New Strategies for Antiretroviral Therapy Financial Relationships With







- Slides: 30

New Strategies for Antiretroviral Therapy

Financial Relationships With Ineligible Companies* Dr Daar has received research support awarded to his institution from Gilead Sciences, Inc. , Merck & Co, Inc. , and Vii. V Healthcare. He also received consultant or advisor fees from Gilead Sciences, Inc. , Merck & Co, Inc. , Vii. V Healthcare, and Genentech. (Updated 02/01/21) Slide 2 of 29 *The ACCME recently updated the term from commercial interests to ineligible companies.

Learning Objectives After attending this presentation, learners will be able to: ▪ Describe evolving issues around starting ARV therapy ▪ Discuss new strategies for those on ARV therapy Slide 3 of 29

Outline on Evolving Issues in ART When to Start What to Start Weight gain with ART Switch options in suppressed patients Pregnancy and ART Slide 4 of 29

ARS Question 1 According to DHHS and IAS-USA guidelines, which of the following is not considered a preferred option for those considering rapid start ART? A. B. C. D. Slide 5 of 29 DTG plus TAF (or TDF) with FTC (or 3 TC) BIC/FTC/TAF DTG/3 TC DRV plus RTV plus TAF (or TDF) with FTC (or 3 TC)

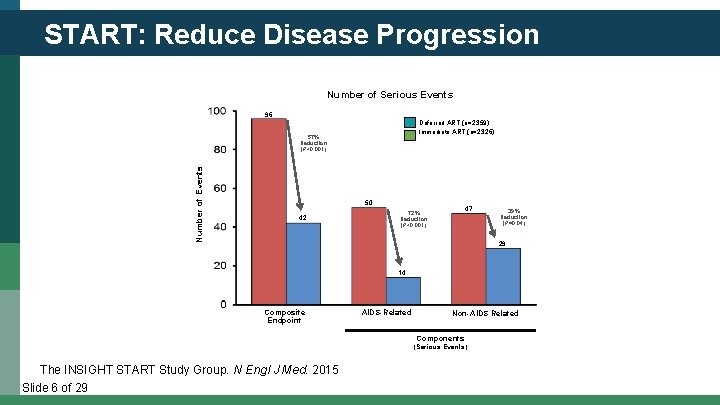
START: Reduce Disease Progression Number of Serious Events 96 Deferred ART (n=2359) Immediate ART (n=2326) Number of Events 57% Reduction (P<0. 001) 50 42 47 72% Reduction (P<0. 001) 39% Reduction (P=0. 04) 29 14 Composite Endpoint AIDS-Related Non-AIDS Related Components (Serious Events) The INSIGHT START Study Group. N Engl J Med. 2015 Slide 6 of 29

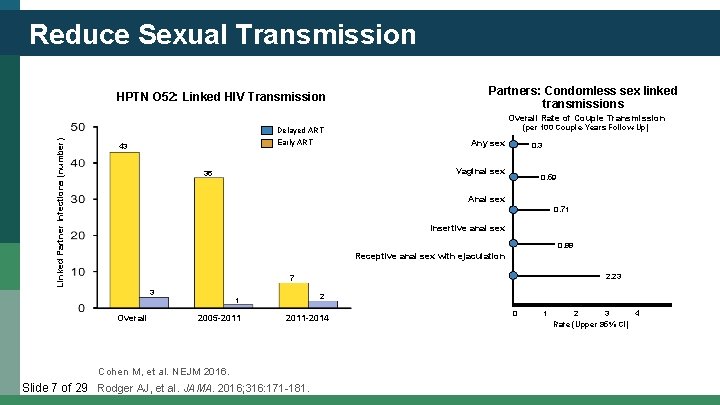
Reduce Sexual Transmission HPTN O 52: Linked HIV Transmission Partners: Condomless sex linked transmissions Linked Partner Infections (number) Overall Rate of Couple Transmission Delayed ART Early ART 43 (per 100 Couple-Years Follow-Up) Any sex 0. 3 Vaginal sex 36 0. 59 Anal sex 0. 71 Insertive anal sex 0. 88 Receptive anal sex with ejaculation 2. 23 7 3 Overall 2 1 2005 -2011 -2014 Cohen M, et al. NEJM 2016. Slide 7 of 29. Rodger AJ, et al. JAMA. 2016; 316: 171 -181. 0 1 2 3 4 Rate (Upper 95% CI)

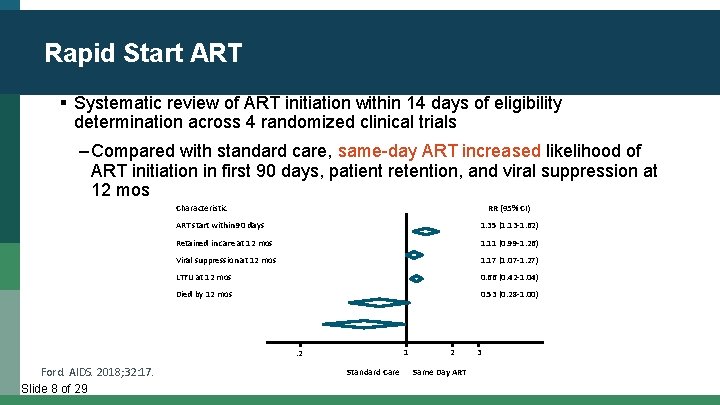
Rapid Start ART § Systematic review of ART initiation within 14 days of eligibility determination across 4 randomized clinical trials ‒ Compared with standard care, same-day ART increased likelihood of ART initiation in first 90 days, patient retention, and viral suppression at 12 mos Characteristic RR (95% CI) ART start within 90 days 1. 35 (1. 13 -1. 62) Retained in care at 12 mos 1. 11 (0. 99 -1. 26) Viral suppression at 12 mos 1. 17 (1. 07 -1. 27) LTFU at 12 mos 0. 66 (0. 42 -1. 04) Died by 12 mos 0. 53 (0. 28 -1. 00) 1 . 2 Ford. AIDS. 2018; 32: 17. Slide 8 of 29 Standard Care 2 Same Day ART 3

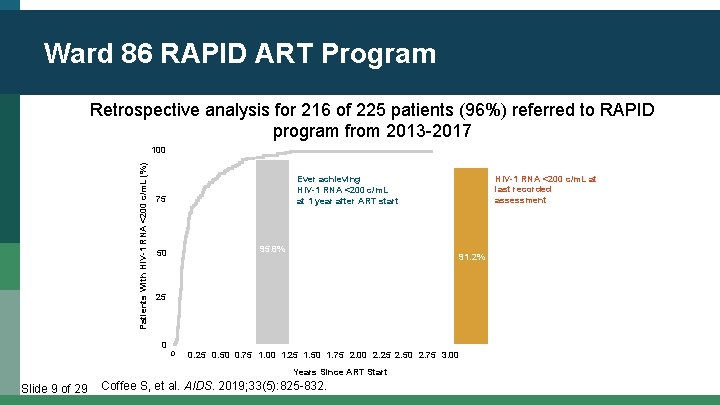
Ward 86 RAPID ART Program Retrospective analysis for 216 of 225 patients (96%) referred to RAPID program from 2013 -2017 Patients With HIV-1 RNA <200 c/m. L (%) 100 75 95. 8% 50 91. 2% 25 0 0 0. 25 0. 50 0. 75 1. 00 1. 25 1. 50 1. 75 2. 00 2. 25 2. 50 2. 75 3. 00 Years Since ART Start Slide 9 of 29 HIV-1 RNA <200 c/m. L at last recorded assessment Ever achieving HIV-1 RNA <200 c/m. L at 1 year after ART start Coffee S, et al. AIDS. 2019; 33(5): 825 -832.

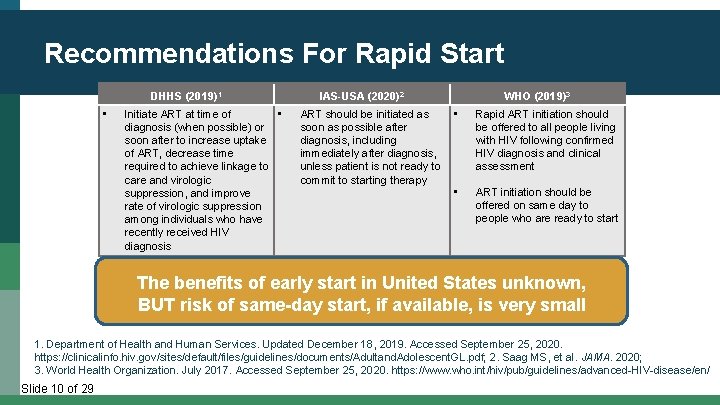
Recommendations For Rapid Start DHHS (2019)1 • Initiate ART at time of • diagnosis (when possible) or soon after to increase uptake of ART, decrease time required to achieve linkage to care and virologic suppression, and improve rate of virologic suppression among individuals who have recently received HIV diagnosis IAS-USA (2020)2 ART should be initiated as soon as possible after diagnosis, including immediately after diagnosis, unless patient is not ready to commit to starting therapy WHO (2019)3 • Rapid ART initiation should be offered to all people living with HIV following confirmed HIV diagnosis and clinical assessment • ART initiation should be offered on same day to people who are ready to start The benefits of early start in United States unknown, BUT risk of same-day start, if available, is very small 1. Department of Health and Human Services. Updated December 18, 2019. Accessed September 25, 2020. https: //clinicalinfo. hiv. gov/sites/default/files/guidelines/documents/Adultand. Adolescent. GL. pdf; 2. Saag MS, et al. JAMA. 2020; 3. World Health Organization. July 2017. Accessed September 25, 2020. https: //www. who. int/hiv/pub/guidelines/advanced-HIV-disease/en/ Slide 10 of 29

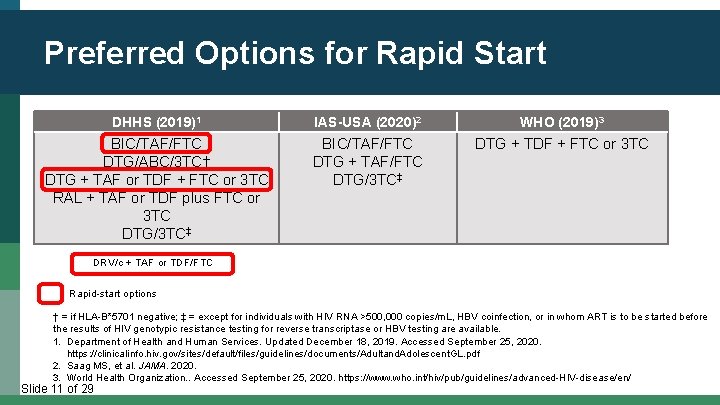
Preferred Options for Rapid Start DHHS (2019)1 IAS-USA (2020)2 WHO (2019)3 BIC/TAF/FTC DTG/ABC/3 TC† DTG + TAF or TDF + FTC or 3 TC RAL + TAF or TDF plus FTC or 3 TC DTG/3 TC‡ BIC/TAF/FTC DTG + TAF/FTC DTG/3 TC‡ DTG + TDF + FTC or 3 TC DRV/c + TAF or TDF/FTC Rapid-start options † = if HLA-B*5701 negative; ‡ = except for individuals with HIV RNA >500, 000 copies/m. L, HBV coinfection, or in whom ART is to be started before the results of HIV genotypic resistance testing for reverse transcriptase or HBV testing are available. 1. Department of Health and Human Services. Updated December 18, 2019. Accessed September 25, 2020. https: //clinicalinfo. hiv. gov/sites/default/files/guidelines/documents/Adultand. Adolescent. GL. pdf 2. Saag MS, et al. JAMA. 2020. 3. World Health Organization. . Accessed September 25, 2020. https: //www. who. int/hiv/pub/guidelines/advanced-HIV-disease/en/ Slide 11 of 29

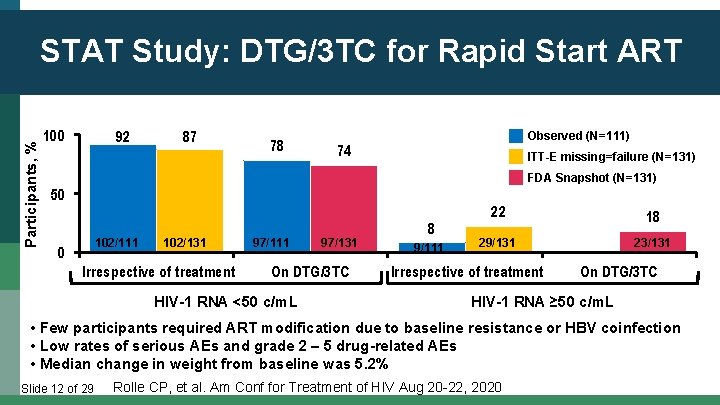
Participants, % STAT Study: DTG/3 TC for Rapid Start ART 100 92 87 78 Observed (N=111) 74 ITT-E missing=failure (N=131) FDA Snapshot (N=131) 50 102/111 0 102/131 Irrespective of treatment 97/111 97/131 On DTG/3 TC HIV-1 RNA <50 c/m. L 8 9/111 22 18 29/131 23/131 Irrespective of treatment On DTG/3 TC HIV-1 RNA ≥ 50 c/m. L • Few participants required ART modification due to baseline resistance or HBV coinfection • Low rates of serious AEs and grade 2 – 5 drug-related AEs • Median change in weight from baseline was 5. 2% Slide 12 of 29 Rolle CP, et al. Am Conf for Treatment of HIV Aug 20 -22, 2020

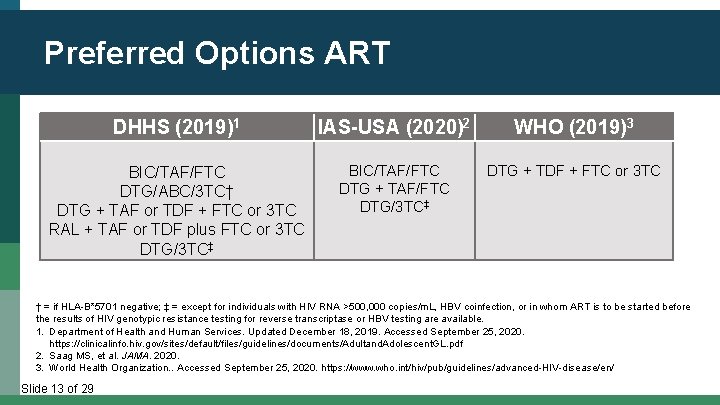
Preferred Options ART DHHS (2019)1 IAS-USA (2020)2 WHO (2019)3 BIC/TAF/FTC DTG/ABC/3 TC† DTG + TAF or TDF + FTC or 3 TC RAL + TAF or TDF plus FTC or 3 TC DTG/3 TC‡ BIC/TAF/FTC DTG + TAF/FTC DTG/3 TC‡ DTG + TDF + FTC or 3 TC † = if HLA-B*5701 negative; ‡ = except for individuals with HIV RNA >500, 000 copies/m. L, HBV coinfection, or in whom ART is to be started before the results of HIV genotypic resistance testing for reverse transcriptase or HBV testing are available. 1. Department of Health and Human Services. Updated December 18, 2019. Accessed September 25, 2020. https: //clinicalinfo. hiv. gov/sites/default/files/guidelines/documents/Adultand. Adolescent. GL. pdf 2. Saag MS, et al. JAMA. 2020. 3. World Health Organization. . Accessed September 25, 2020. https: //www. who. int/hiv/pub/guidelines/advanced-HIV-disease/en/ Slide 13 of 29

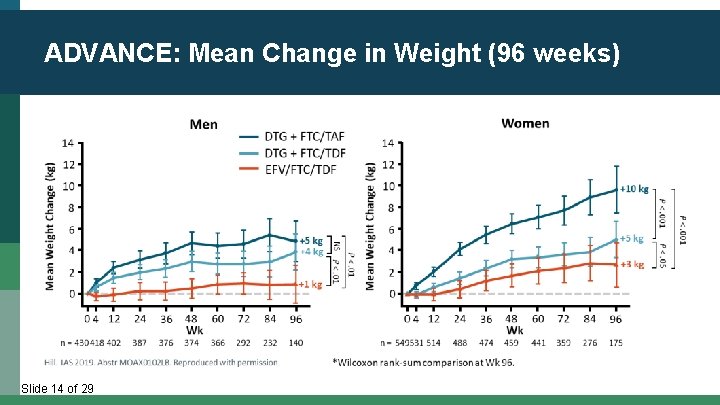
ADVANCE: Mean Change in Weight (96 weeks) Slide 14 of 29

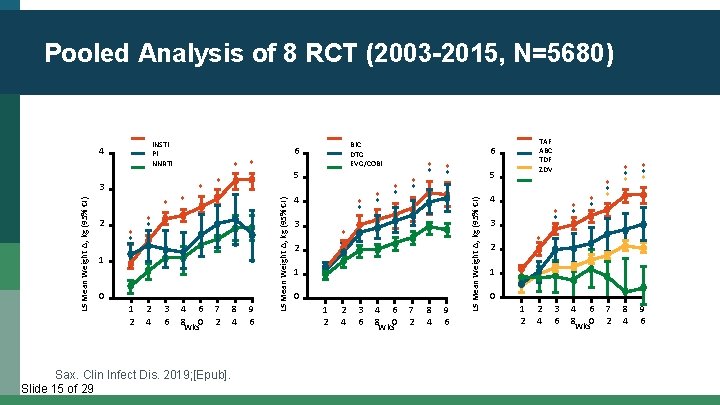
Pooled Analysis of 8 RCT (2003 -2015, N=5680) * 2 * * * * 1 0 1 2 2 4 3 6 4 6 8 Wks 0 7 2 Sax. Clin Infect Dis. 2019; [Epub]. Slide 15 of 29 8 4 9 6 4 * * 3 * * * 2 1 0 1 2 2 4 3 6 4 8 TAF ABC TDF ZDV 6 5 LS Mean Weight Δ, kg (95% CI) 3 * BIC DTG EVG/COBI 6 6 0 Wks 7 2 8 4 9 6 5 LS Mean Weight Δ, kg (95% CI) INSTI PI NNRTI 4 4 * * 3 * * * * 8 4 9 6 * 2 1 0 1 2 2 4 3 6 4 8 6 0 Wks 7 2

Strategies for Managing ART-Associated Weight Gain • Amount of weight gain varies based upon ART use • This may be multifactorial, e. g. some drugs increasing and others attenuating weight gain • Strategies for managing weight gain under investigation ACTG 5391 (The Do IT Study): Doravirine for Persons with Excessive Weight Gain on INSTIs and TAF Slide 16 of 29

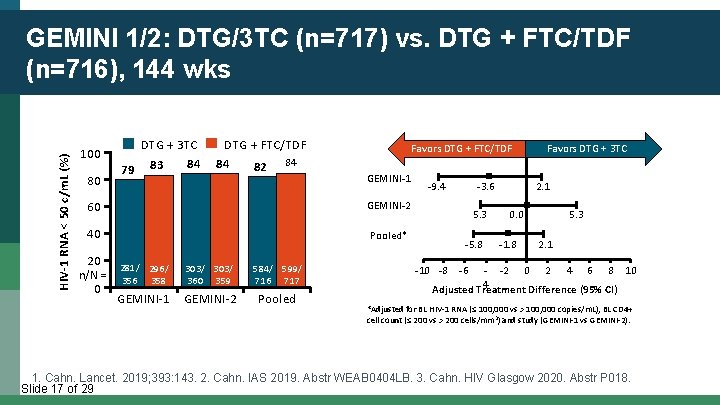
HIV-1 RNA < 50 c/m. L (%) GEMINI 1/2: DTG/3 TC (n=717) vs. DTG + FTC/TDF (n=716), 144 wks 100 80 DTG + 3 TC 84 79 83 DTG + FTC/TDF 84 82 84 Favors DTG + FTC/TDF GEMINI-1 60 GEMINI-2 40 Pooled* 20 n/N = 0 281/ 296/ 356 358 303/ 360 359 GEMINI-1 GEMINI-2 584/ 599/ 716 717 Pooled -9. 4 -3. 6 5. 3 -5. 8 -10 -8 Favors DTG + 3 TC 2. 1 0. 0 -1. 8 5. 3 2. 1 -6 - -2 0 2 4 6 8 10 4 Adjusted Treatment Difference (95% CI) *Adjusted for BL HIV-1 RNA (≤ 100, 000 vs > 100, 000 copies/m. L), BL CD 4+ cell count (≤ 200 vs > 200 cells/mm 3) and study (GEMINI-1 vs GEMINI-2). 1. Cahn. Lancet. 2019; 393: 143. 2. Cahn. IAS 2019. Abstr WEAB 0404 LB. 3. Cahn. HIV Glasgow 2020. Abstr P 018. Slide 17 of 29

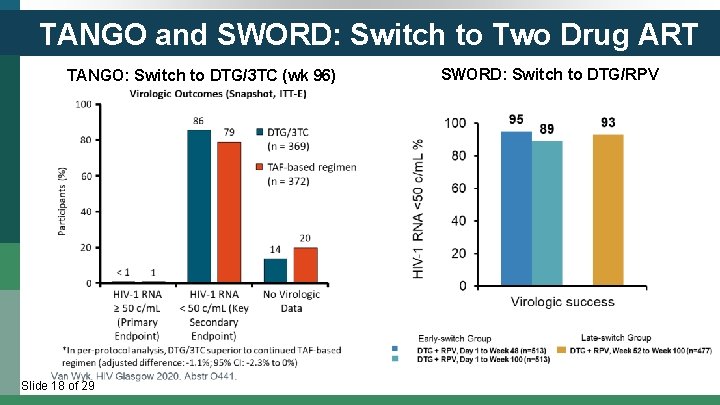
TANGO and SWORD: Switch to Two Drug ART TANGO: Switch to DTG/3 TC (wk 96) Slide 18 of 29 SWORD: Switch to DTG/RPV

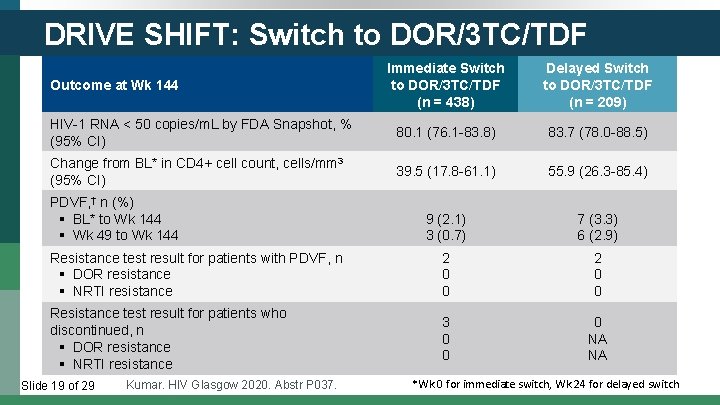
DRIVE SHIFT: Switch to DOR/3 TC/TDF Immediate Switch to DOR/3 TC/TDF (n = 438) Delayed Switch to DOR/3 TC/TDF (n = 209) HIV-1 RNA < 50 copies/m. L by FDA Snapshot, % (95% CI) 80. 1 (76. 1 -83. 8) 83. 7 (78. 0 -88. 5) Change from BL* in CD 4+ cell count, cells/mm 3 (95% CI) 39. 5 (17. 8 -61. 1) 55. 9 (26. 3 -85. 4) 9 (2. 1) 3 (0. 7) 7 (3. 3) 6 (2. 9) Resistance test result for patients with PDVF, n § DOR resistance § NRTI resistance 2 0 0 Resistance test result for patients who discontinued, n § DOR resistance § NRTI resistance 3 0 0 0 NA NA Outcome at Wk 144 PDVF, † n (%) § BL* to Wk 144 § Wk 49 to Wk 144 Slide 19 of 29 Kumar. HIV Glasgow 2020. Abstr P 037. *Wk 0 for immediate switch, Wk 24 for delayed switch

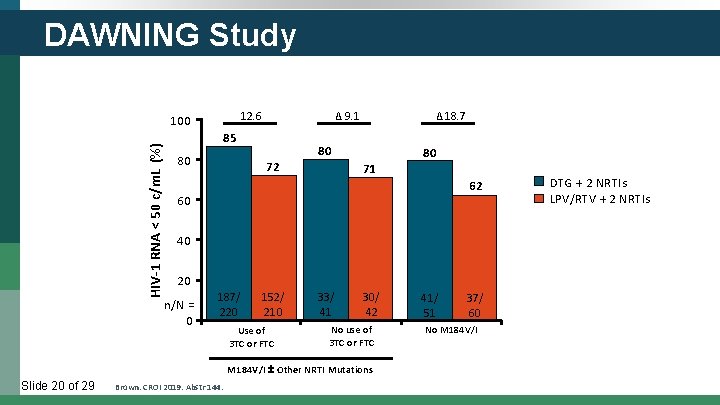
DAWNING Study HIV-1 RNA < 50 c/m. L (%) 100 12. 6 Δ 9. 1 85 80 72 Δ 18. 7 80 71 62 60 40 20 n/N = 0 187/ 220 152/ 210 Use of 3 TC or FTC 33/ 41 219/ 30/ 312 42 No use of 3 TC or FTC M 184 V/I Other NRTI Mutations Slide 20 of 29 80 Brown. CROI 2019. Abstr 144. 41/ 51 37/ 60 No M 184 V/I DTG + 2 NRTIs LPV/RTV + 2 NRTIs

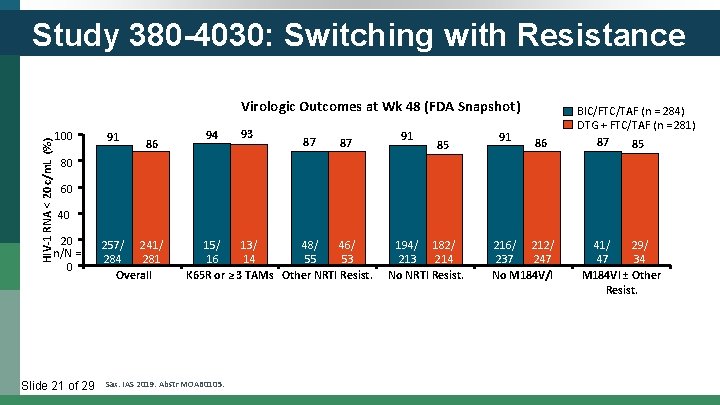
Study 380 -4030: Switching with Resistance HIV-1 RNA < 20 c/m. L (%) Virologic Outcomes at Wk 48 (FDA Snapshot) 100 91 86 94 93 87 87 91 85 91 86 BIC/FTC/TAF (n = 284) DTG + FTC/TAF (n = 281) 87 85 80 60 40 20 n/N = 0 Slide 21 of 29 257/ 241/ 284 281 Overall 15/ 13/ 48/ 46/ 16 14 55 53 K 65 R or ≥ 3 TAMs Other NRTI Resist. Sax. IAS 2019. Abstr MOAB 0105. 194/ 182/ 213 214 No NRTI Resist. 216/ 212/ 237 247 No M 184 V/I 41/ 29/ 47 34 M 184 VI ± Other Resist.

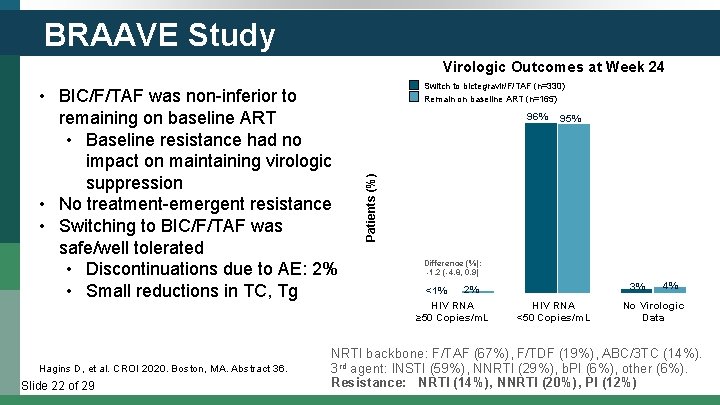
BRAAVE Study Virologic Outcomes at Week 24 Hagins D, et al. CROI 2020. Boston, MA. Abstract 36. Slide 22 of 29 Switch to bictegravir/F/TAF (n=330) Remain on baseline ART (n=165) 100 96% 95% 80 Patients (%) • BIC/F/TAF was non-inferior to remaining on baseline ART • Baseline resistance had no impact on maintaining virologic suppression • No treatment-emergent resistance • Switching to BIC/F/TAF was safe/well tolerated • Discontinuations due to AE: 2% • Small reductions in TC, Tg 60 40 20 0 Difference (%): -1. 2 (-4. 8, 0. 9) 2% <1% HIV RNA ≥ 50 Copies/m. L 3% HIV RNA <50 Copies/m. L 4% No Virologic Data NRTI backbone: F/TAF (67%), F/TDF (19%), ABC/3 TC (14%). 3 rd agent: INSTI (59%), NNRTI (29%), b. PI (6%), other (6%). Resistance: NRTI (14%), NNRTI (20%), PI (12%)

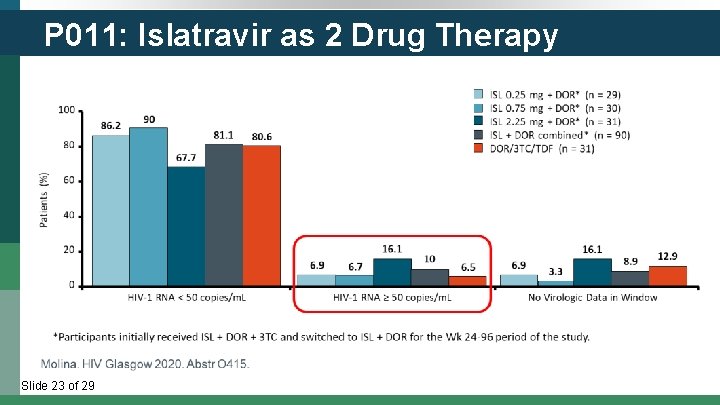
P 011: Islatravir as 2 Drug Therapy Slide 23 of 29

ARS Question 2 According to DHHS guidelines, which of the following is not considered a preferred option for those who might become pregnant on ART? A. B. C. D. Raltegravir Bictegravir Dolutegravir Darunavir/ritonavir Slide 24 of 29

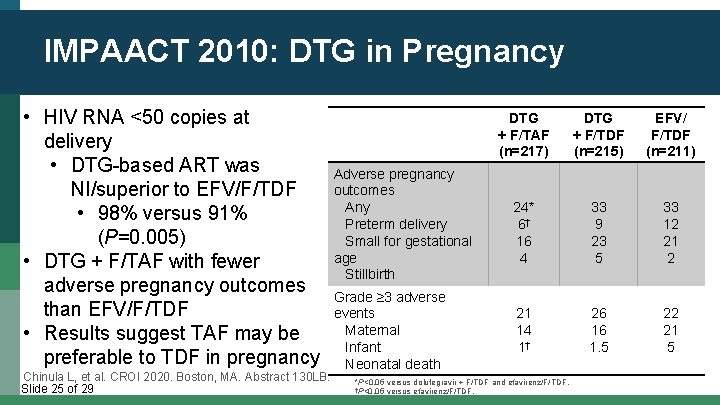
IMPAACT 2010: DTG in Pregnancy • HIV RNA <50 copies at delivery • DTG-based ART was NI/superior to EFV/F/TDF • 98% versus 91% (P=0. 005) • DTG + F/TAF with fewer adverse pregnancy outcomes than EFV/F/TDF • Results suggest TAF may be preferable to TDF in pregnancy Chinula L, et al. CROI 2020. Boston, MA. Abstract 130 LB. Slide 25 of 29 Adverse pregnancy outcomes Any Preterm delivery Small for gestational age Stillbirth Grade ≥ 3 adverse events Maternal Infant Neonatal death DTG + F/TAF (n=217) DTG + F/TDF (n=215) EFV/ F/TDF (n=211) 24* 6† 16 4 33 9 23 5 33 12 21 14 1† 26 16 1. 5 22 21 5 *P<0. 05 versus dolutegravir + F/TDF and efavirenz/F/TDF. †P<0. 05 versus efavirenz/F/TDF.

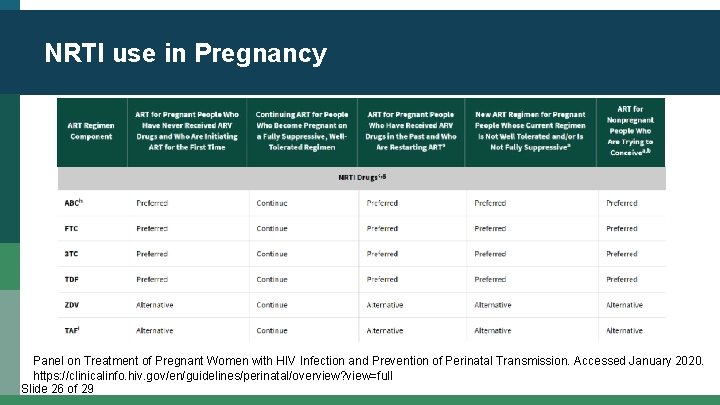
NRTI use in Pregnancy Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Accessed January 2020. https: //clinicalinfo. hiv. gov/en/guidelines/perinatal/overview? view=full Slide 26 of 29

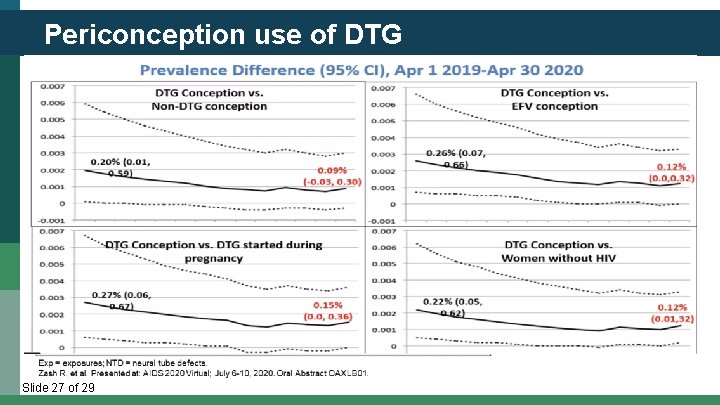
Periconception use of DTG Slide 27 of 29

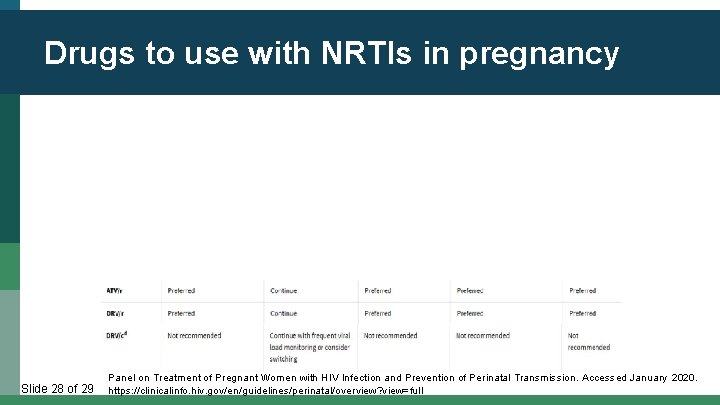
Drugs to use with NRTIs in pregnancy Slide 28 of 29 Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Accessed January 2020. https: //clinicalinfo. hiv. gov/en/guidelines/perinatal/overview? view=full

Summary § Rapid Start ART is increasingly the standard of care for those willing § Weight gain continues to get attention in context of ART § Dual therapy ART is getting increasing traction for first-line and switch options § Simple INSTI-based regimens are options even for those with some underlying resistance. § DTG and TAF emerging as options for those who are or might get pregnant Slide 29 of 29

Question-and-Answer Session
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Bioness integrated therapy system price
Bioness integrated therapy system price Humanistic therapies aim to boost
Humanistic therapies aim to boost Retail financial management
Retail financial management Wii hab
Wii hab Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Adressändring ideell förening
Adressändring ideell förening Tidböcker
Tidböcker Sura för anatom
Sura för anatom Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Debattartikel mall
Debattartikel mall Delegerande ledarstil
Delegerande ledarstil Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Vätsketryck formel
Vätsketryck formel Offentlig förvaltning
Offentlig förvaltning Jag har nigit för nymånens skära text
Jag har nigit för nymånens skära text Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Plats för toran ark
Plats för toran ark Klassificeringsstruktur för kommunala verksamheter
Klassificeringsstruktur för kommunala verksamheter Epiteltyper
Epiteltyper