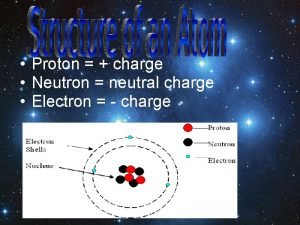
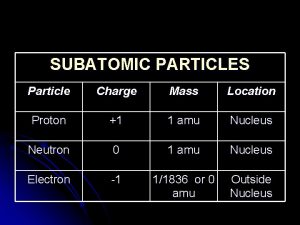
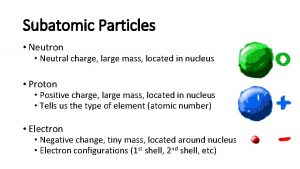
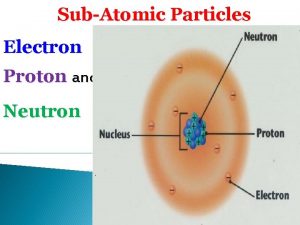
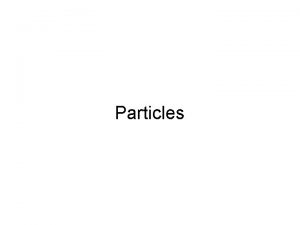
NUCLEAR CHEMISTRY Nuclear Particles PROTON NEUTRON Mass Charge















- Slides: 33

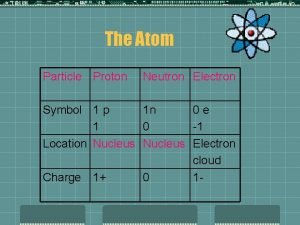
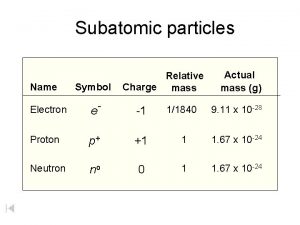
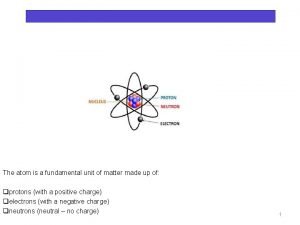
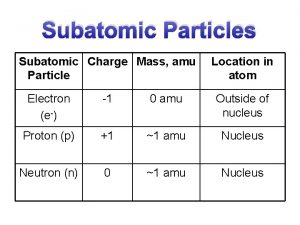
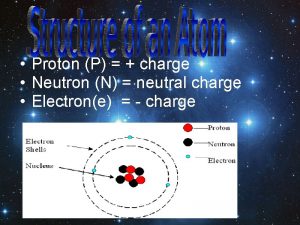
NUCLEAR CHEMISTRY Nuclear Particles: PROTON NEUTRON Mass Charge Symbol 1 amu +1 H+, H, p 1 amu © Copyright 1994 -2001 R. J. Rusay 0 n


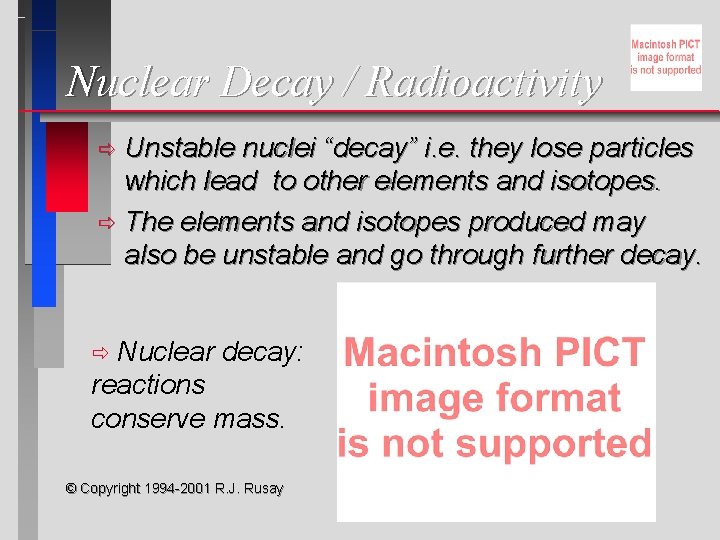
Nuclear Decay / Radioactivity Unstable nuclei “decay” i. e. they lose particles which lead to other elements and isotopes. ð The elements and isotopes produced may also be unstable and go through further decay. ð Nuclear decay: reactions conserve mass. ð © Copyright 1994 -2001 R. J. Rusay

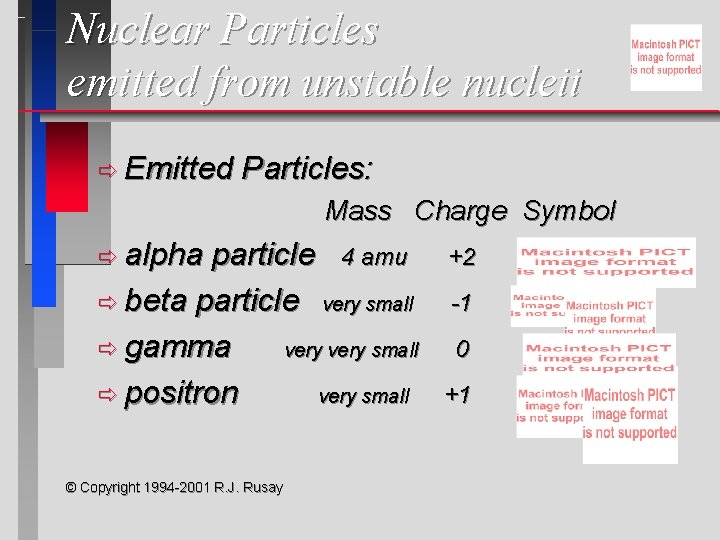
Nuclear Particles emitted from unstable nucleii ð Emitted Particles: Mass Charge Symbol ð alpha particle 4 amu ð beta particle very small ð gamma very small ð positron very small © Copyright 1994 -2001 R. J. Rusay +2 -1 0 +1

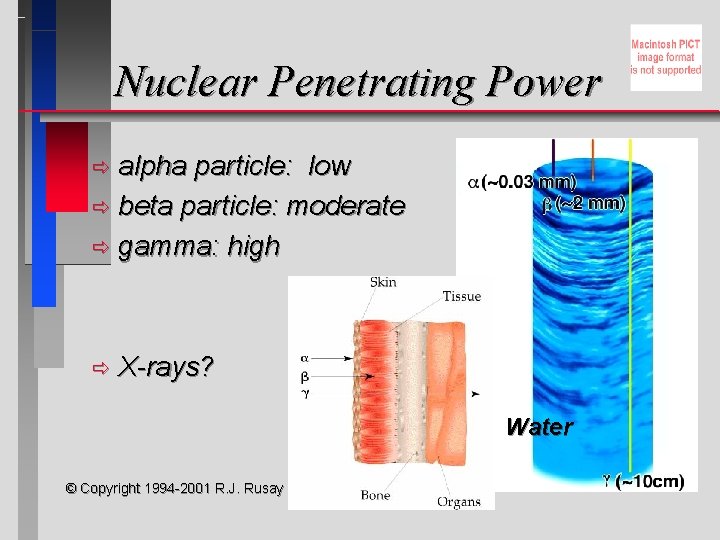
Nuclear Penetrating Power alpha particle: low ð beta particle: moderate ð gamma: high ð ð X-rays? Water © Copyright 1994 -2001 R. J. Rusay

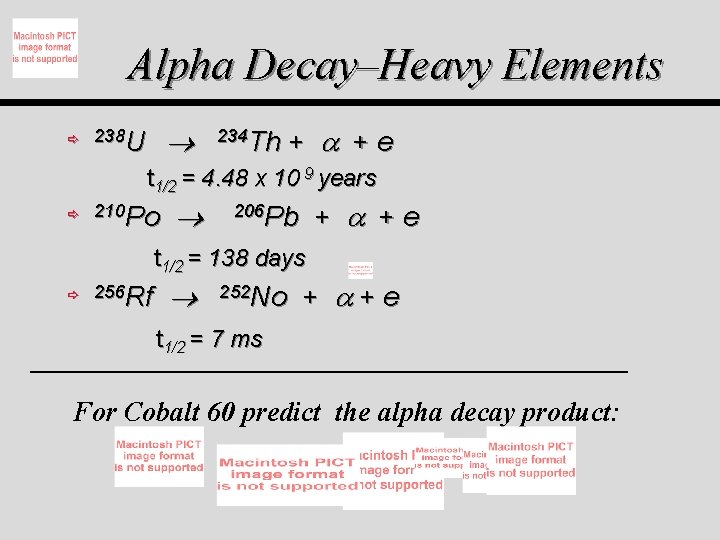
Alpha Decay–Heavy Elements ð 238 U 234 Th + + e t 1/2 = 4. 48 x 10 9 years ð 210 Po + + e 206 Pb t 1/2 = 138 days ð 256 Rf 252 No + +e t 1/2 = 7 ms For Cobalt 60 predict the alpha decay product:

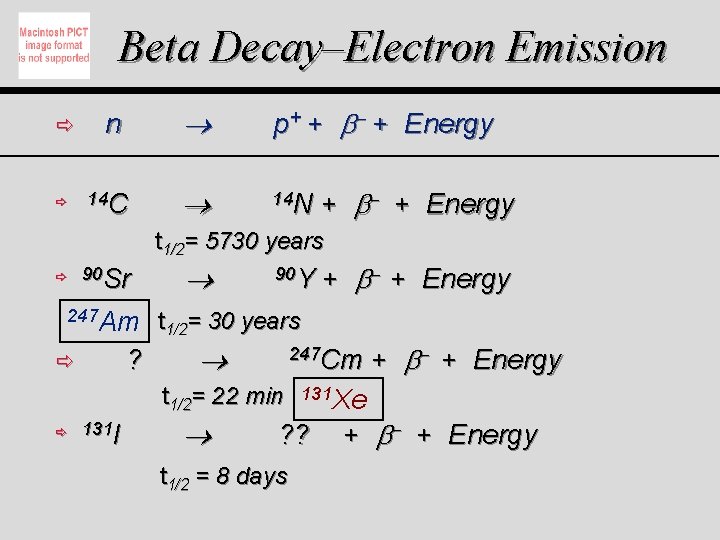
Beta Decay–Electron Emission ð ð n 14 C p+ + + Energy 14 N + + Energy t 1/2= 5730 years ð 90 Sr 247 Am ? ð 90 Y t 1/2= 30 years 247 Cm t 1/2= 22 min ð 131 I + + Energy 131 Xe ? ? t 1/2 = 8 days + + Energy + + Energy

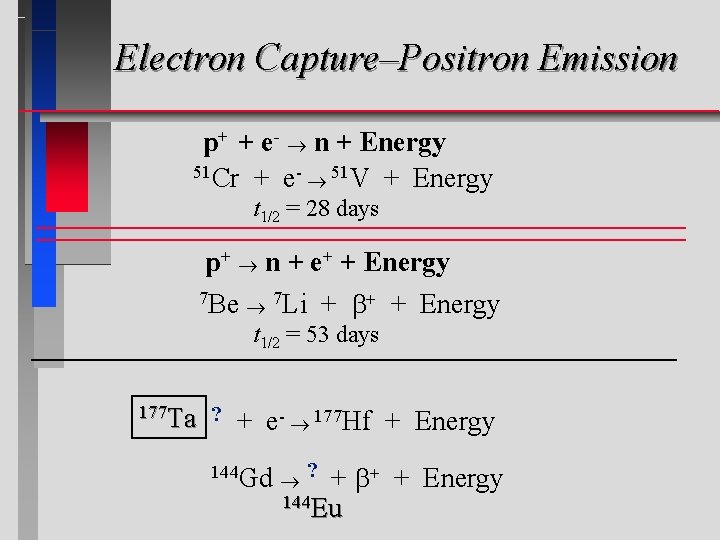
Electron Capture–Positron Emission p+ + e- n + Energy = Electron capture 51 Cr + e- 51 V + Energy t 1/2 = 28 days p+ n + e+ + Energy = 7 Positron Be 7 Li emission + + Energy t 1/2 = 53 days 177 Ta ? + e- 177 Hf + Energy 144 Gd ? + 144 Eu + Energy

Nuclear Decay Predict the Particle or element: ð Thallium 206 decays to Lead 206. What particle is emitted? ð Cesium 137 goes through Beta decay. What element is produced and what is its mass? ð Thorium 230 decays to Radium 226. What particle is emitted? © Copyright 1994 -2001 R. J. Rusay

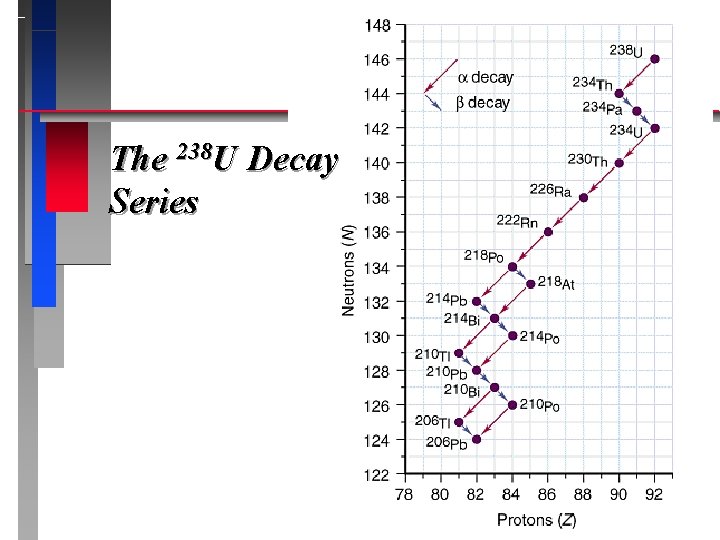
Nuclear Decay Series ð If the nuclei produced from radioactive decay are unstable, they continue to decay until a stable isotope results. ð An example is Radium which produces Lead © Copyright 1994 -2001 R. J. Rusay

The 238 U Decay Series

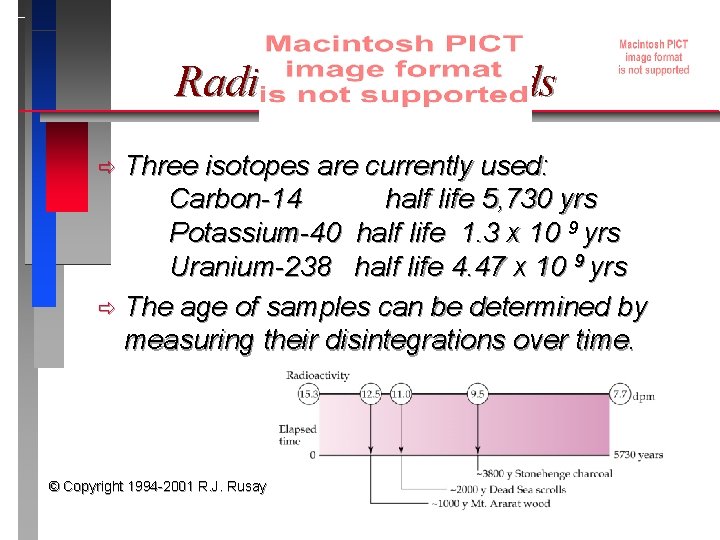
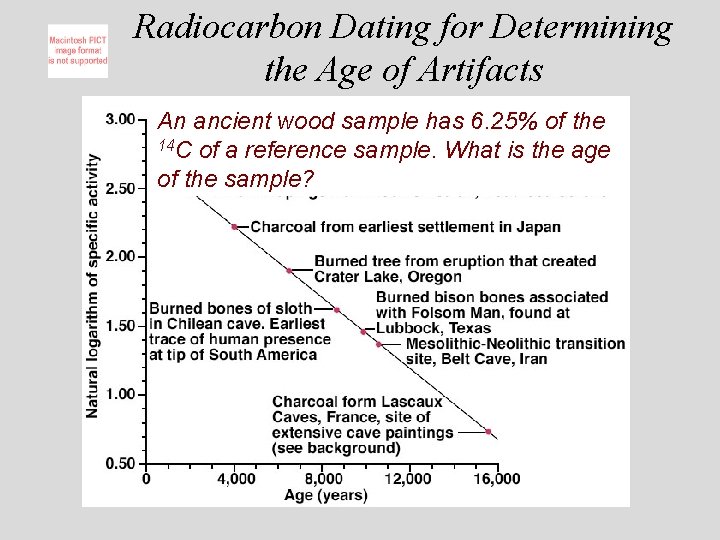
Radiodating Methods Three isotopes are currently used: Carbon-14 half life 5, 730 yrs Potassium-40 half life 1. 3 x 10 9 yrs Uranium-238 half life 4. 47 x 10 9 yrs ð The age of samples can be determined by measuring their disintegrations over time. ð © Copyright 1994 -2001 R. J. Rusay


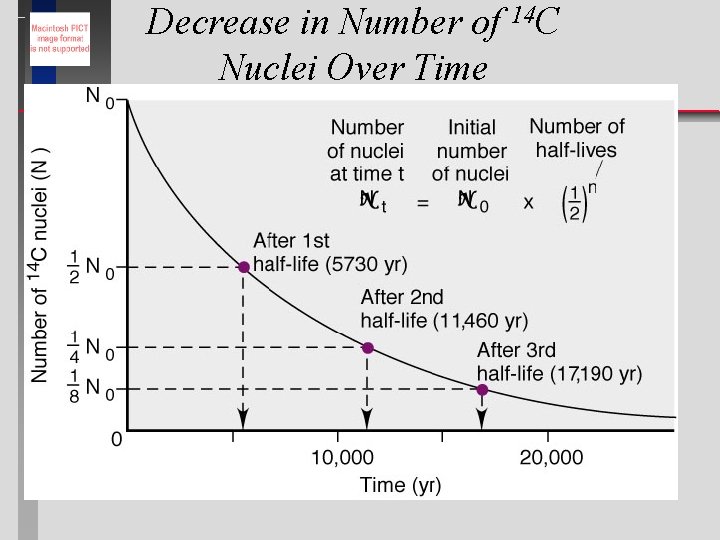
Decrease in Number of 14 C Nuclei Over Time

Radiocarbon Dating for Determining the Age of Artifacts An ancient wood sample has 6. 25% of the 14 C of a reference sample. What is the age of the sample?

Nuclear Reactions The mass of the visible universe is 73% H 2 and 25% He. The remaining 2%, “heavy” elements, have atomic masses >4. ð The “heavy” elements are formed at very high temperatures (T>10 6 o. C) by FUSION, i. e. nuclei combining to form new elements. ð There is an upper limit to the production of heavy nuclei at A=92, Uranium. ð Heavy nuclei split to lighter ones by FISSION ð © Copyright 1994 -2001 R. J. Rusay

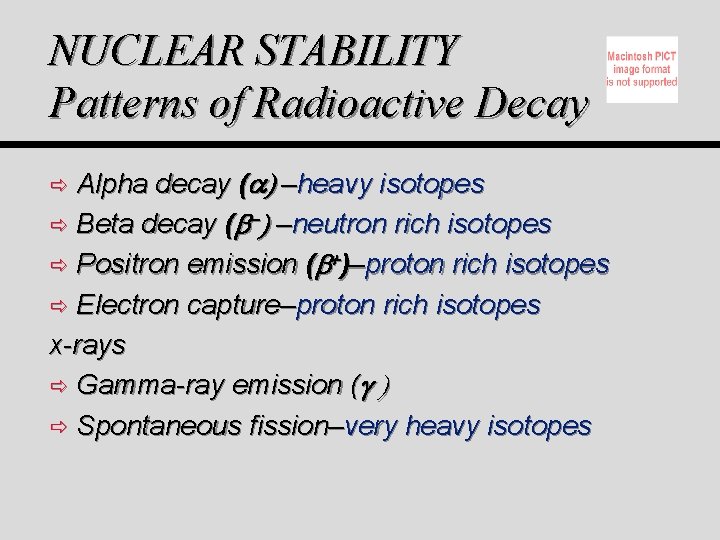
NUCLEAR STABILITY Patterns of Radioactive Decay Alpha decay ( ) –heavy isotopes ð Beta decay ( ) –neutron rich isotopes ð Positron emission ( )–proton rich isotopes ð Electron capture–proton rich isotopes x-rays ð Gamma-ray emission ( ð Spontaneous fission–very heavy isotopes ð


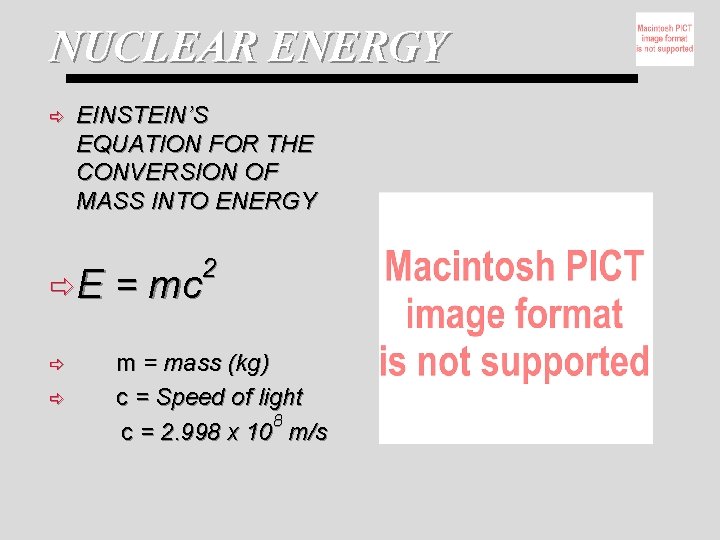
NUCLEAR ENERGY ð EINSTEIN’S EQUATION FOR THE CONVERSION OF MASS INTO ENERGY 2 ðE = mc ð m = mass (kg) c = Speed of light 8 c = 2. 998 x 10 m/s ð

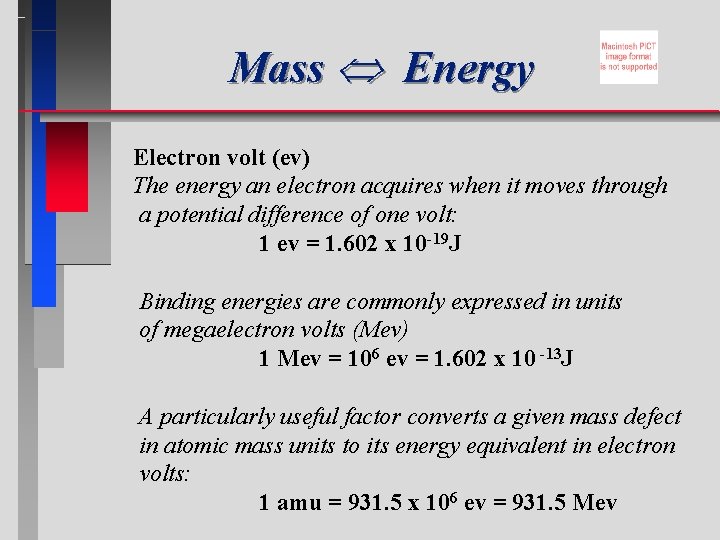
Mass Energy Electron volt (ev) The energy an electron acquires when it moves through a potential difference of one volt: 1 ev = 1. 602 x 10 -19 J Binding energies are commonly expressed in units of megaelectron volts (Mev) 1 Mev = 106 ev = 1. 602 x 10 -13 J A particularly useful factor converts a given mass defect in atomic mass units to its energy equivalent in electron volts: 1 amu = 931. 5 x 106 ev = 931. 5 Mev

Nuclear Reactions ð ð ð Fission and Fusion reactions are highly exothermic (1 Mev / nucleon). This is 10 6 times larger than “chemical” reactions which are about 1 ev / atom. Nuclear fission was first used in a chain reaction: © Copyright 1994 -2001 R. J. Rusay

Nuclear Reactions: Fission: ð ð Fusion: Fission and Fusion reactions are highly exothermic (1 Mev / nucleon). This is 10 6 times larger than “chemical” reactions which are about 1 ev / atom.

Nuclear Reactions ð Nuclear fission was first used in a chain reaction:



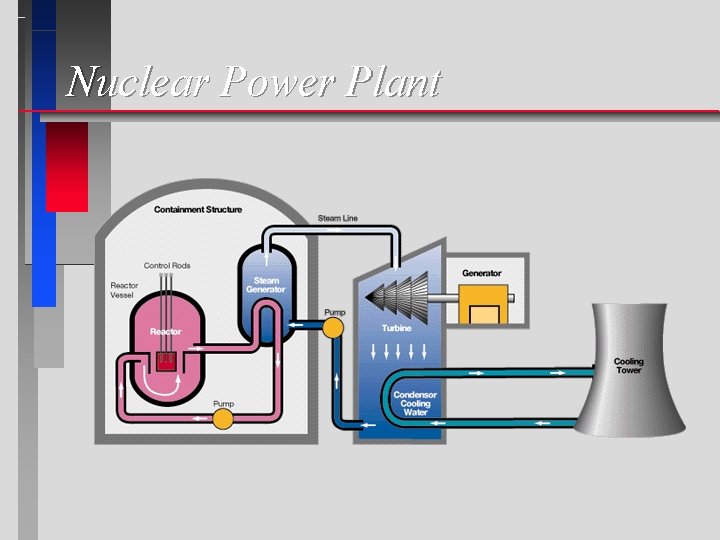
Nuclear Reactions / Fission The Fission Chain Reaction proceeds geometrically: 1 neutron -> 3 -> 9 -> 27 -> 81 etc. ð 1 Mole of U-235 (about 1/2 lb) produces 2 x 1010 k. J which is equivalent to the combustion of 800 tons of Coal! ð Commercial nuclear reactors use fission to produce electricity. . Fission bombs were used in the destruction of Hiroshima and Nagasaki, Japan, in August 1945. ð © Copyright R. J. Rusay 1994 -2001

The Nuclear Dawn August 6, 1945

Nuclear Power Plant

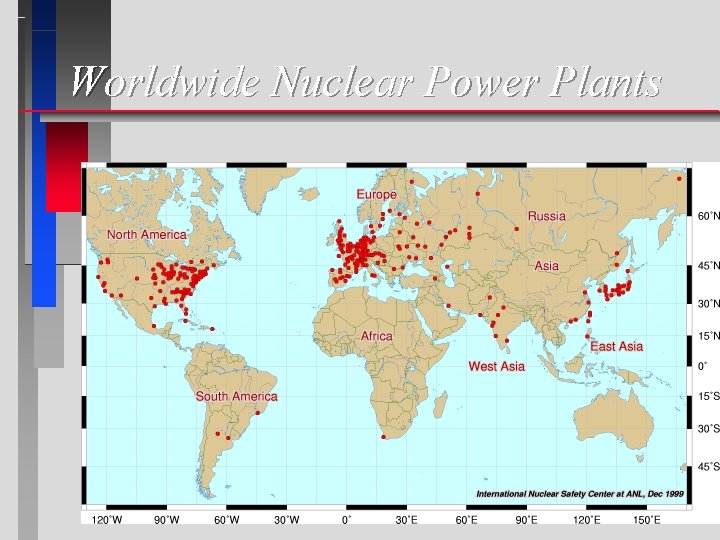
Worldwide Nuclear Power Plants

Chernobyl, Ukraine April, 1986 and April, 2001

Nuclear Reactions / Fusion ð ð Fusion has been described as the chemistry of the sun and stars. It too has been used in weapons. It has not yet found a peaceful commercial application ðThe application has great promise in producing relatively “clean” abundant energy through the combination of Hydrogen isotopes particularly from 2 H, deuterium and 3 H, tritium: (NIF/National Ignition Facility, LLNL) © Copyright 1994 -2001 R. J. Rusay

National Ignition Facility Lawrence Livermore National Laboratory

National Ignition Facility Lawrence Livermore National Laboratory © Copyright 1994 -2001 R. J. Rusay
 Symbole proton neutron electron
Symbole proton neutron electron How to find electrons
How to find electrons Elektron neutron proton
Elektron neutron proton Atom proton neutron electron
Atom proton neutron electron Proton neutron quarks
Proton neutron quarks Proton neutron
Proton neutron Proton neutron quarks
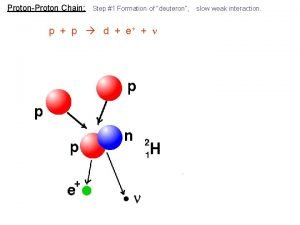
Proton neutron quarks Proton proton chain
Proton proton chain Proton proton chain
Proton proton chain Proton proton chain
Proton proton chain Effective nuclear charge trend
Effective nuclear charge trend Electron charge
Electron charge Proton charge.
Proton charge. Symbol for relative
Symbol for relative Proton charge radius
Proton charge radius Charge of a proton
Charge of a proton Charge on proton
Charge on proton Charge on proton
Charge on proton Mass of neutron
Mass of neutron Mass of subatomic particles in amu
Mass of subatomic particles in amu Mass of subatomic particles
Mass of subatomic particles Mass of subatomic particles
Mass of subatomic particles Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
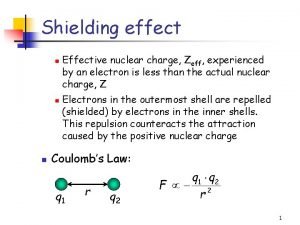
Fisión nuclear vs fision nuclear Shielding effect definition
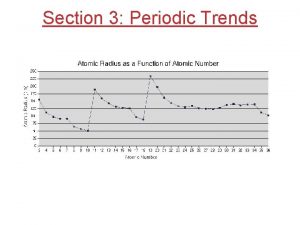
Shielding effect definition Atomic radius increases when
Atomic radius increases when Nuclear charge across a period
Nuclear charge across a period Slaters rule
Slaters rule Pharmaceutical uses of group 1 elements
Pharmaceutical uses of group 1 elements Periodic trends in elemental properties
Periodic trends in elemental properties Increasing electron affinity
Increasing electron affinity Effective nuclear charge trend
Effective nuclear charge trend Effective nuclear charge trend
Effective nuclear charge trend Carbon atom charge
Carbon atom charge