Electric charge and electric forces Chapter 1 The


- Slides: 27

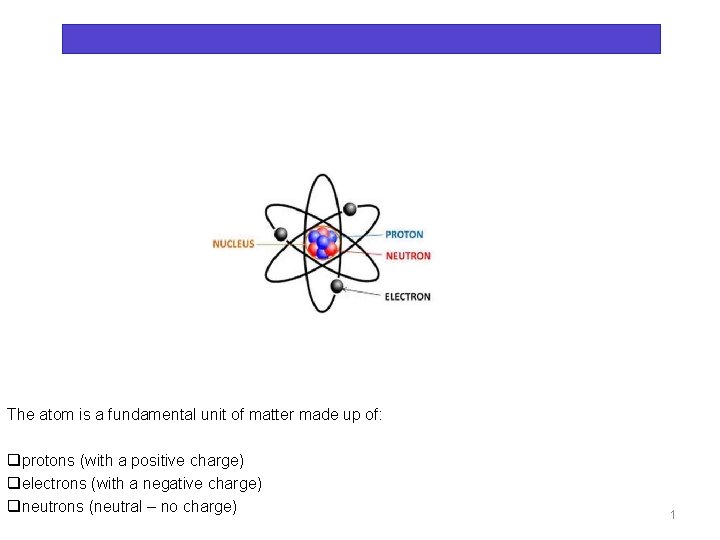
Electric charge and electric forces Chapter 1 The atom is a fundamental unit of matter made up of: qprotons (with a positive charge) qelectrons (with a negative charge) qneutrons (neutral – no charge) 1

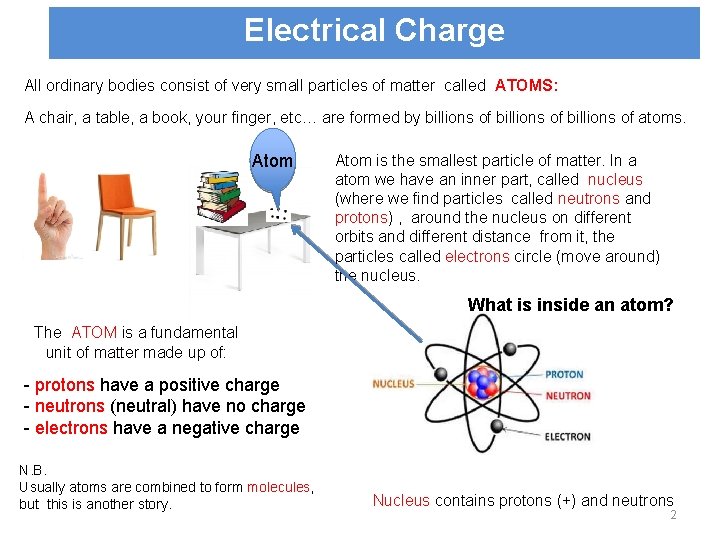
Electrical Charge All ordinary bodies consist of very small particles of matter called ATOMS: A chair, a table, a book, your finger, etc… are formed by billions of atoms. Atom is the smallest particle of matter. In a atom we have an inner part, called nucleus (where we find particles called neutrons and protons) , around the nucleus on different orbits and different distance from it, the particles called electrons circle (move around) the nucleus. What is inside an atom? The ATOM is a fundamental unit of matter made up of: - protons have a positive charge - neutrons (neutral) have no charge - electrons have a negative charge N. B. Usually atoms are combined to form molecules, but this is another story. Nucleus contains protons (+) and neutrons 2

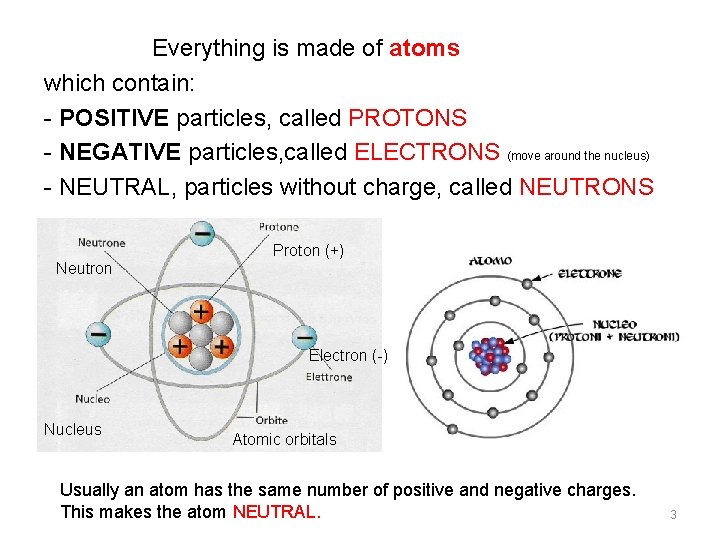
Everything is made of atoms which contain: - POSITIVE particles, called PROTONS - NEGATIVE particles, called ELECTRONS (move around the nucleus) - NEUTRAL, particles without charge, called NEUTRONS Neutron Proton (+) Electron (-) Nucleus Atomic orbitals Usually an atom has the same number of positive and negative charges. This makes the atom NEUTRAL. 3

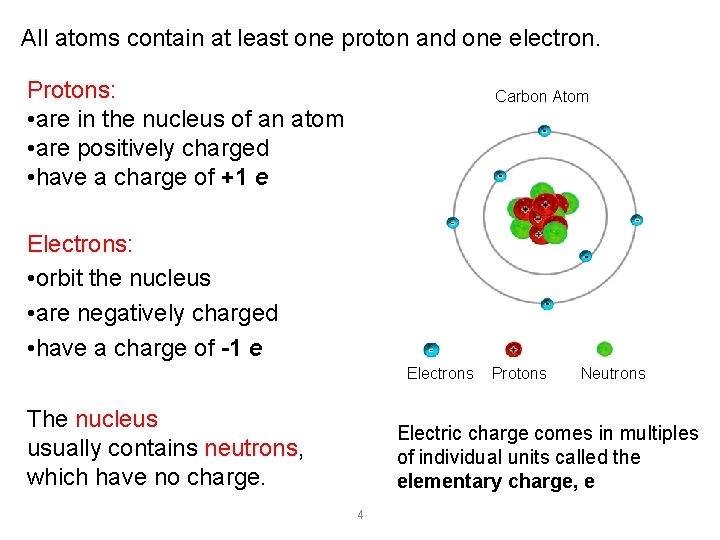
All atoms contain at least one proton and one electron. Protons: • are in the nucleus of an atom • are positively charged • have a charge of +1 e Carbon Atom Electrons: • orbit the nucleus • are negatively charged • have a charge of -1 e Electrons The nucleus usually contains neutrons, which have no charge. Protons Neutrons Electric charge comes in multiples of individual units called the elementary charge, e 4

Electric charge (Electricity) is a property acquired by certain materials (such as amber, called élektron in Greek) when they have been rubbed Electric charge is a fundamental property of matter. There are two types of electric charges • Positive charge - every proton has a single positive charge. • Negative charge - every electron has a single negative charge. • An object with an excess of electrons is negatively charged. • An object with too few electrons (too many protons) is positively charged. • An object with the same number of electrons and protons is neutral. • An object may be negatively charged, which indicates an excess of electrons • An object may be positively charged, which indicates a loss of electrons Since they carry equal but opposite charge, the net charge on a neutral atom is zero. 5

An object, even if discharged, has always negative and positive charges in equal numbers, che si annullano a vicenda, per cui l’oggetto risulta essere scarico/non carico. If an object isn’t charged it is said discharged or uncharged or neutral. The elementery electric charge is equal to. Electrical Charge The elementary electric charge on a electron is equal to: e=1. 6 x -19 10 C which is the same size as the charge on an proton. 6

Electrical Charge All the bodies, from an electrical point of view, can be divided in charged and discharged (or neutral ). Usually, all the bodies are discharged (or neutral), that is they are without electricity, to be more exact the positive charges are equal to the negatives ones. A body that is not discharged , it is charged of elecricity. A body charged of elecricity, it is charged positively or negatively. We say that a body is negatively charged, if it has got an excess of negative charges; if instead it has got an excess of positive charges, it would be better to say a defecit of negative charges, we say it is a posivetely charged. To indicate that a body is charged, we use symbol: Q or q The SI unit of electric charge is the Coulomb indicated with “ C “ Q+ Q- For example: Q = 4 C As the Coulomb is a big unit of measurement, we can use millic oulomb, micro Coulomb, nano coulomb. 1 m C = 10 -3 C 1 C = 10 -6 C 1 n C = 10 -9 C The unit of measurment of electric charge is the Coulomb (C). 7

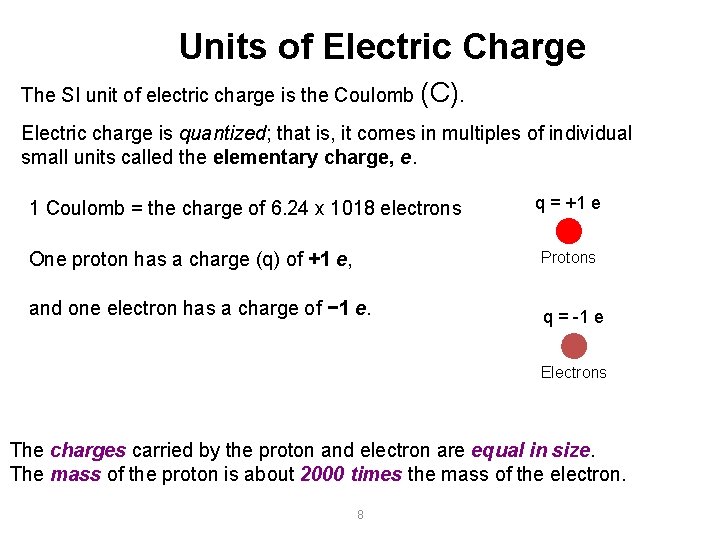
Units of Electric Charge The SI unit of electric charge is the Coulomb (C). Electric charge is quantized; that is, it comes in multiples of individual small units called the elementary charge, e. 1 Coulomb = the charge of 6. 24 x 1018 electrons q = +1 e One proton has a charge (q) of +1 e, Protons and one electron has a charge of − 1 e. q = -1 e Electrons The charges carried by the proton and electron are equal in size. The mass of the proton is about 2000 times the mass of the electron. 8

“Creating” an Electric Charge • When you “create” an electric charge you are actually separating existing charges - not creating charges. • One object ends up with an excess of electrons (- charge), and the other a deficit of electrons (+ charge). The law of conservation of electric charge Don’t forget: Charge cannot be created or destroyed only transferred from one object to another. Making a body to acquire property of attracting small objects is called charging (or electrification or electricity. A body can by charged by three different methods (ways), which we will see later. 9

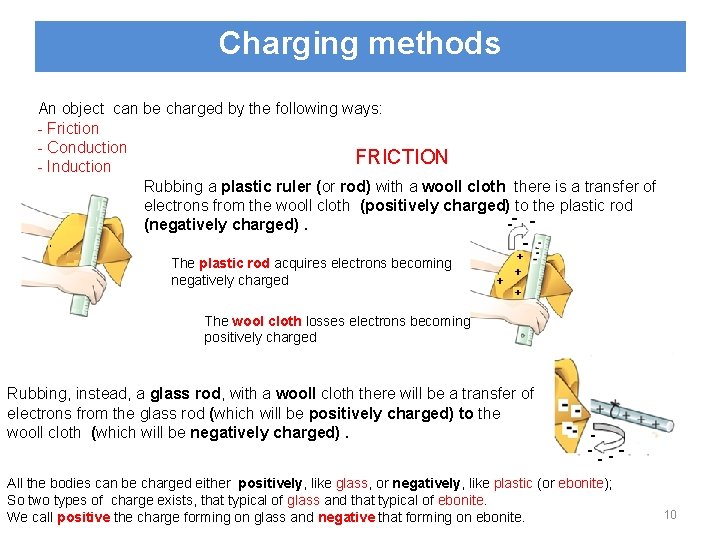
Charging methods An object can be charged by the following ways: - Friction - Conduction FRICTION - Induction Rubbing a plastic ruler (or rod) with a wooll cloth there is a transfer of electrons from the wooll cloth (positively charged) to the plastic rod (negatively charged). The plastic rod acquires electrons becoming negatively charged The wool cloth losses electrons becoming positively charged Rubbing, instead, a glass rod, with a wooll cloth there will be a transfer of electrons from the glass rod (which will be positively charged) to the wooll cloth (which will be negatively charged). All the bodies can be charged either positively, like glass, or negatively, like plastic (or ebonite); So two types of charge exists, that typical of glass and that typical of ebonite. We call positive the charge forming on glass and negative that forming on ebonite. 10

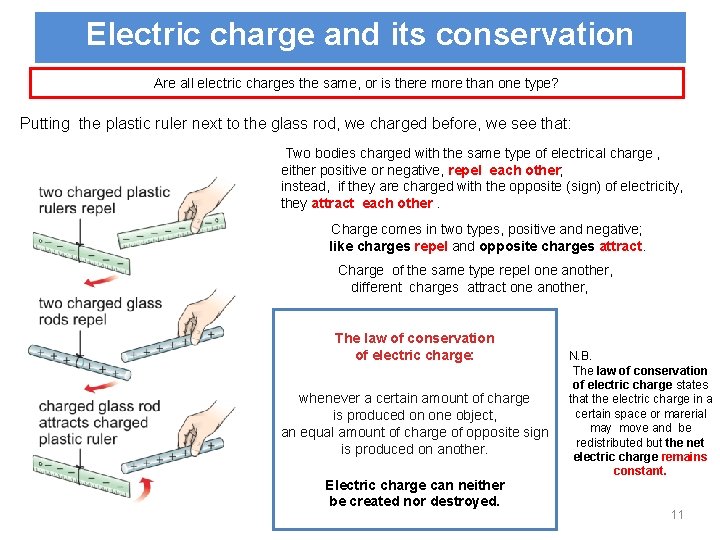
Electric charge and its conservation Are all electric charges the same, or is there more than one type? Putting the plastic ruler next to the glass rod, we charged before, we see that: Two bodies charged with the same type of electrical charge , either positive or negative, repel each other; instead, if they are charged with the opposite (sign) of electricity, they attract each other. Charge comes in two types, positive and negative; like charges repel and opposite charges attract. Charge of the same type repel one another, different charges attract one another, The law of conservation of electric charge: whenever a certain amount of charge is produced on one object, an equal amount of charge of opposite sign is produced on another. Electric charge can neither be created nor destroyed. N. B. The law of conservation of electric charge states that the electric charge in a certain space or marerial may move and be redistributed but the net electric charge remains constant. 11

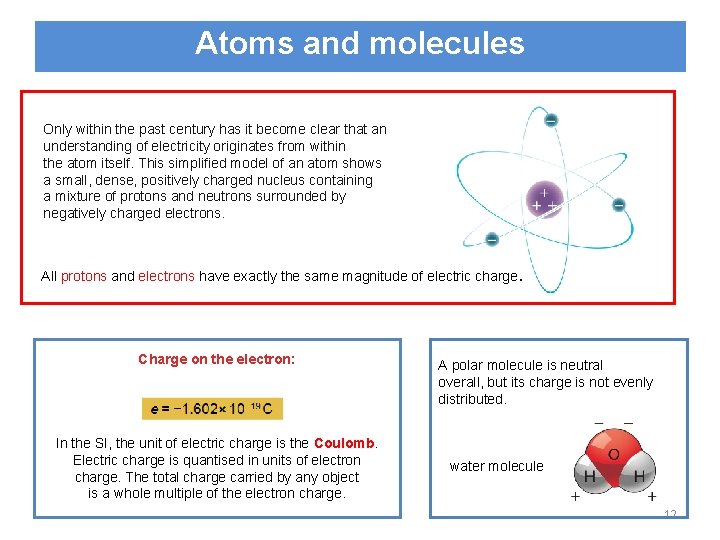
Atoms and molecules Only within the past century has it become clear that an understanding of electricity originates from within the atom itself. This simplified model of an atom shows a small, dense, positively charged nucleus containing a mixture of protons and neutrons surrounded by negatively charged electrons. All protons and electrons have exactly the same magnitude of electric charge. Charge on the electron: In the SI, the unit of electric charge is the Coulomb. Electric charge is quantised in units of electron charge. The total charge carried by any object is a whole multiple of the electron charge. A polar molecule is neutral overall, but its charge is not evenly distributed. water molecule 12

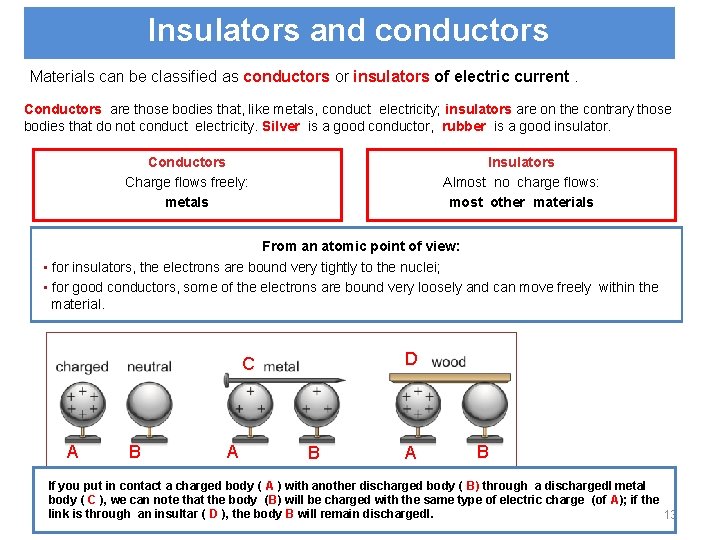
Insulators and conductors Materials can be classified as conductors or insulators of electric current. Conductors are those bodies that, like metals, conduct electricity; insulators are on the contrary those bodies that do not conduct electricity. Silver is a good conductor, rubber is a good insulator. Conductors Charge flows freely: metals Insulators Almost no charge flows: most other materials From an atomic point of view: • for insulators, the electrons are bound very tightly to the nuclei; • for good conductors, some of the electrons are bound very loosely and can move freely within the material. D C A B A B If you put in contact a charged body ( A ) with another discharged body ( B) through a dischargedl metal body ( C ), we can note that the body (B) will be charged with the same type of electric charge (of A); if the link is through an insultar ( D ), the body B will remain dischargedl. 13

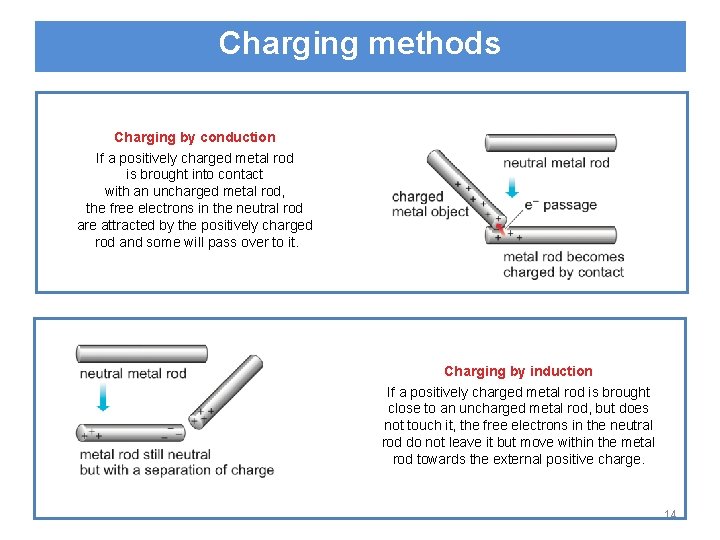
Charging methods Charging by conduction If a positively charged metal rod is brought into contact with an uncharged metal rod, the free electrons in the neutral rod are attracted by the positively charged rod and some will pass over to it. Charging by induction If a positively charged metal rod is brought close to an uncharged metal rod, but does not touch it, the free electrons in the neutral rod do not leave it but move within the metal rod towards the external positive charge. 14

We call insulators substances (or bodies) where the charge stays still in the area which it has been generated in. We call conductors substances where the charge generated in an area speads all over the area of the body. As regards the insulators, the charge has almost nothing mobility; it remains in the area where it has been created. Insulators are plastic, dry wood, glass, porcellain, ebonite; Conductors are all metals and conductor is also the human body. 15

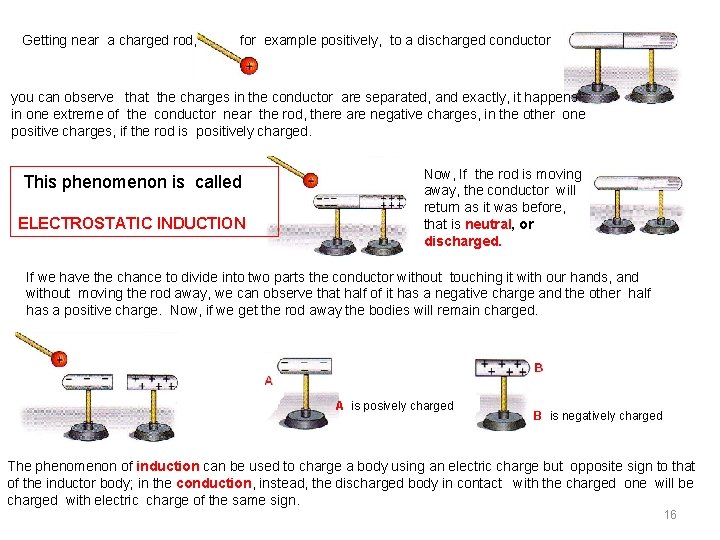
Getting near a charged rod, for example positively, to a discharged conductor you can observe that the charges in the conductor are separated, and exactly, it happens : in one extreme of the conductor near the rod, there are negative charges, in the other one positive charges, if the rod is positively charged. This phenomenon is called ELECTROSTATIC INDUCTION Now, If the rod is moving away, the conductor will return as it was before, that is neutral, or discharged. If we have the chance to divide into two parts the conductor without touching it with our hands, and without moving the rod away, we can observe that half of it has a negative charge and the other half has a positive charge. Now, if we get the rod away the bodies will remain charged. A is posively charged B is negatively charged The phenomenon of induction can be used to charge a body using an electric charge but opposite sign to that of the inductor body; in the conduction, instead, the discharged body in contact with the charged one will be charged with electric charge of the same sign. 16

Approaching a charged rod to a uncharged conductor we observe opposite charges forming on the conductor’s extremities; in the extremity near to the rod charges of opposite sign to that on the rod and in the other extremity identical sign. This phenomenon is called electrostatic induction. To detect whether an object is charged or uncharged, we can use a device: an electroscope. A body can be charged of electricity through: - Friction - Conduction or Contact - Induction Rubbing certain materials (such amber, called elektron, in Greek ), there will be a transfer of electrons, so they acquire the property to attract light bodies, such small paper chunks (pezzeti di carta); this property acquired from amber is called: electricity ). Other methods of charging are through: - contact, by placing the material (or object) in contact with another charged material (or object) - or induction, by placing the material close (next) to a charged material without them actually touching 17

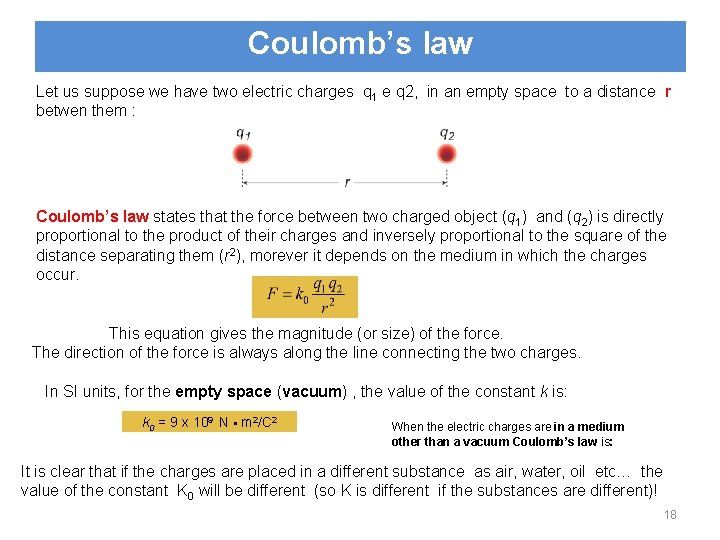
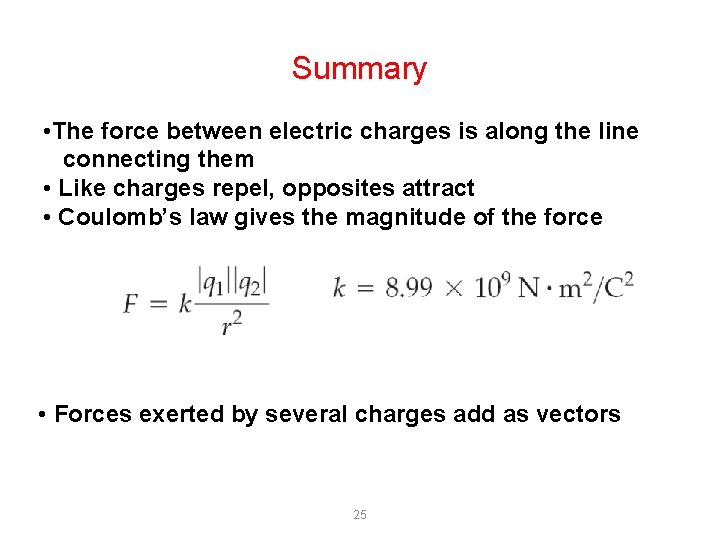
Coulomb’s law Let us suppose we have two electric charges q 1 e q 2, in an empty space to a distance r betwen them : Coulomb’s law states that the force between two charged object (q 1) and (q 2) is directly proportional to the product of their charges and inversely proportional to the square of the distance separating them (r 2), morever it depends on the medium in which the charges occur. This equation gives the magnitude (or size) of the force. The direction of the force is always along the line connecting the two charges. In SI units, for the empty space (vacuum) , the value of the constant k is: k 0 = 9 x 109 N m 2/C 2 When the electric charges are in a medium other than a vacuum Coulomb’s law is: It is clear that if the charges are placed in a different substance as air, water, oil etc… the value of the constant K 0 will be different (so K is different if the substances are different)! 18

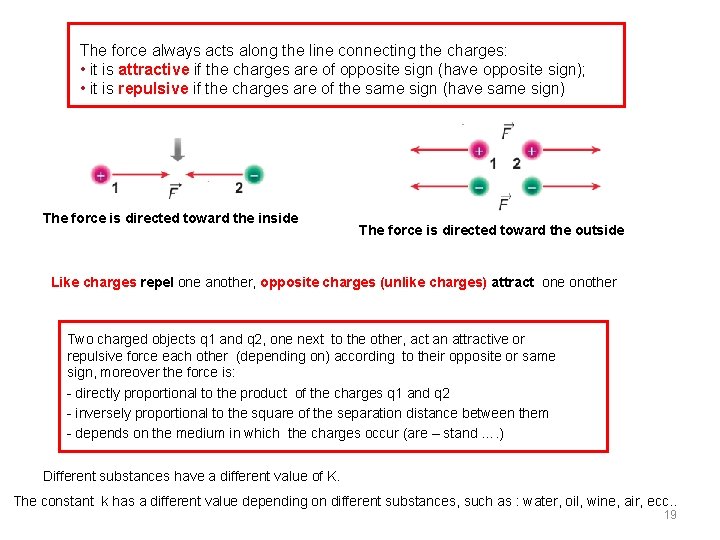
The force always acts along the line connecting the charges: • it is attractive if the charges are of opposite sign (have opposite sign); • it is repulsive if the charges are of the same sign (have same sign) The force is directed toward the inside The force is directed toward the outside Like charges repel one another, opposite charges (unlike charges) attract one onother Two charged objects q 1 and q 2, one next to the other, act an attractive or repulsive force each other (depending on) according to their opposite or same sign, moreover the force is: - directly proportional to the product of the charges q 1 and q 2 - inversely proportional to the square of the separation distance between them - depends on the medium in which the charges occur (are – stand …. ) Different substances have a different value of K. The constant k has a different value depending on different substances, such as : water, oil, wine, air, ecc. . 19

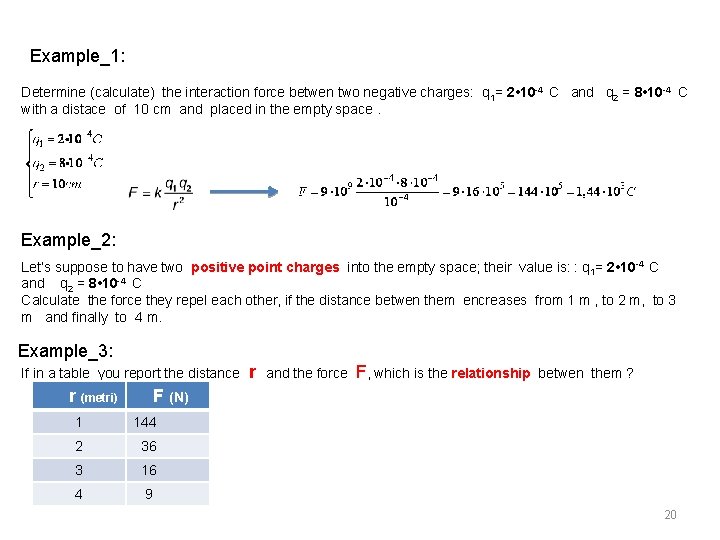
Example_1: Determine (calculate) the interaction force betwen two negative charges: q 1= 2 • 10 -4 C and q 2 = 8 • 10 -4 C with a distace of 10 cm and placed in the empty space. Example_2: Let’s suppose to have two positive point charges into the empty space; their value is: : q 1= 2 • 10 -4 C and q 2 = 8 • 10 -4 C Calculate the force they repel each other, if the distance betwen them encreases from 1 m , to 2 m, to 3 m and finally to 4 m. Example_3: If in a table you report the distance r (metri) r and the force F, which is the relationship betwen them ? F (N) 1 144 2 36 3 16 4 9 20

Exercises: 1) Two positive point charges q and 2 q are separeted by a distance r. If between the charges there is a force of magnitude F when their seperation is r, what is the magnitude between the force when their separetion is 2 r ? . 2) At what seperation will two charges, each of magnitude 6. 0 C, exert a force of 1. 4 N on each other? . 3) Two point charges q 1 and q 2 of magnitude, respectively -4, 0 C and +6. 0 C are separeted by a distance r = 0. 3 m. What is the magnitude and the direction of the electrostatic force on each other? . 21

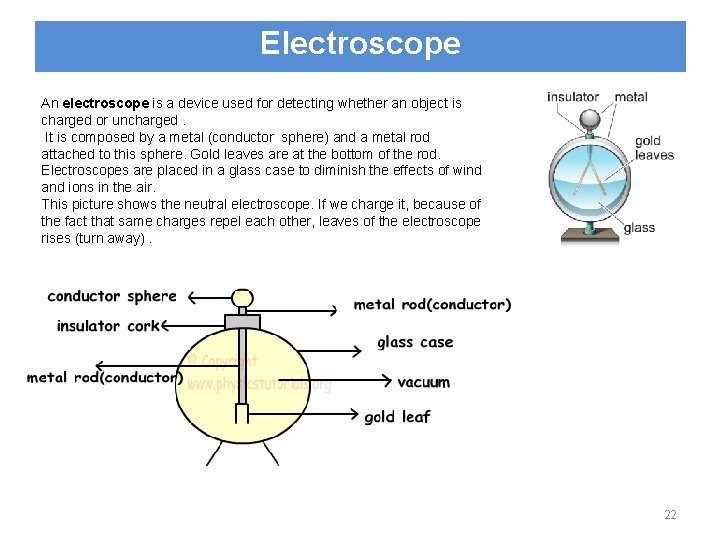
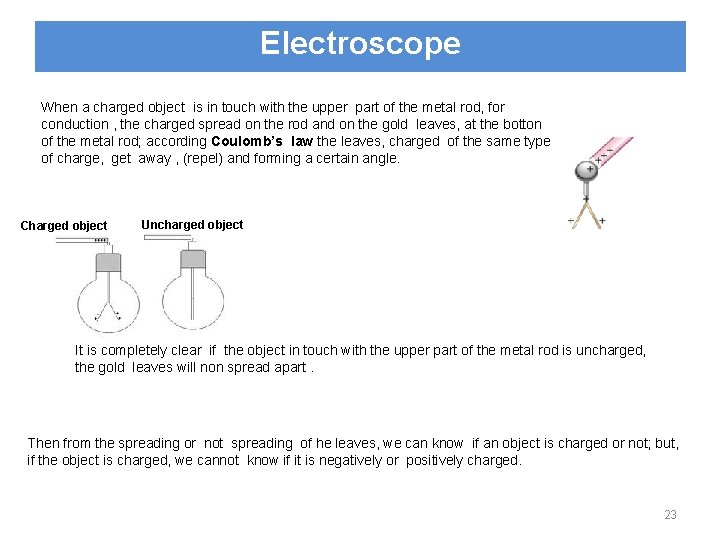
Electroscope An electroscope is a device used for detecting whether an object is charged or uncharged. It is composed by a metal (conductor sphere) and a metal rod attached to this sphere. Gold leaves are at the bottom of the rod. Electroscopes are placed in a glass case to diminish the effects of wind and ions in the air. This picture shows the neutral electroscope. If we charge it, because of the fact that same charges repel each other, leaves of the electroscope rises (turn away). 22

Electroscope When a charged object is in touch with the upper part of the metal rod, for conduction , the charged spread on the rod and on the gold leaves, at the botton of the metal rod; according Coulomb’s law the leaves, charged of the same type of charge, get away , (repel) and forming a certain angle. Charged object Uncharged object It is completely clear if the object in touch with the upper part of the metal rod is uncharged, the gold leaves will non spread apart. Then from the spreading or not spreading of he leaves, we can know if an object is charged or not; but, if the object is charged, we cannot know if it is negatively or positively charged. 23

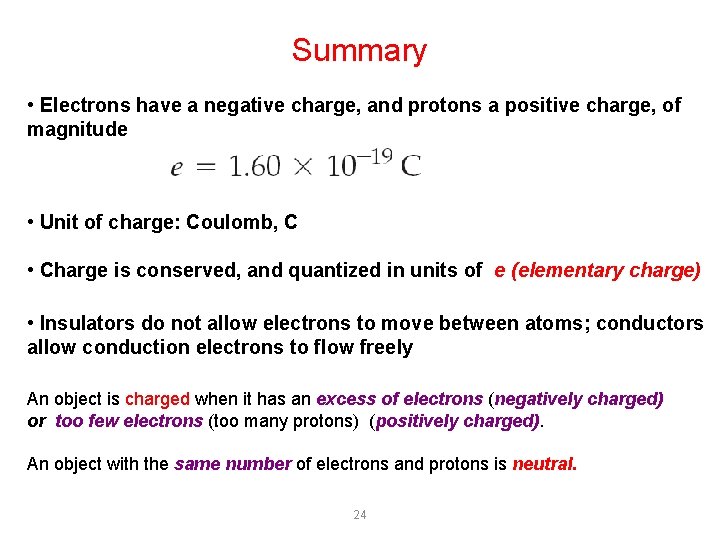
Summary • Electrons have a negative charge, and protons a positive charge, of magnitude • Unit of charge: Coulomb, C • Charge is conserved, and quantized in units of e (elementary charge) • Insulators do not allow electrons to move between atoms; conductors allow conduction electrons to flow freely An object is charged when it has an excess of electrons (negatively charged) or too few electrons (too many protons) (positively charged). An object with the same number of electrons and protons is neutral. 24

Summary • The force between electric charges is along the line connecting them • Like charges repel, opposites attract • Coulomb’s law gives the magnitude of the force • Forces exerted by several charges add as vectors 25

learning the basics 1. 2. 3. In a good conductor, the electrons are bound very tightly to the nuclei. T j. F j When an electroscope is charged, the two leaves repel each other and remain at an angle. T j. F j Tj Fj Coulomb’s law states that the force between two point charges is proportional to the square of the distance separating the charges. 26

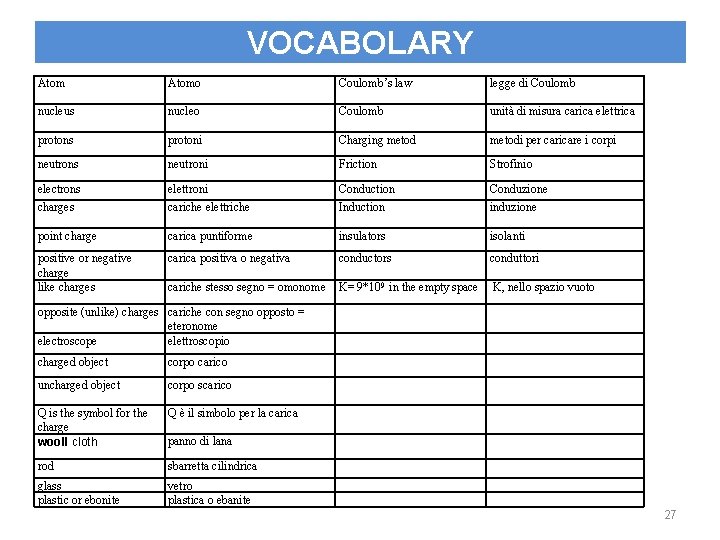
VOCABOLARY Atomo Coulomb’s law legge di Coulomb nucleus nucleo Coulomb unità di misura carica elettrica protons protoni Charging metodi per caricare i corpi neutrons neutroni Friction Strofinio electrons elettroni Conduction Conduzione charges cariche elettriche Induction induzione point charge carica puntiforme insulators isolanti positive or negative charge like charges carica positiva o negativa conductors conduttori cariche stesso segno = omonome K= 9*109 in the empty space K, nello spazio vuoto opposite (unlike) charges cariche con segno opposto = eteronome electroscope elettroscopio charged object corpo carico uncharged object corpo scarico Q is the symbol for the charge wooll cloth Q è il simbolo per la carica rod sbarretta cilindrica glass plastic or ebonite vetro plastica o ebanite panno di lana 27