Nuclear Intro Nuclear Reactions vs Normal Chemical Changes





- Slides: 31

Nuclear Intro

Nuclear Reactions vs. Normal Chemical Changes Nuclear reactions involve the nucleus The nucleus opens, and protons and neutrons are rearranged The opening of the nucleus releases a tremendous amount of energy that holds the nucleus together – called binding energy “Normal” Chemical Reactions involve electrons, not protons and neutrons

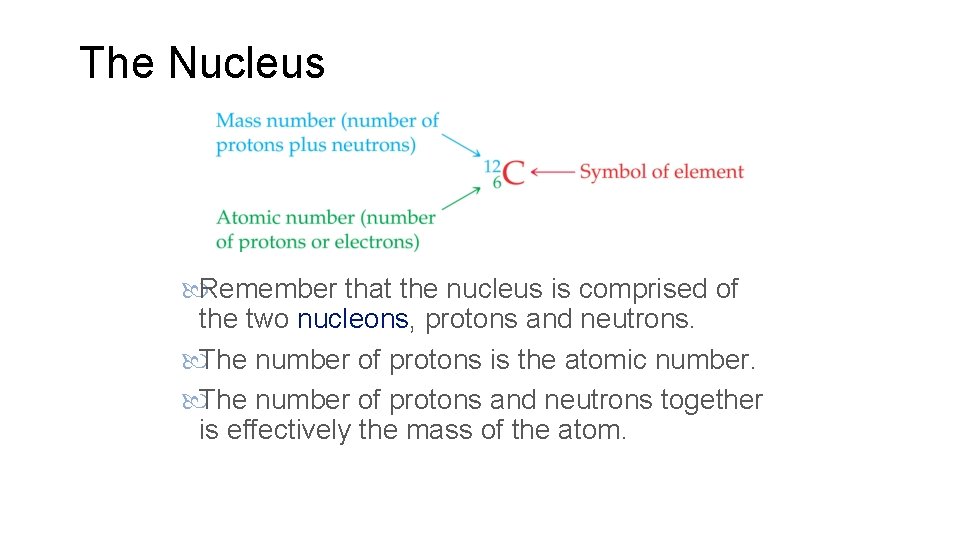
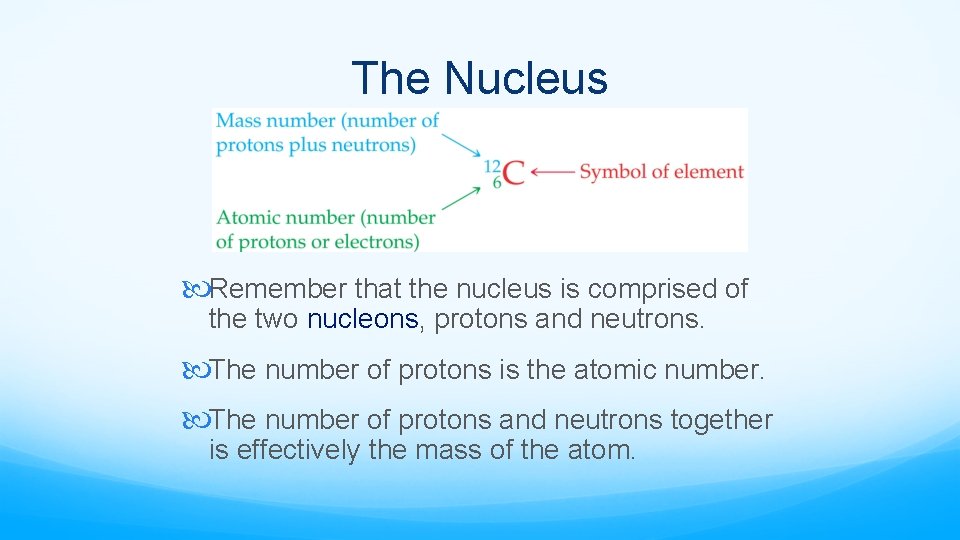
The Nucleus Remember that the nucleus is comprised of the two nucleons, protons and neutrons. The number of protons is the atomic number. The number of protons and neutrons together is effectively the mass of the atom.

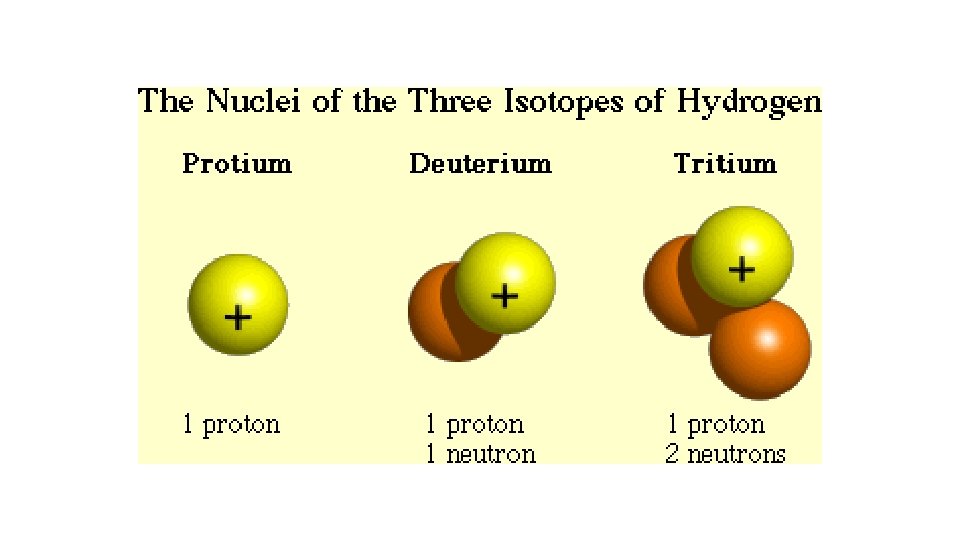
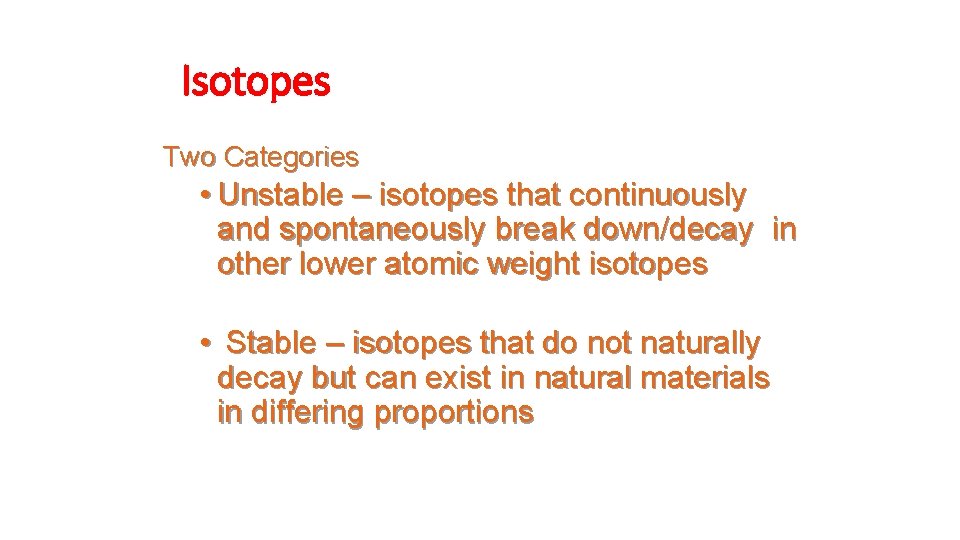
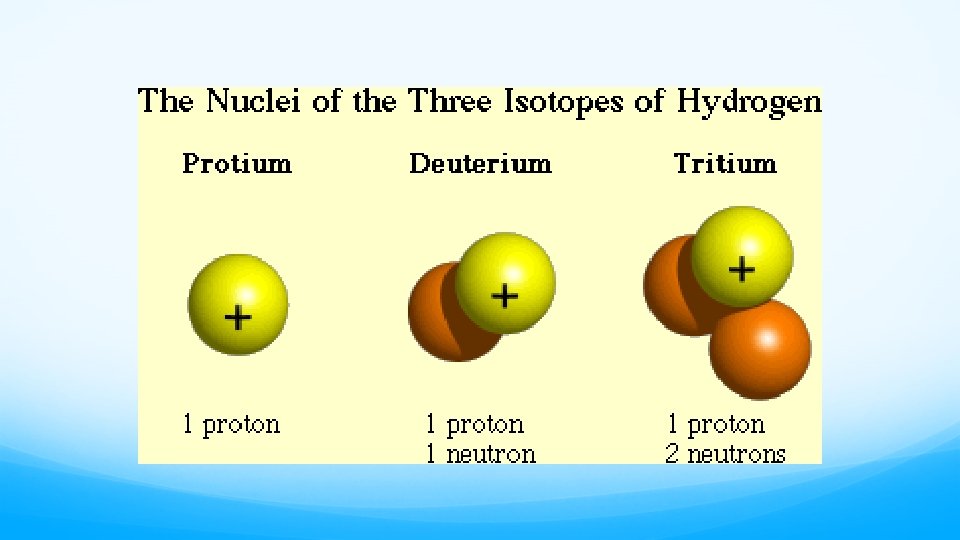
Isotopes • Not all atoms of the same element have the same mass due to different numbers of neutrons in those atoms. • There are three naturally occurring isotopes of uranium: • Uranium-234 • Uranium-235* • Uranium-238


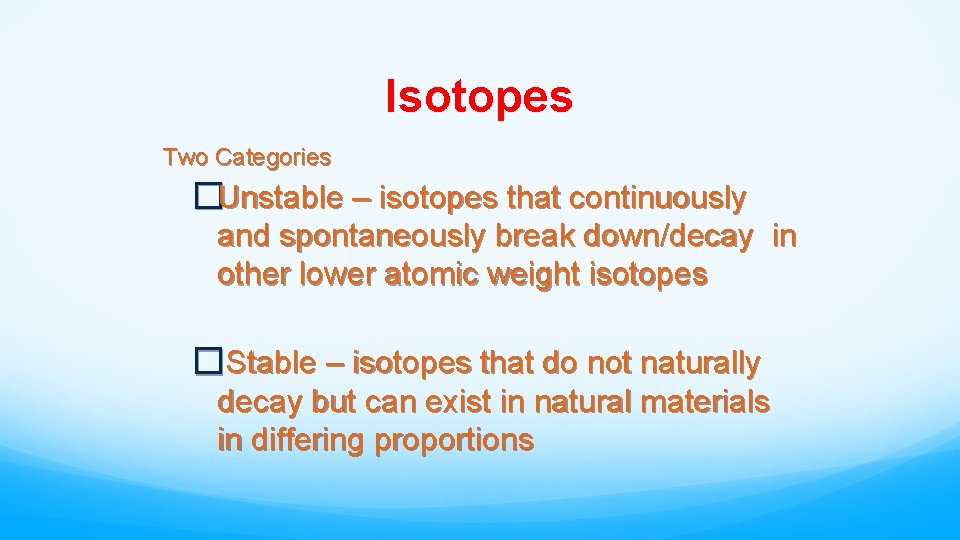
Isotopes Two Categories • Unstable – isotopes that continuously and spontaneously break down/decay in other lower atomic weight isotopes • Stable – isotopes that do not naturally decay but can exist in natural materials in differing proportions

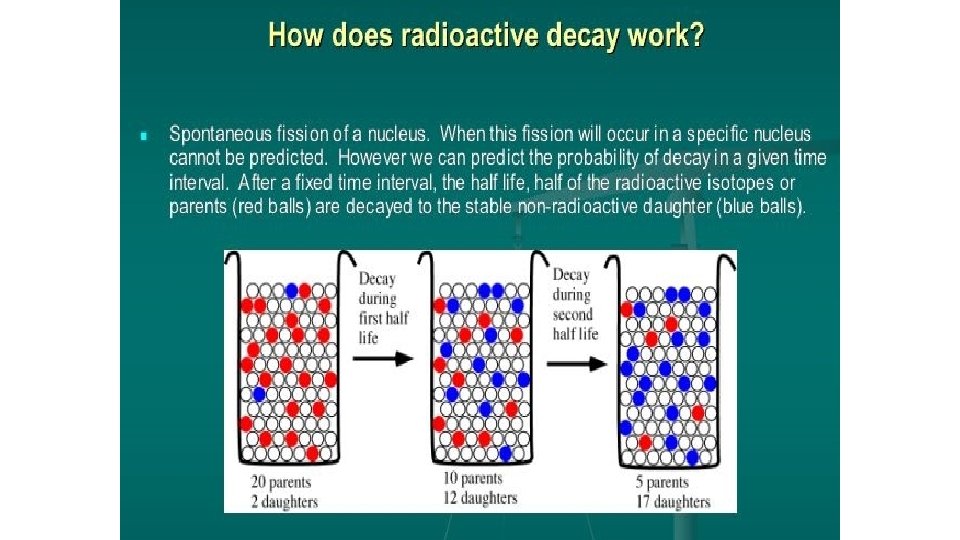
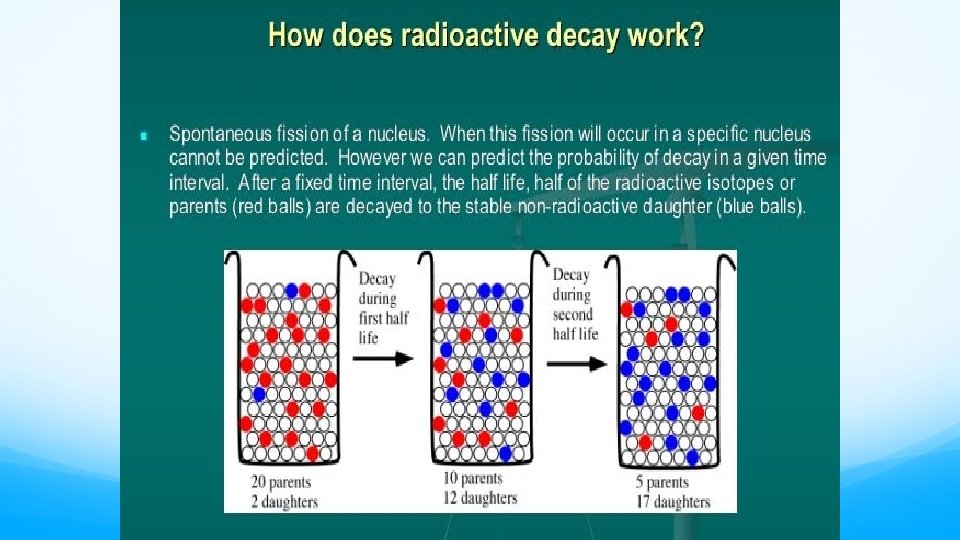
Radioactivity • It is not uncommon for some isotopes of an element to be unstable, or radioactive. • We refer to these as radioisotopes. • There are several ways radioisotopes can decay and give off energy known as radiation.


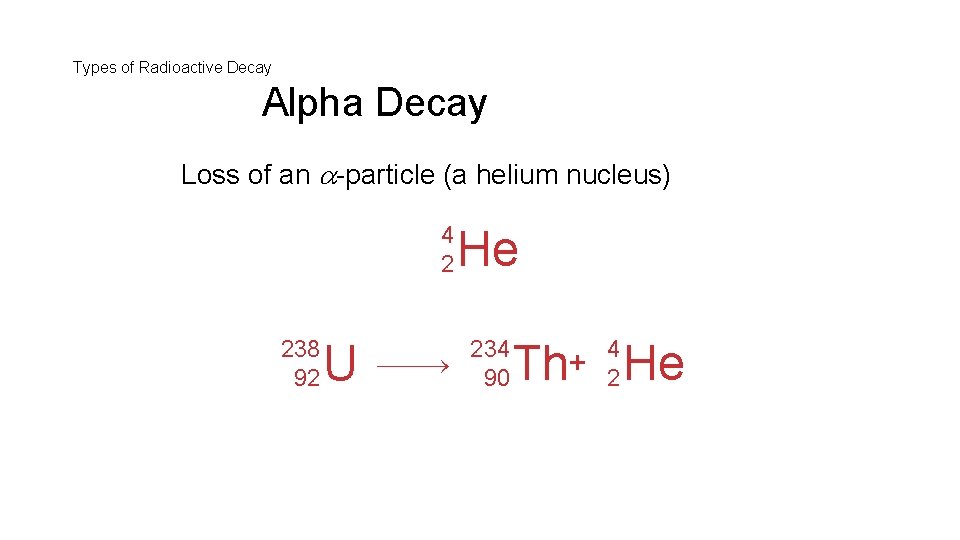
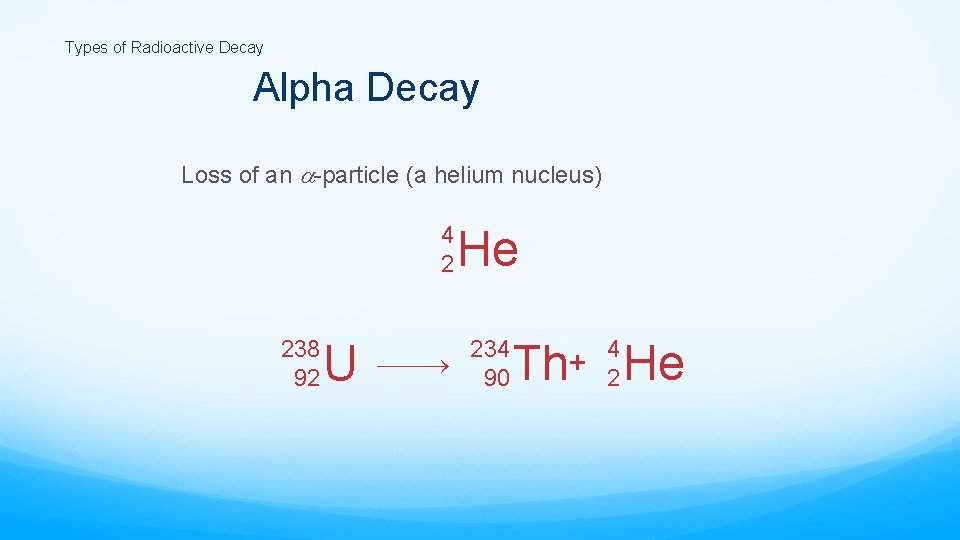
Types of Radioactive Decay Alpha Decay Loss of an -particle (a helium nucleus) 4 2 238 92 U He 234 90 4 2 Th+ He

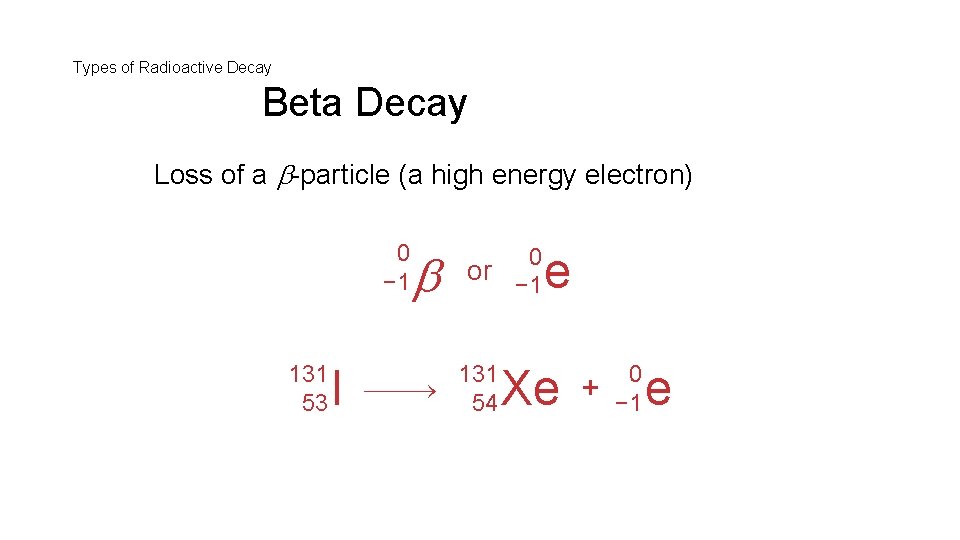
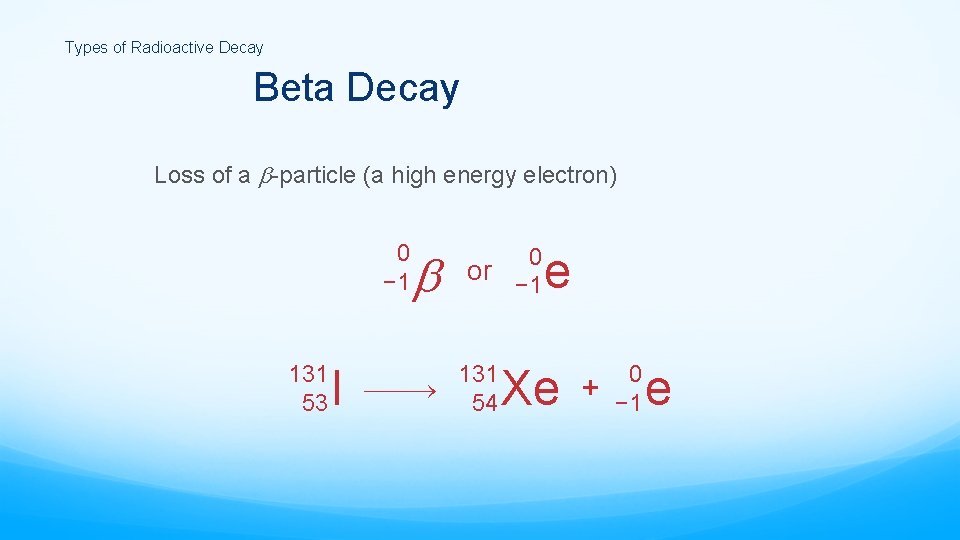
Types of Radioactive Decay Beta Decay Loss of a -particle (a high energy electron) 0 − 1 131 53 I 0 or − 1 131 54 e Xe + 0 − 1 e

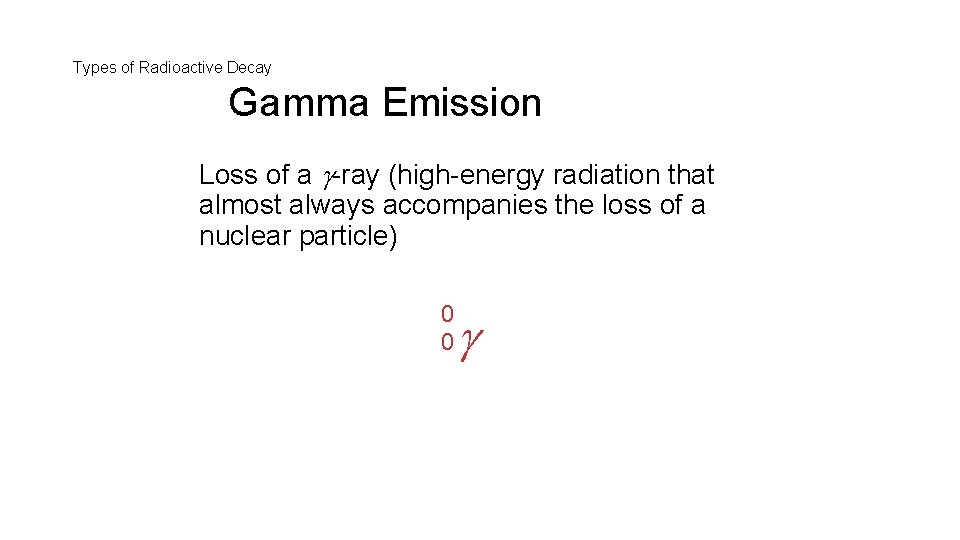
Types of Radioactive Decay Gamma Emission Loss of a -ray (high-energy radiation that almost always accompanies the loss of a nuclear particle) 0 0

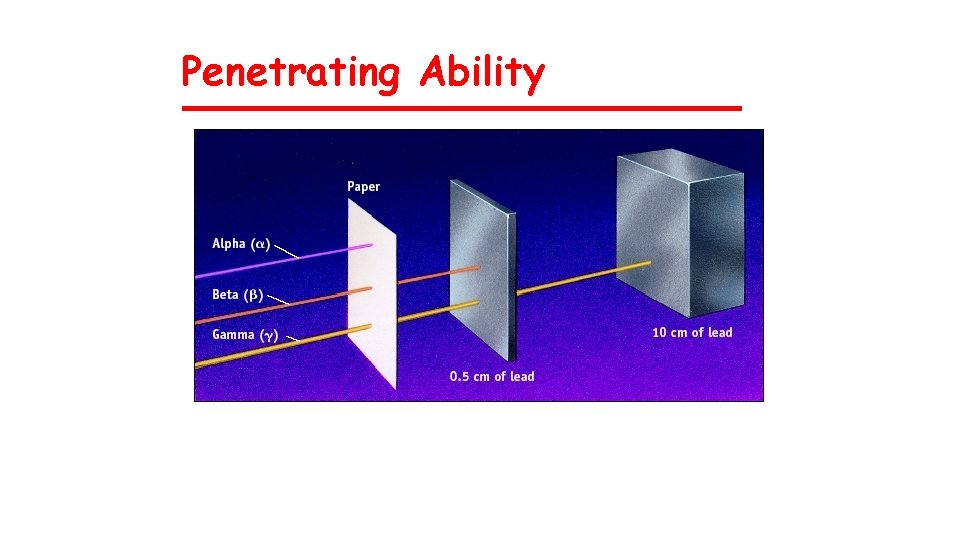
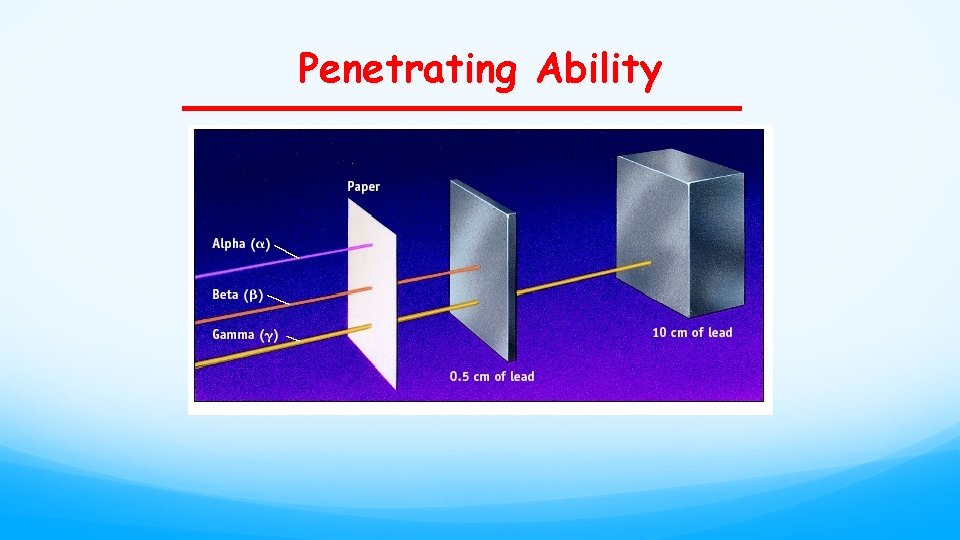
Penetrating Ability

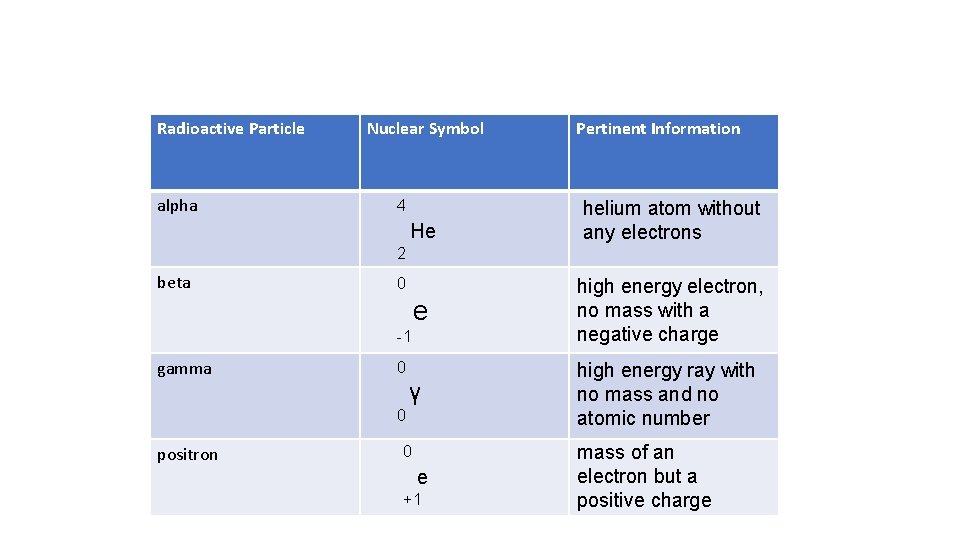
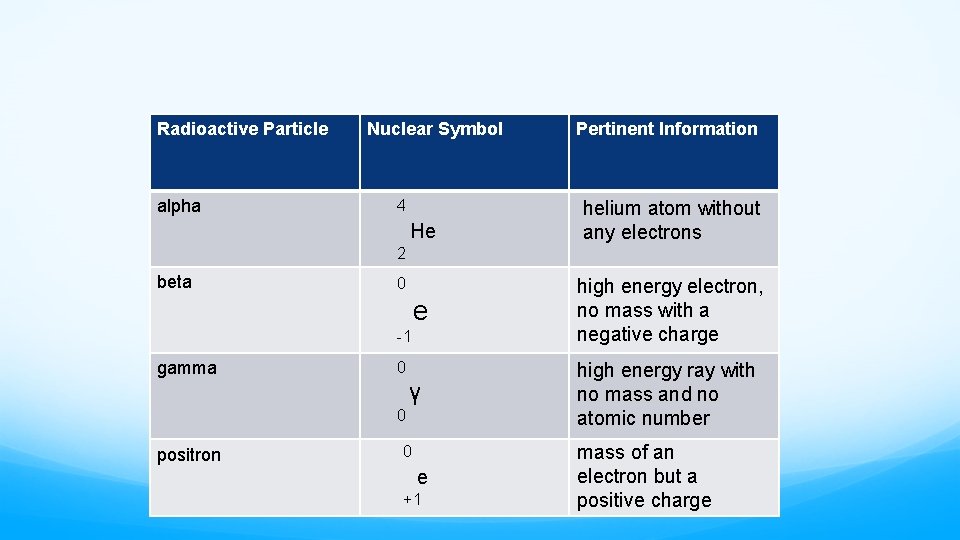
Radioactive Particle alpha Nuclear Symbol 4 He Pertinent Information helium atom without any electrons 2 beta 0 e high energy electron, no mass with a negative charge γ high energy ray with no mass and no atomic number -1 gamma 0 0 positron 0 e +1 mass of an electron but a positive charge

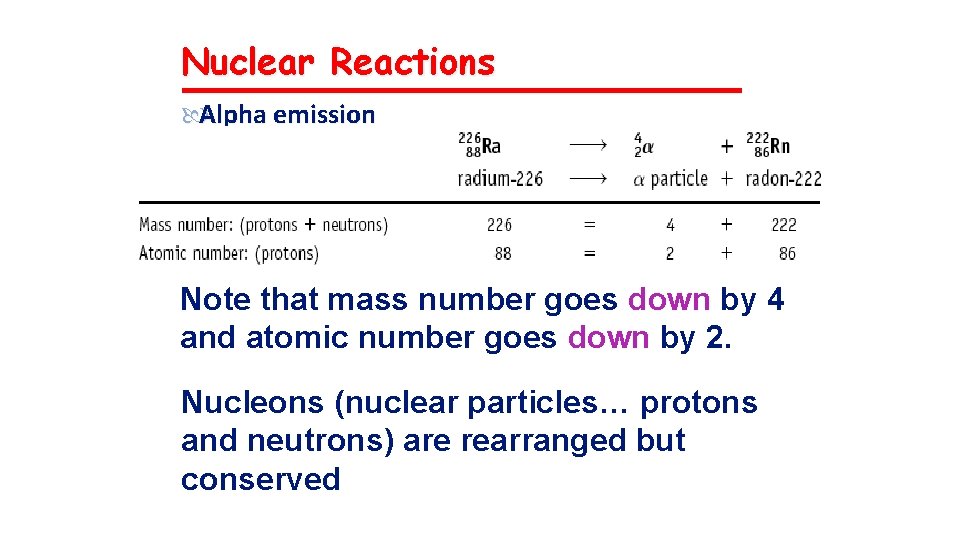
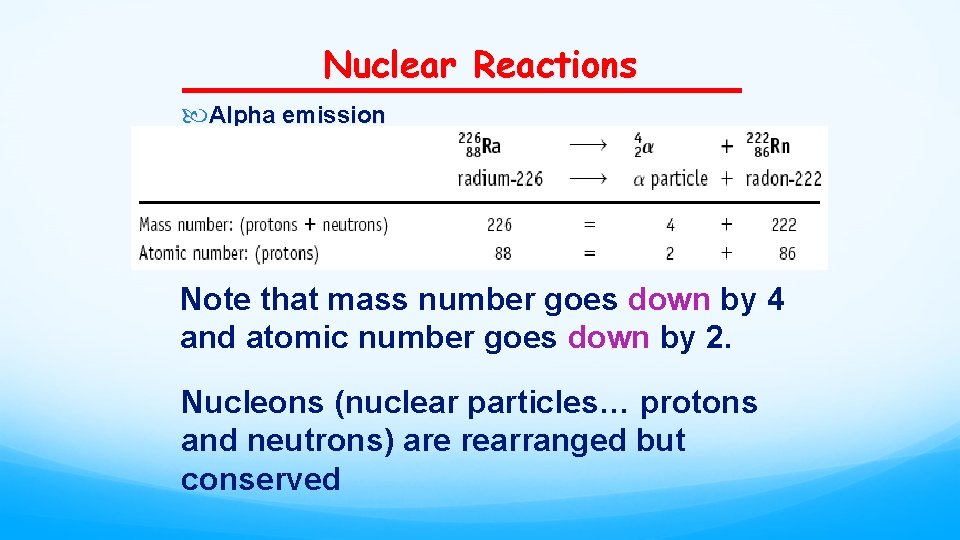
Nuclear Reactions Alpha emission Note that mass number goes down by 4 and atomic number goes down by 2. Nucleons (nuclear particles… protons and neutrons) are rearranged but conserved

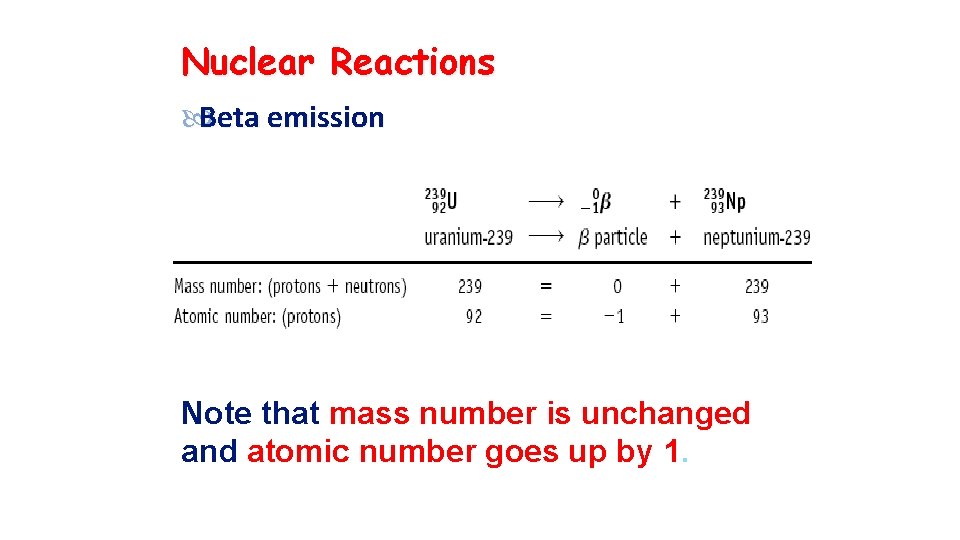
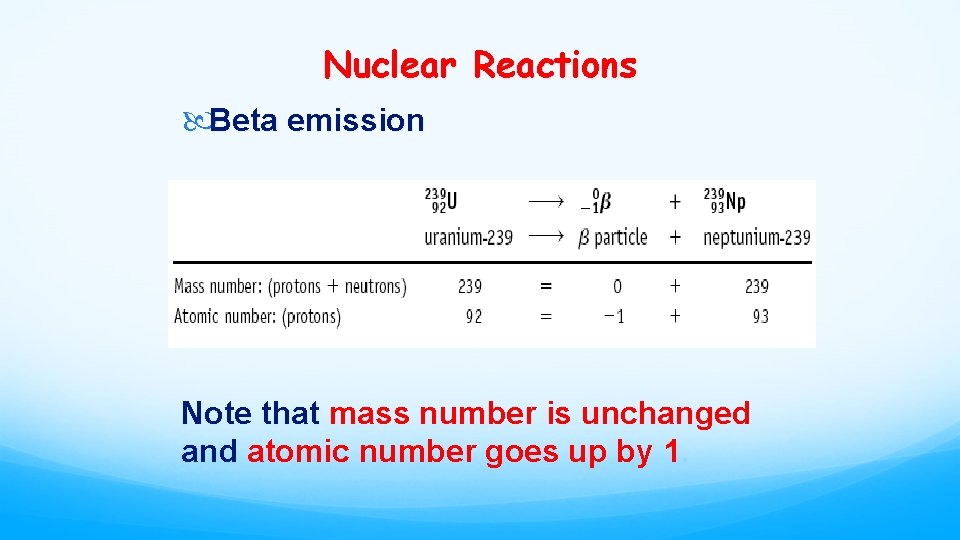
Nuclear Reactions Beta emission Note that mass number is unchanged and atomic number goes up by 1.

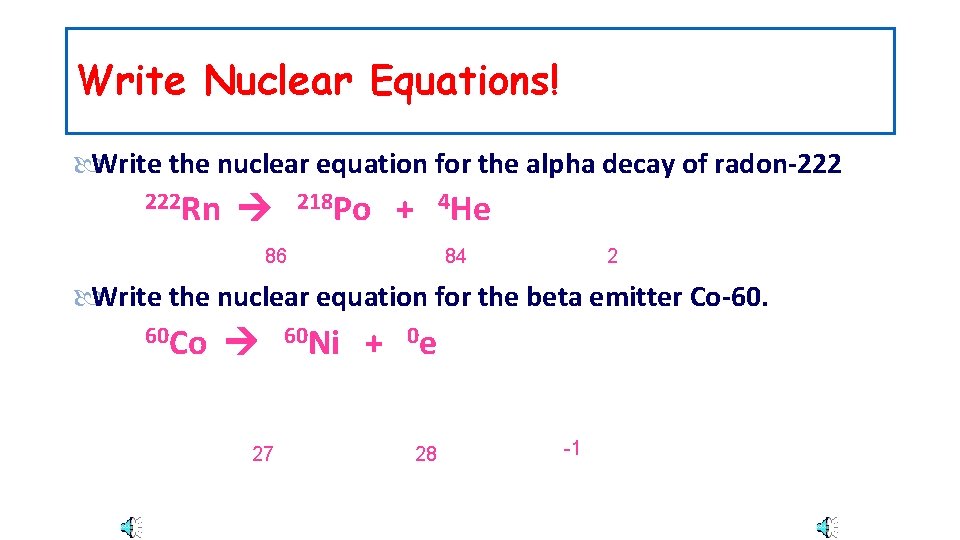
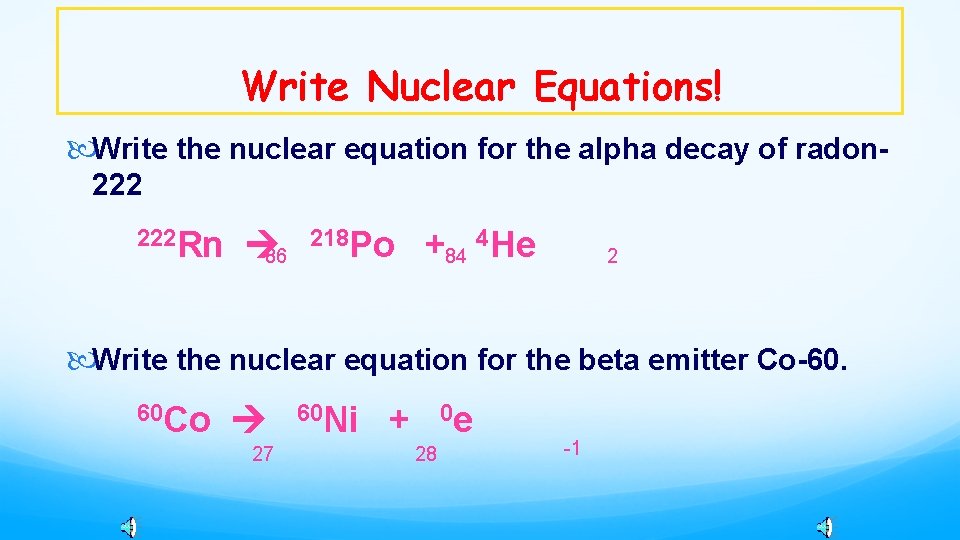
Write Nuclear Equations! Write the nuclear equation for the alpha decay of radon-222 222 Rn 218 Po + 4 He 86 84 2 Write the nuclear equation for the beta emitter Co-60. 60 Co 27 60 Ni + 0 e 28 -1

Nuclear Reactions vs. Normal Chemical Changes Nuclear reactions involve the nucleus The nucleus opens, and protons and neutrons are rearranged The opening of the nucleus releases a tremendous amount of energy that holds the nucleus together – called binding energy “Normal” Chemical Reactions involve electrons, not protons and neutrons

The Nucleus Remember that the nucleus is comprised of the two nucleons, protons and neutrons. The number of protons is the atomic number. The number of protons and neutrons together is effectively the mass of the atom.

Isotopes �Not all atoms of the same element have the same mass due to different numbers of neutrons in those atoms. �There are three naturally occurring isotopes of uranium: � Uranium-234 � Uranium-235* � Uranium-238


Isotopes Two Categories �Unstable – isotopes that continuously and spontaneously break down/decay in other lower atomic weight isotopes �Stable – isotopes that do not naturally decay but can exist in natural materials in differing proportions

Radioactivity �It is not uncommon for some isotopes of an element to be unstable, or radioactive. �We refer to these as radioisotopes. �There are several ways radioisotopes can decay and give off energy known as radiation.


Types of Radioactive Decay Alpha Decay Loss of an -particle (a helium nucleus) 4 2 238 92 U He 234 90 4 2 Th+ He

Types of Radioactive Decay Beta Decay Loss of a -particle (a high energy electron) 0 − 1 131 53 I 0 or − 1 131 54 e Xe + 0 − 1 e

Types of Radioactive Decay Gamma Emission Loss of a -ray (high-energy radiation that almost always accompanies the loss of a nuclear particle) 0 0

Penetrating Ability

Radioactive Particle alpha Nuclear Symbol 4 He Pertinent Information helium atom without any electrons 2 beta 0 e high energy electron, no mass with a negative charge γ high energy ray with no mass and no atomic number -1 gamma 0 0 positron 0 e +1 mass of an electron but a positive charge

Nuclear Reactions Alpha emission Note that mass number goes down by 4 and atomic number goes down by 2. Nucleons (nuclear particles… protons and neutrons) are rearranged but conserved

Nuclear Reactions Beta emission Note that mass number is unchanged and atomic number goes up by 1.

Write Nuclear Equations! Write the nuclear equation for the alpha decay of radon 222 Rn 86 218 Po +84 4 He 2 Write the nuclear equation for the beta emitter Co-60. 60 Co 27 60 Ni + 0 e 28 -1