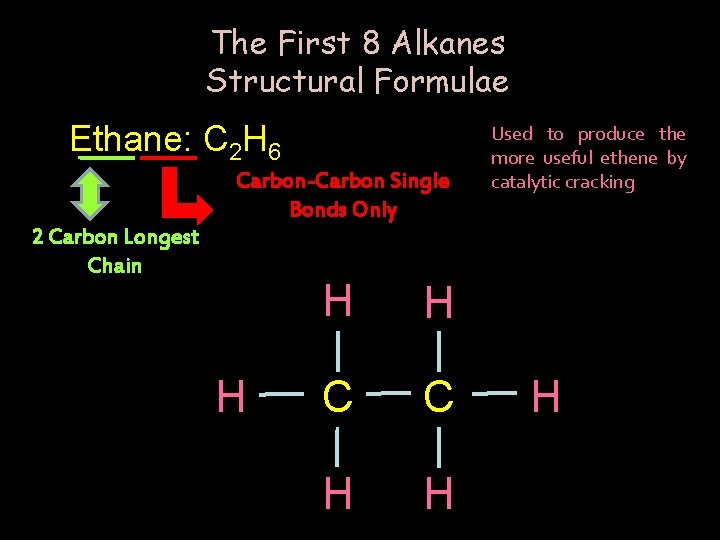
The First 8 Alkanes Structural Formulae Ethane: C 2 H 6 Carbon-Carbon Single Bonds Only 2 Carbon Longest Chain H H H C C H H Used to produce the more useful ethene by catalytic cracking H

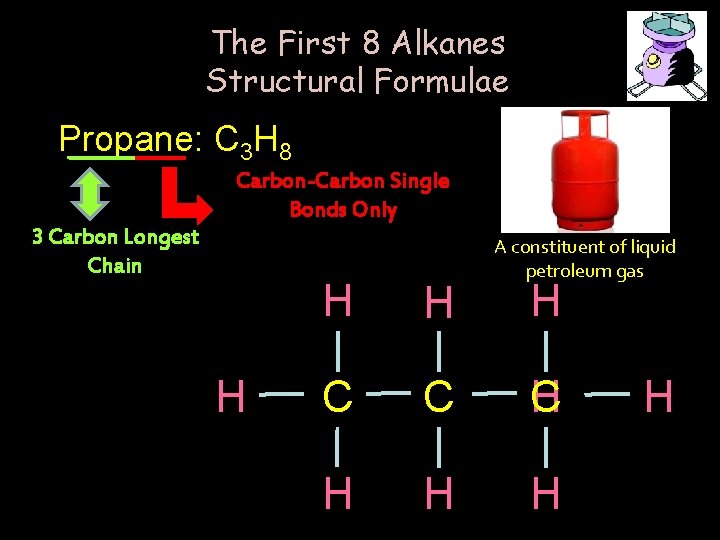
The First 8 Alkanes Structural Formulae Propane: C 3 H 8 Carbon-Carbon Single Bonds Only 3 Carbon Longest Chain H A constituent of liquid petroleum gas H H H C C H H H H

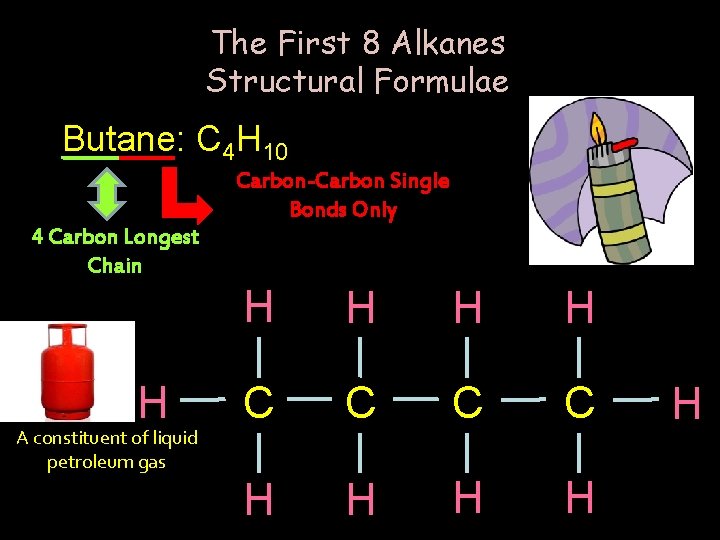
The First 8 Alkanes Structural Formulae Butane: C 4 H 10 Carbon-Carbon Single Bonds Only 4 Carbon Longest Chain H A constituent of liquid petroleum gas H H C C H H H

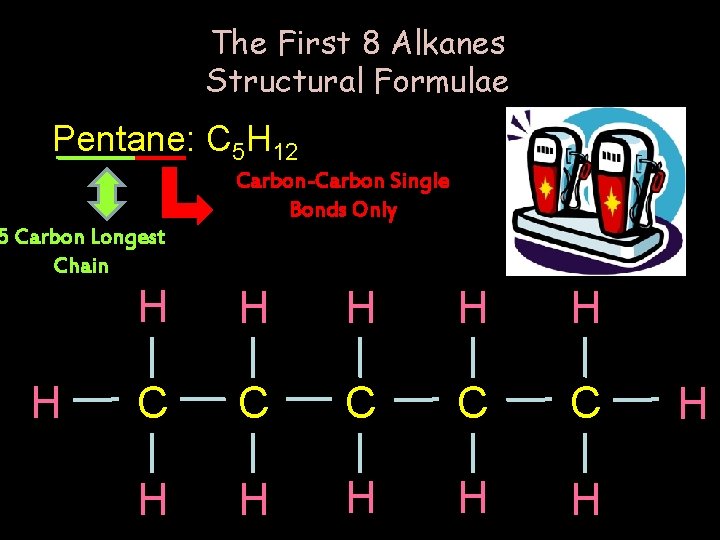
The First 8 Alkanes Structural Formulae Pentane: C 5 H 12 Carbon-Carbon Single Bonds Only 5 Carbon Longest Chain H H H C C C H H H

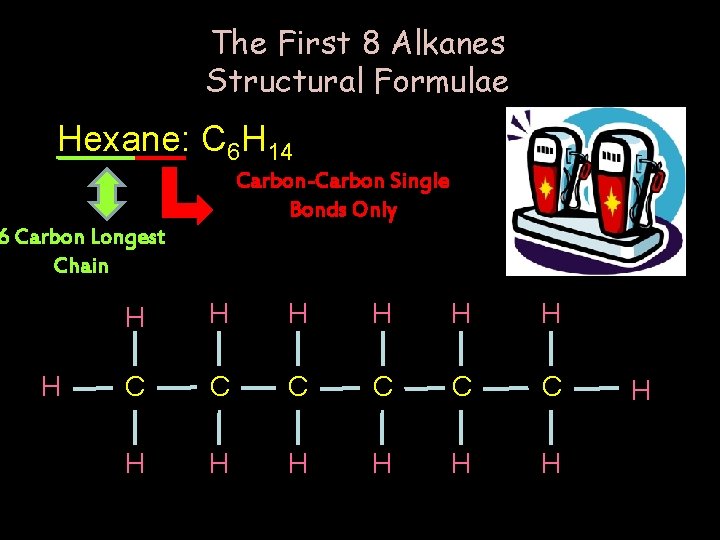
The First 8 Alkanes Structural Formulae Hexane: C 6 H 14 Carbon-Carbon Single Bonds Only 6 Carbon Longest Chain H H H H C C C H H H H

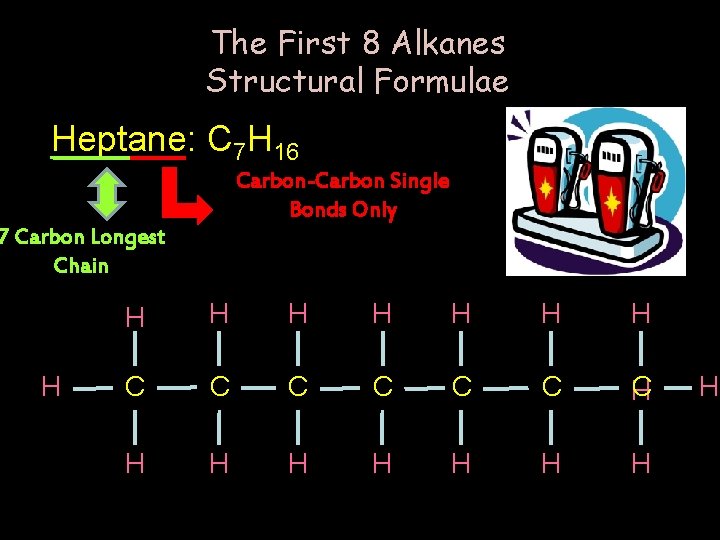
The First 8 Alkanes Structural Formulae Heptane: C 7 H 16 Carbon-Carbon Single Bonds Only 7 Carbon Longest Chain H H H H C C C C H H H H H

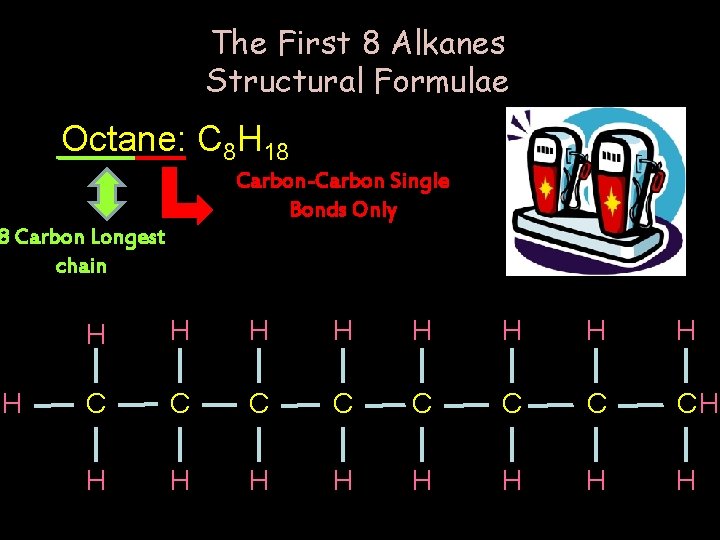
The First 8 Alkanes Structural Formulae Octane: C 8 H 18 Carbon-Carbon Single Bonds Only 8 Carbon Longest chain H H H H H C C C CH H H H H

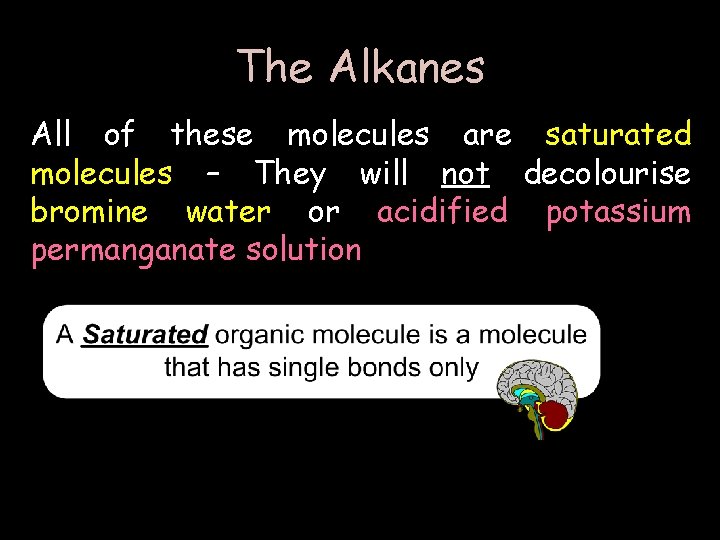
The Alkanes All of these molecules are saturated molecules – They will not decolourise bromine water or acidified potassium permanganate solution

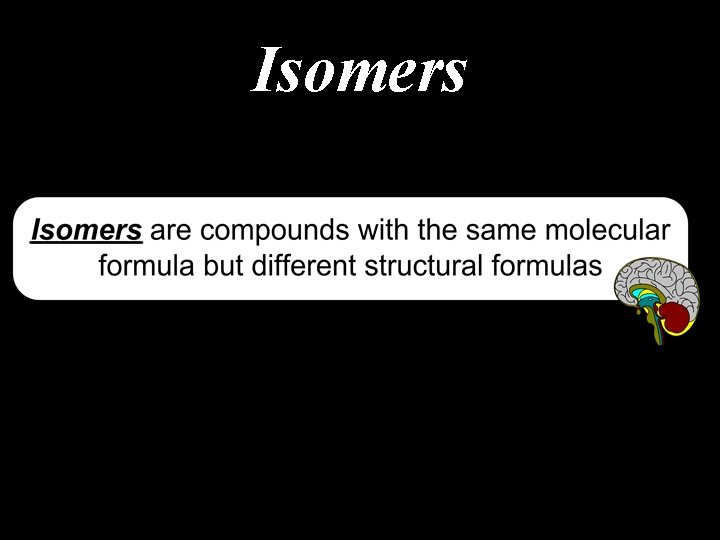
Isomers

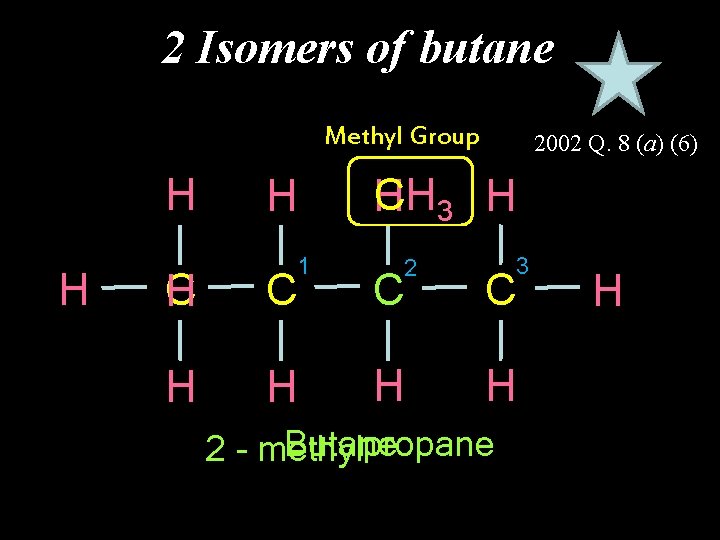
2 Isomers of butane Methyl Group H H H 1 2002 Q. 8 (a) (6) CH 3 H H 2 3 C H C C C H H Butane propane 2 - methyl H

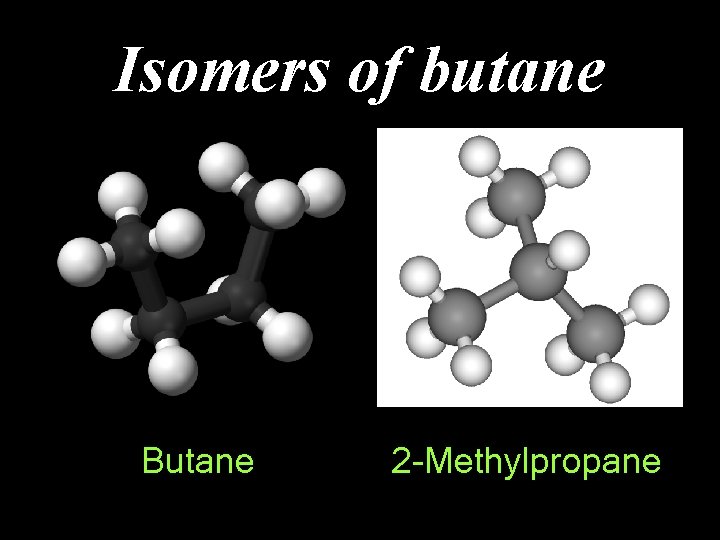
Isomers of butane Butane 2 -Methylpropane

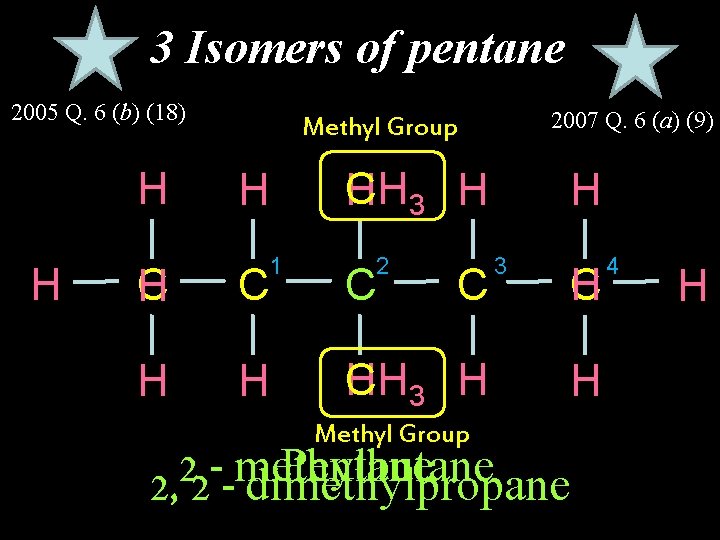
3 Isomers of pentane 2005 Q. 6 (b) (18) H H 2007 Q. 6 (a) (9) Methyl Group H 1 CH 3 H H 2 C H C C C H H H CH 3 H Methyl Group H 3 C H H 2 methyl Pentane butane 2, 2 - dimethylpropane 4 H

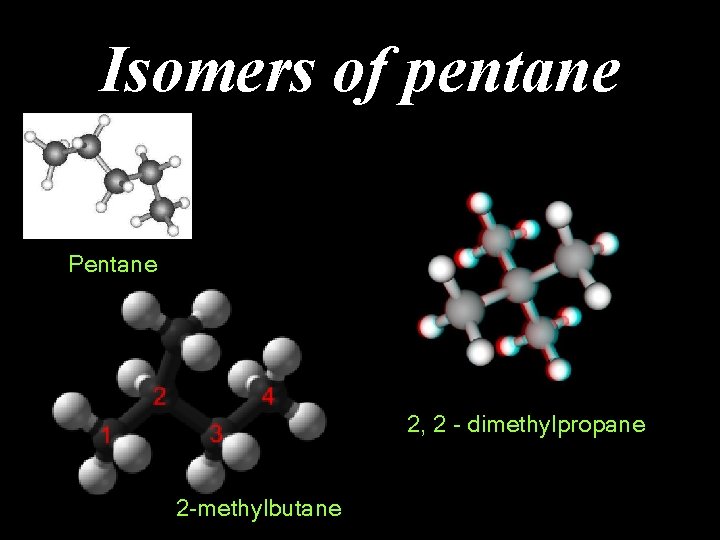
Isomers of pentane Pentane 2, 2 - dimethylpropane 2 -methylbutane

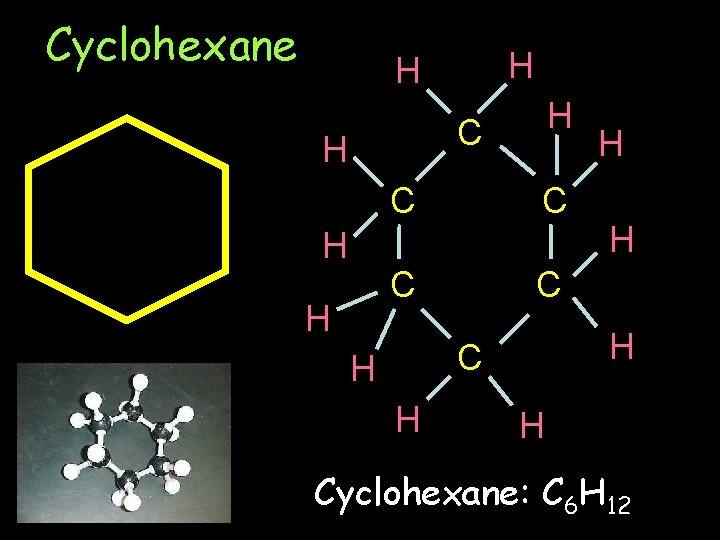
Cyclohexane H H H C C C C H H H Cyclohexane: C 6 H 12

Check your learning. . Do you know ? • What a homologous series is • What saturated means and how to test for it • What an isomer is and how to name isomers of alkanes

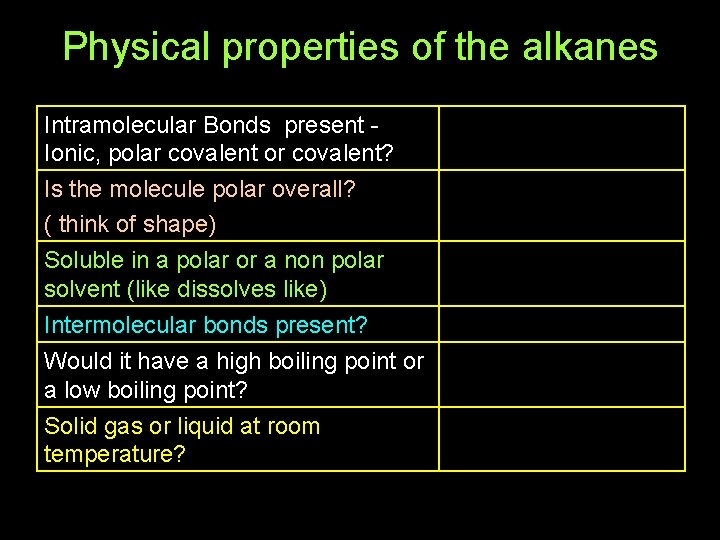
Physical properties of the alkanes Intramolecular Bonds present Ionic, polar covalent or covalent? Is the molecule polar overall? ( think of shape) Soluble in a polar or a non polar solvent (like dissolves like) Intermolecular bonds present? Would it have a high boiling point or a low boiling point? Solid gas or liquid at room temperature?

Physical Properties of the Alkanes They are all excellent fuels No taste or smell They all burn in air to produce carbon dioxide and water -For Example Pentane C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O

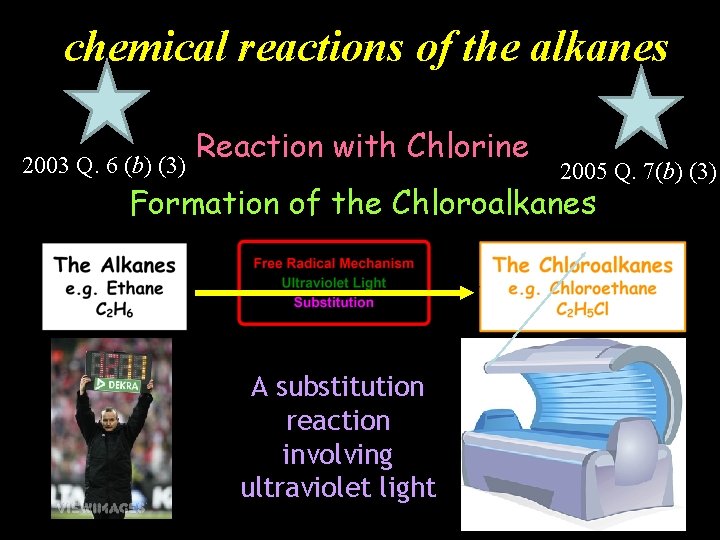
chemical reactions of the alkanes 2003 Q. 6 (b) (3) Reaction with Chlorine 2005 Q. 7(b) (3) Formation of the Chloroalkanes A substitution reaction involving ultraviolet light

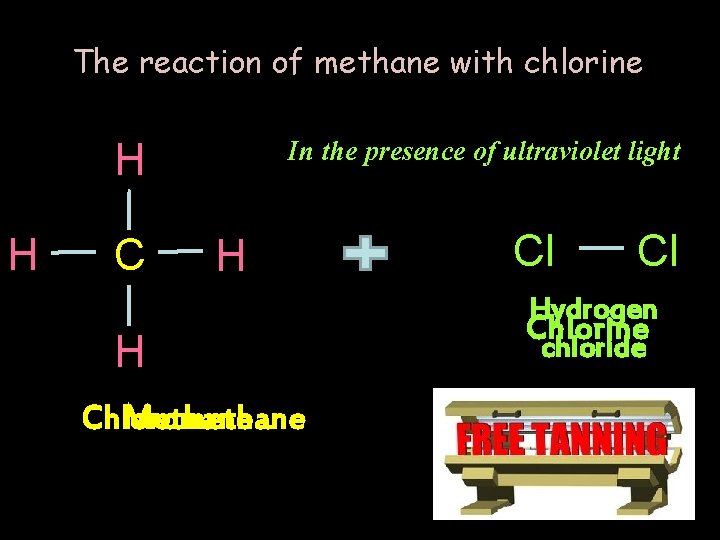
The reaction of methane with chlorine H H C In the presence of ultraviolet light H H Chloromethane Methane Cl Cl Hydrogen Chlorine chloride

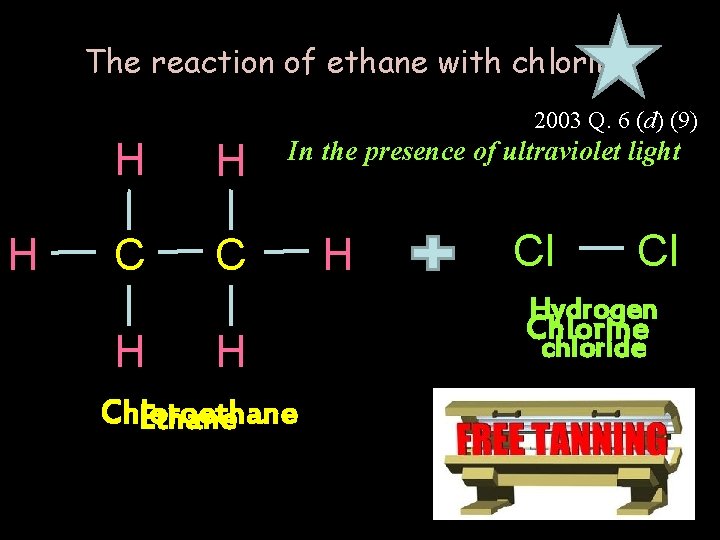
The reaction of ethane with chlorine 2003 Q. 6 (d) (9) H H H C C H In the presence of ultraviolet light H Chloroethane Ethane H Cl Cl Hydrogen Chlorine chloride

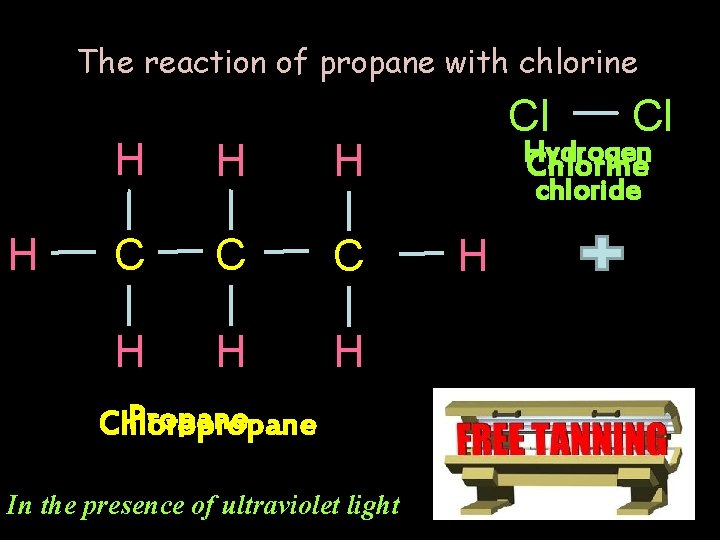
The reaction of propane with chlorine H H C C C H H H Propane Chloropropane In the presence of ultraviolet light Cl Cl Hydrogen Chlorine chloride H

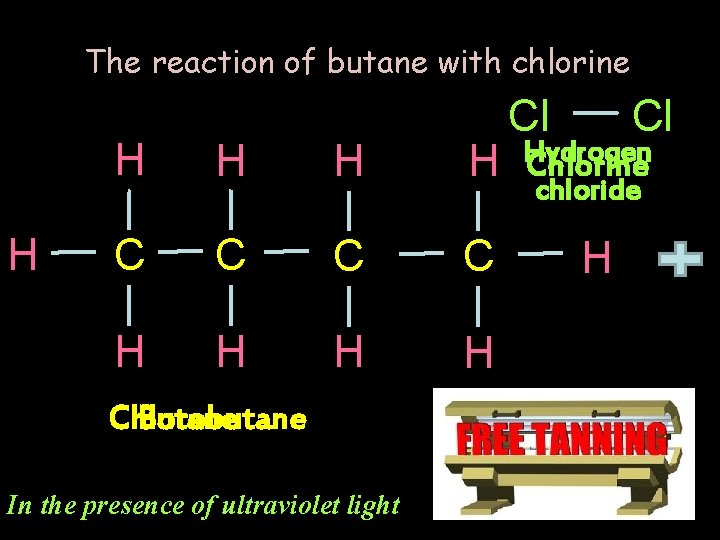
The reaction of butane with chlorine H Cl Cl H H H Chlorine H Hydrogen C C H H Chlorobutane Butane In the presence of ultraviolet light chloride H

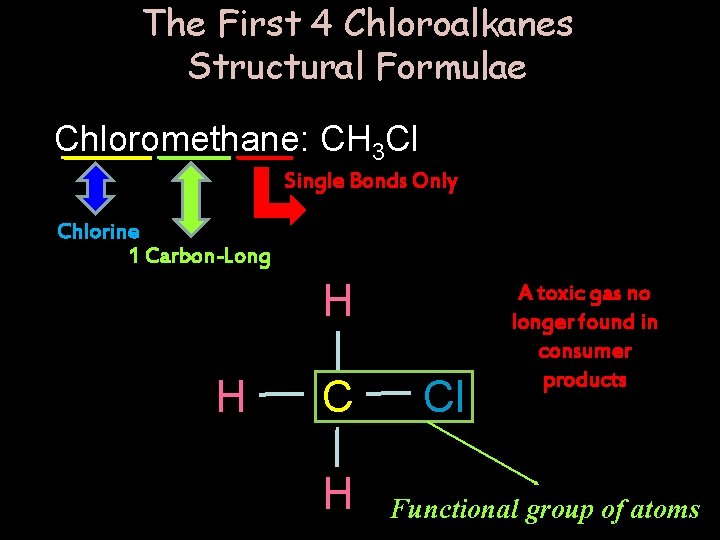
The First 4 Chloroalkanes Structural Formulae Chloromethane: CH 3 Cl Single Bonds Only Chlorine 1 Carbon-Long H H Cl A toxic gas no longer found in consumer products Functional group of atoms

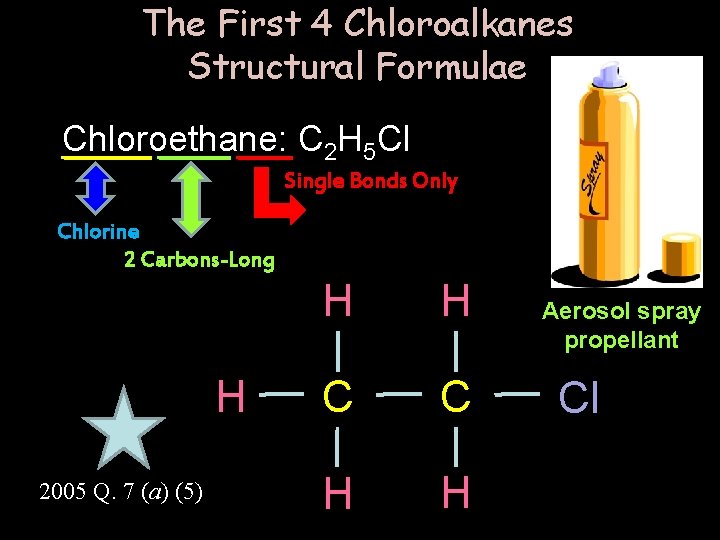
The First 4 Chloroalkanes Structural Formulae Chloroethane: C 2 H 5 Cl Single Bonds Only Chlorine 2 Carbons-Long H 2005 Q. 7 (a) (5) H H C C H H Aerosol spray propellant Cl

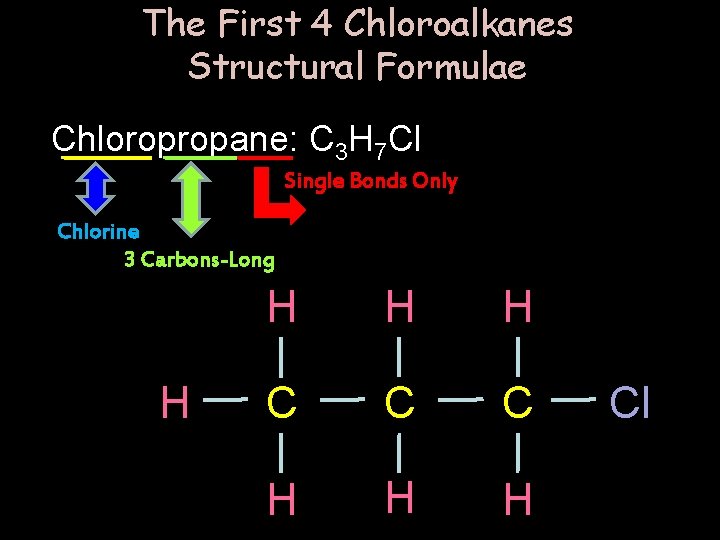
The First 4 Chloroalkanes Structural Formulae Chloropropane: C 3 H 7 Cl Single Bonds Only Chlorine 3 Carbons-Long H H C C C H H H Cl

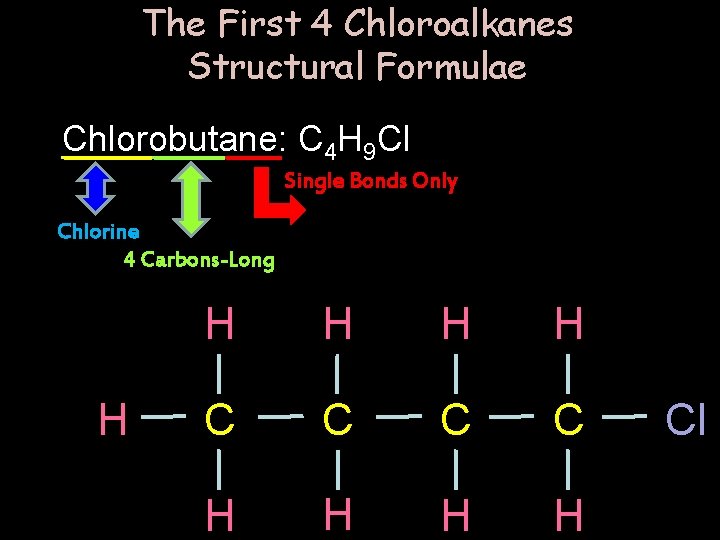
The First 4 Chloroalkanes Structural Formulae Chlorobutane: C 4 H 9 Cl Single Bonds Only Chlorine 4 Carbons-Long H H H C C H H Cl
 Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Primary school leaving examination in chinese
Primary school leaving examination in chinese Leaving certificate
Leaving certificate History syllabus leaving cert
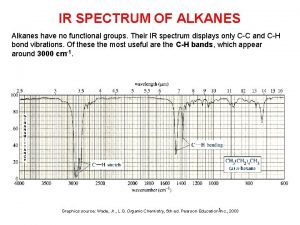
History syllabus leaving cert Ir spectrum of alkanes
Ir spectrum of alkanes Uses of alkanes
Uses of alkanes Isomers of pentane
Isomers of pentane Viscosity marble experiment
Viscosity marble experiment Uses of alkanes
Uses of alkanes Test for alkenes
Test for alkenes Combustion of alkanes equations
Combustion of alkanes equations Combustion of alkynes
Combustion of alkynes Cycloalkanes
Cycloalkanes Relative stability of isomeric alkanes
Relative stability of isomeric alkanes Alkanes alkenes alkynes
Alkanes alkenes alkynes Iupac suffix table
Iupac suffix table Alkanes def
Alkanes def Uses of alkanes
Uses of alkanes Sources of alkanes
Sources of alkanes 3-butyl-3-propyl-1-pentyne
3-butyl-3-propyl-1-pentyne Met et prop but
Met et prop but Alkanes solubility
Alkanes solubility Alkylation of alkanes
Alkylation of alkanes Alkanes list
Alkanes list Gauche conformation
Gauche conformation Leaving group
Leaving group Mindup mind map
Mindup mind map Intro to organic chemistry
Intro to organic chemistry Organic and biochemistry
Organic and biochemistry Carbohydrates organic chemistry
Carbohydrates organic chemistry Organic chemistry
Organic chemistry David klein organic chemistry
David klein organic chemistry Analytical chemistry chapter 1
Analytical chemistry chapter 1 Condensed formula
Condensed formula Organic chemistry nomenclature
Organic chemistry nomenclature Radicals
Radicals