Antiretroviral Therapy What to Start and New Drugs














- Slides: 40

Antiretroviral Therapy: What to Start and New Drugs Roy M. Gulick, MD, MPH Gladys and Roland Harriman Professor of Medicine Chief, Division of Infectious Diseases Weill Medical College of Cornell University New York, New York FORMATTED: 03/08/16 New York, New York: March 23, 2016 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

Learning Objectives After attending this presentation, participants will be able to: �List the current recommended and alternative ART regimens �Describe research data to support these recommendations �Describe at least 3 investigational antiretroviral drugs in the pipeline Slide 2 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

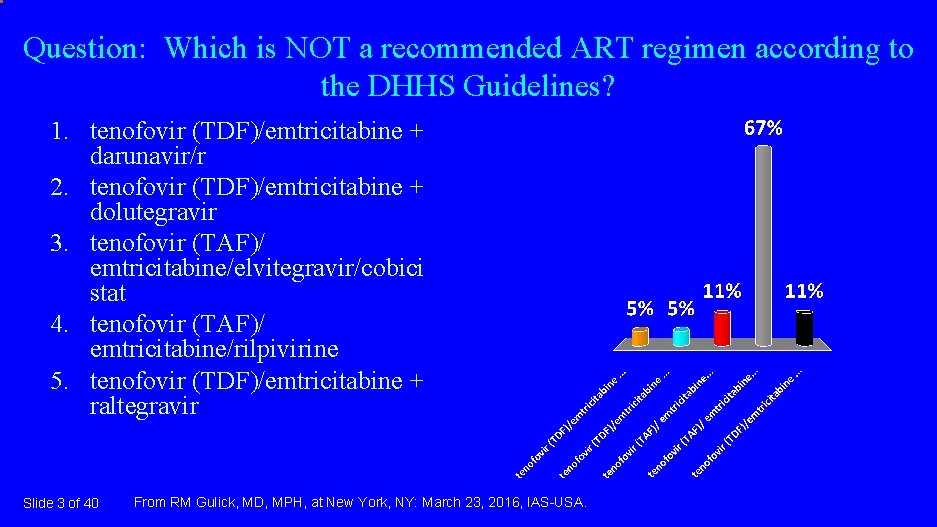
Question: Which is NOT a recommended ART regimen according to the DHHS Guidelines? 1. tenofovir (TDF)/emtricitabine + darunavir/r 2. tenofovir (TDF)/emtricitabine + dolutegravir 3. tenofovir (TAF)/ emtricitabine/elvitegravir/cobici stat 4. tenofovir (TAF)/ emtricitabine/rilpivirine 5. tenofovir (TDF)/emtricitabine + raltegravir Slide 3 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

Recommended ART Regimens (2 NRTI + 3 rd drug) • Protease Inhibitor-based – tenofovir (TDF)/emtricitabine + darunavir/r • Integrase Inhibitor-based – abacavir/lamivudine/dolutegravir – tenofovir (TDF)/emtricitabine + dolutegravir – tenofovir (TAF)/ emtricitabine/elvitegravir/cobicistat – tenofovir (TDF)/emtricitabine + raltegravir Slide 4 of 40 U. S. DHHS Guidelines 1/28/16 www. aidsinfo. nih. gov From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

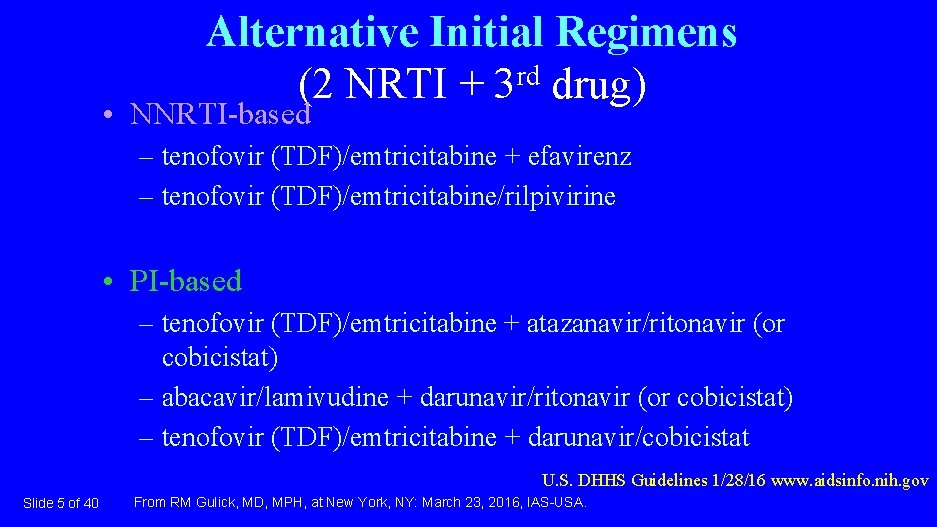
Alternative Initial Regimens (2 NRTI + 3 rd drug) • NNRTI-based – tenofovir (TDF)/emtricitabine + efavirenz – tenofovir (TDF)/emtricitabine/rilpivirine • PI-based – tenofovir (TDF)/emtricitabine + atazanavir/ritonavir (or cobicistat) – abacavir/lamivudine + darunavir/ritonavir (or cobicistat) – tenofovir (TDF)/emtricitabine + darunavir/cobicistat U. S. DHHS Guidelines 1/28/16 www. aidsinfo. nih. gov Slide 5 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

Newer ART Rx-Naïve Studies Study (reference) N Regimen ACTG 5257 605 2 NRTI + ATV/r VL <50 (96 wks) 88% Lennox Ann Intern Med 2014; 161: 461 SINGLE 601 2 NRTI + DRV/r 89% 603 2 NRTI + RAL 94%* 414 ABC/3 TC + DTG 80%* Walmsley NEJM 2013; 369: 1807 + JAIDS 2015; 70: 515 FLAMINGO 419 TDF/FTC/EFV 242 2 NRTI + DTG 72% 80%* 242 2 NRTI + DRV/r 68% Molina Lancet 2014; 383: 2222 + Lancet HIV 2015; 2: e 127 Slide 6 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. * = significant difference

Two drugs? • PADDLE Study Figueroa EACS 2015 #LBPS 4/1 – Treatment-naïve individuals with HIV RNA 5 -100 K (N=20) – 2 -drug regimen of DTG + 3 TC – Results: All suppressed VL <50 by week 8 week 24 – HIV RNA decline Sued CROI 2016 #947 • -2. 75 log cps/ml (PADDLE) vs. -2. 5 (SPRING-1) and -2. 6 (SINGLE) • ACTG 5353 Slide 7 of 40 – Pilot study enrolling (N=120) – Treatment-Naïve Individuals with HIV RNA <500 K From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

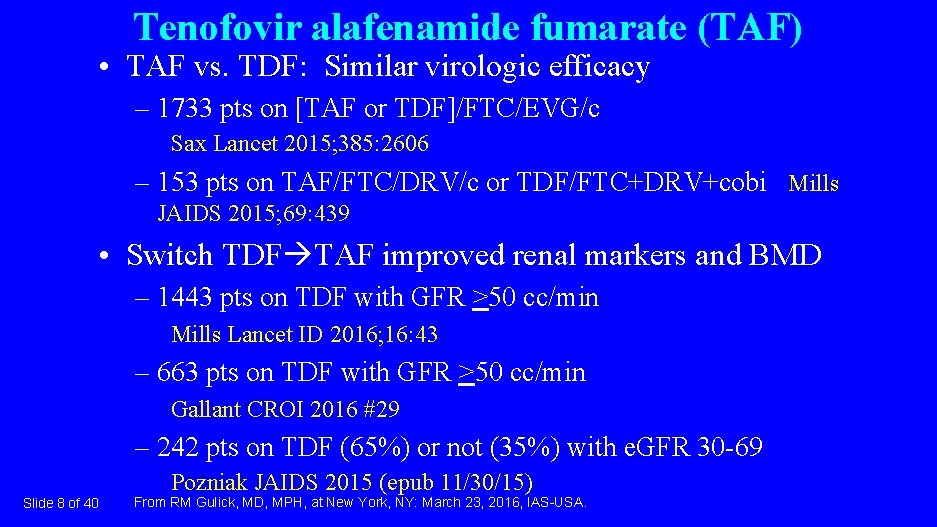
Tenofovir alafenamide fumarate (TAF) • TAF vs. TDF: Similar virologic efficacy – 1733 pts on [TAF or TDF]/FTC/EVG/c Sax Lancet 2015; 385: 2606 – 153 pts on TAF/FTC/DRV/c or TDF/FTC+DRV+cobi Mills JAIDS 2015; 69: 439 • Switch TDF TAF improved renal markers and BMD – 1443 pts on TDF with GFR >50 cc/min Mills Lancet ID 2016; 16: 43 – 663 pts on TDF with GFR >50 cc/min Gallant CROI 2016 #29 – 242 pts on TDF (65%) or not (35%) with e. GFR 30 -69 Pozniak JAIDS 2015 (epub 11/30/15) Slide 8 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

Tenofovir alafenamide (TAF) • TAF dose will be 25 mg, despite other drugs Lawson ICAAC 2014 #H-1012 • Co-formulations – TAF/FTC/EVG/c (FDA approved 11/5/15) – TAF/FTC/RPV (FDA approved 3/1/16) – TAF/FTC (FDA target action date: 4/7/16) – TAF/FTC/DRV/c (in clinical trials) Slide 9 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

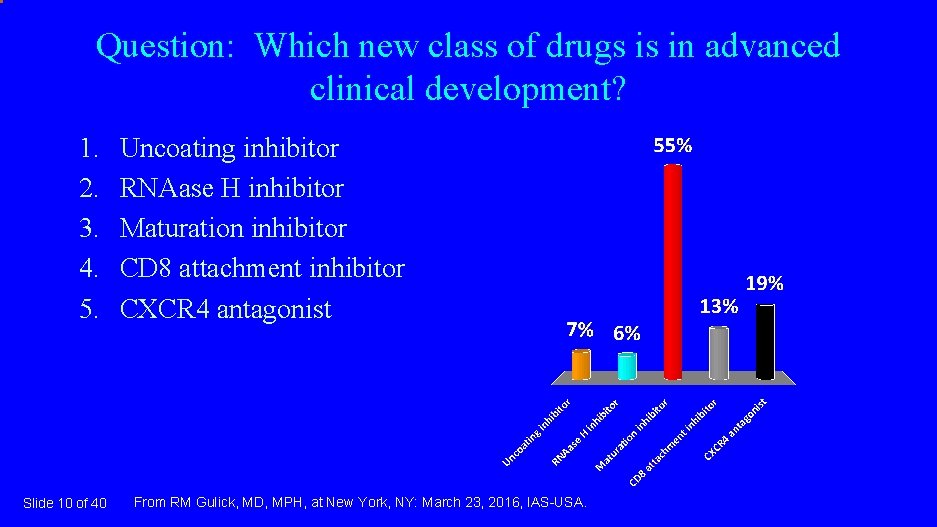
Question: Which new class of drugs is in advanced clinical development? 1. 2. 3. 4. 5. Slide 10 of 40 Uncoating inhibitor RNAase H inhibitor Maturation inhibitor CD 8 attachment inhibitor CXCR 4 antagonist From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

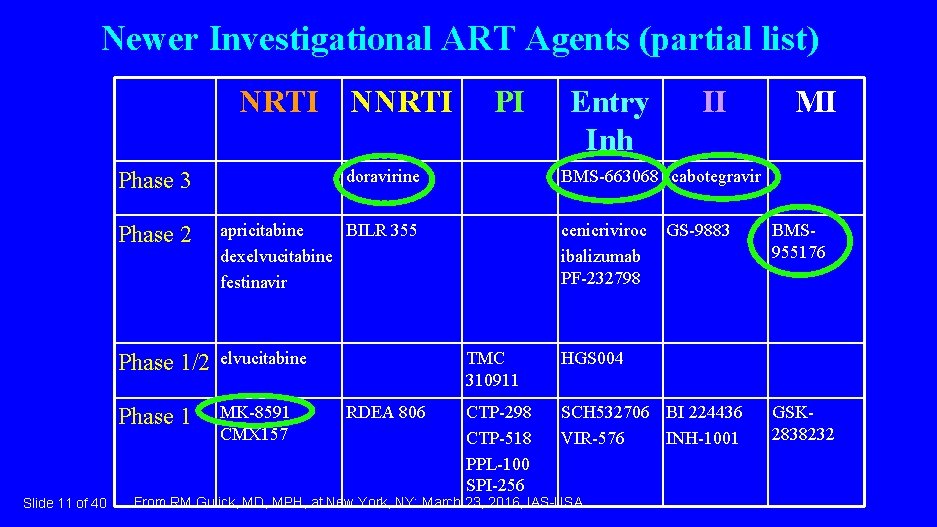
Newer Investigational ART Agents (partial list) NRTI NNRTI Slide 11 of 40 Phase 3 doravirine Phase 2 apricitabine BILR 355 dexelvucitabine festinavir Phase 1/2 elvucitabine Phase 1 MK-8591 CMX 157 RDEA 806 PI Entry Inh II MI BMS-663068 cabotegravir cenicriviroc ibalizumab PF-232798 GS-9883 TMC 310911 HGS 004 CTP-298 CTP-518 PPL-100 SPI-256 SCH 532706 BI 224436 VIR-576 INH-1001 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. BMS 955176 GSK 2838232

NRTI Needs: • More convenient Slide 12 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

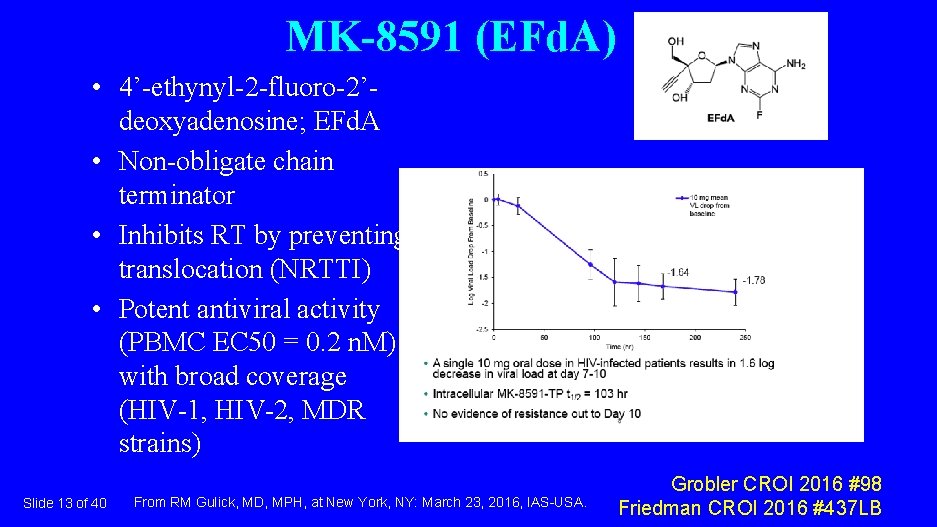
MK-8591 (EFd. A) • 4’-ethynyl-2 -fluoro-2’deoxyadenosine; EFd. A • Non-obligate chain terminator • Inhibits RT by preventing translocation (NRTTI) • Potent antiviral activity (PBMC EC 50 = 0. 2 n. M) with broad coverage (HIV-1, HIV-2, MDR strains) Slide 13 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Grobler CROI 2016 #98 Friedman CROI 2016 #437 LB

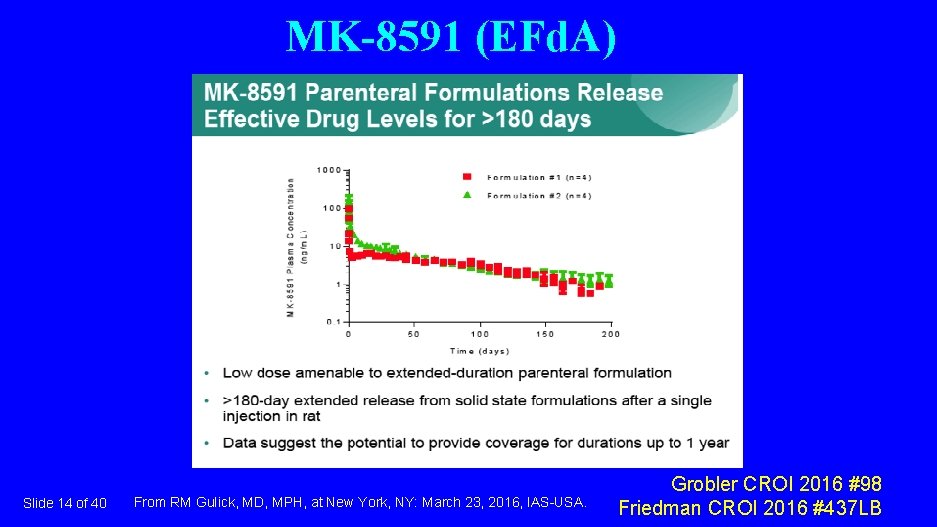
MK-8591 (EFd. A) Slide 14 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Grobler CROI 2016 #98 Friedman CROI 2016 #437 LB

NNRTI Needs: • Less toxicity and better tolerability • Active against resistant viral strains • Fewer drug interactions Slide 15 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

Doravirine (DOR; MK-1439) • Investigational NNRTI • Pre-clinical –Potent at low milligram dose –Metabolized by CYP 3 A 4; not a CYP 450 inhibitor or inducer –Active in vitro against viral strains with: • K 103 N • Y 181 C • G 190 A • E 101 K • E 138 K • K 103 N/Y 181 C Lai AAC 2014; 58: 1652 -1663 Slide 16 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

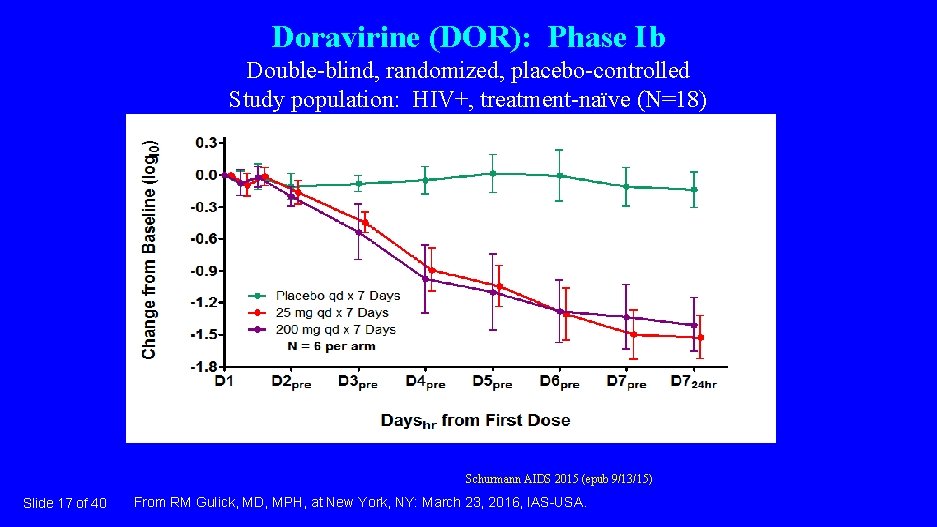
Doravirine (DOR): Phase Ib Double-blind, randomized, placebo-controlled Study population: HIV+, treatment-naïve (N=18) Schurmann AIDS 2015 (epub 9/13/15) Slide 17 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

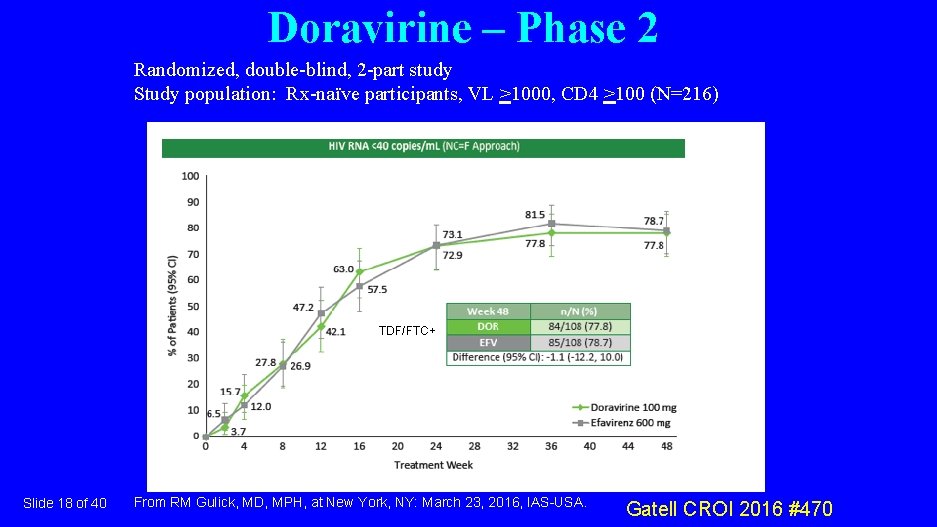
Doravirine – Phase 2 Randomized, double-blind, 2 -part study Study population: Rx-naïve participants, VL >1000, CD 4 >100 (N=216) TDF/FTC+ Slide 18 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Gatell CROI 2016 #470

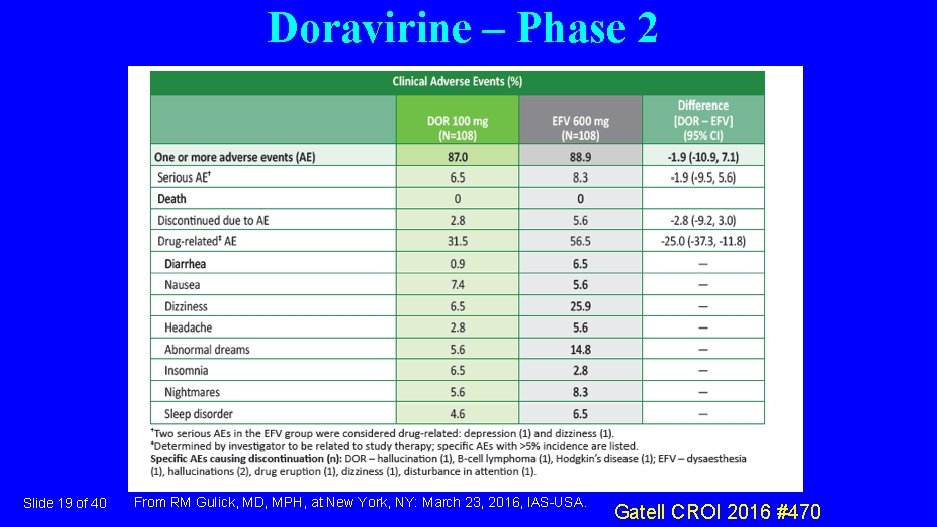
Doravirine – Phase 2 Slide 19 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Gatell CROI 2016 #470

INSTI Needs: • More convenient Slide 20 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

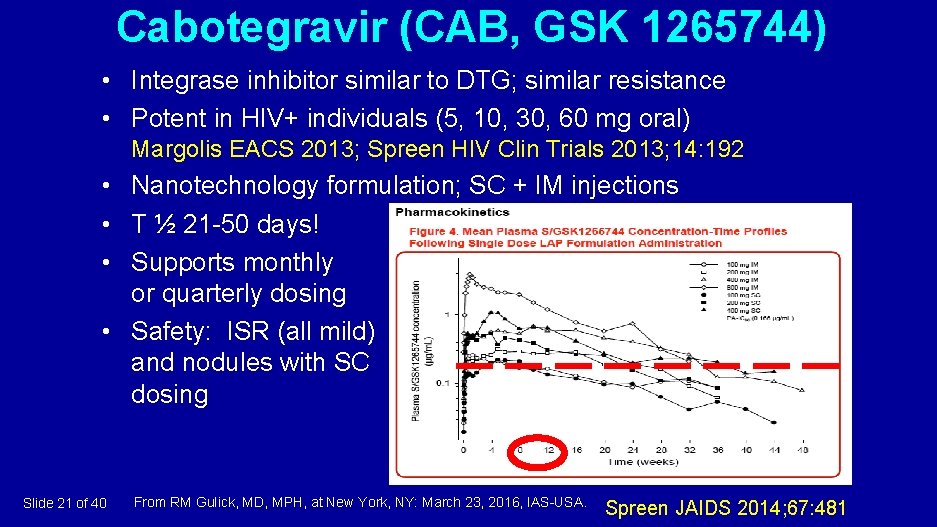
Cabotegravir (CAB, GSK 1265744) • Integrase inhibitor similar to DTG; similar resistance • Potent in HIV+ individuals (5, 10, 30, 60 mg oral) Margolis EACS 2013; Spreen HIV Clin Trials 2013; 14: 192 • Nanotechnology formulation; SC + IM injections • T ½ 21 -50 days! • Supports monthly or quarterly dosing • Safety: ISR (all mild) and nodules with SC dosing Slide 21 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Spreen JAIDS 2014; 67: 481

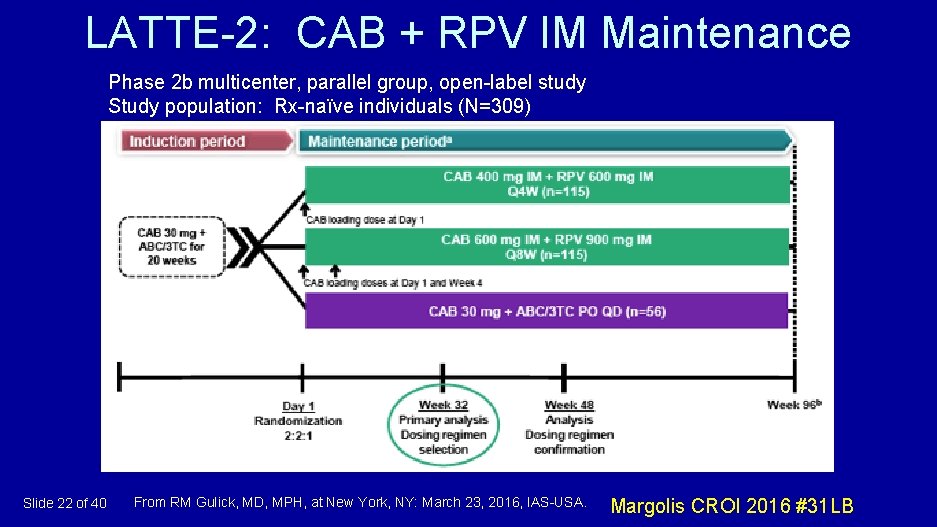
LATTE-2: CAB + RPV IM Maintenance Phase 2 b multicenter, parallel group, open-label study Study population: Rx-naïve individuals (N=309) Slide 22 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Margolis CROI 2016 #31 LB

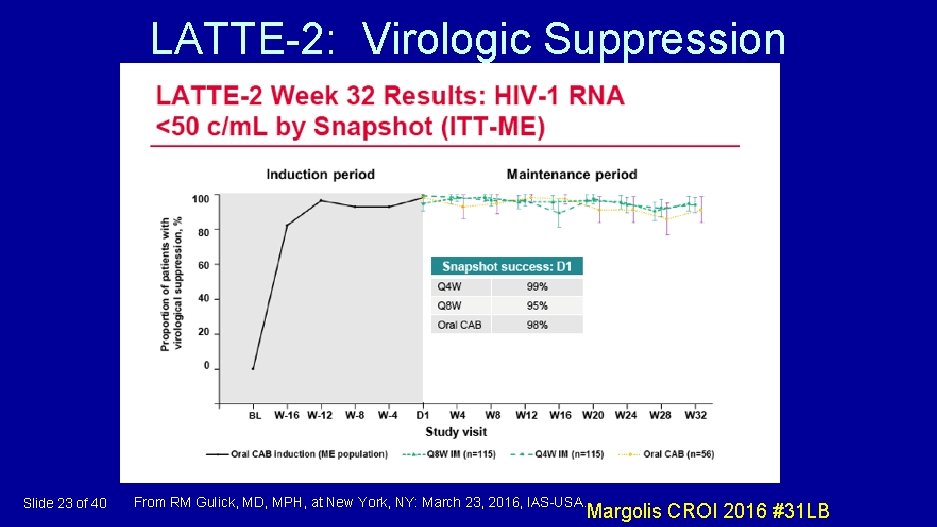
LATTE-2: Virologic Suppression Slide 23 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Margolis CROI 2016 #31 LB

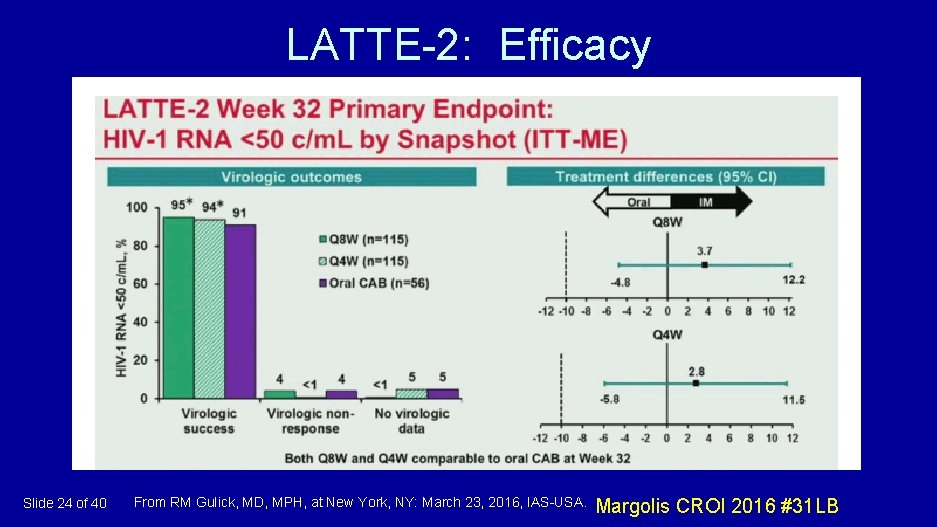
LATTE-2: Efficacy Slide 24 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Margolis CROI 2016 #31 LB

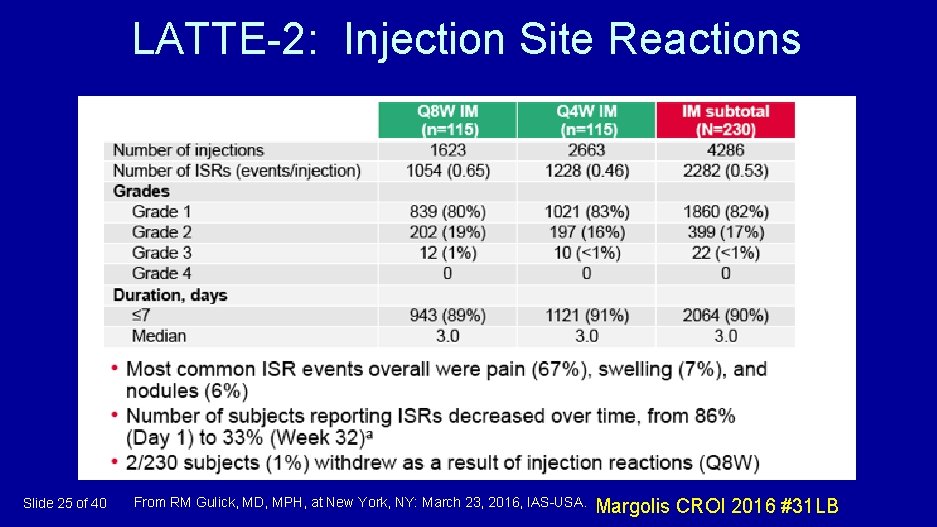
LATTE-2: Injection Site Reactions Slide 25 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Margolis CROI 2016 #31 LB

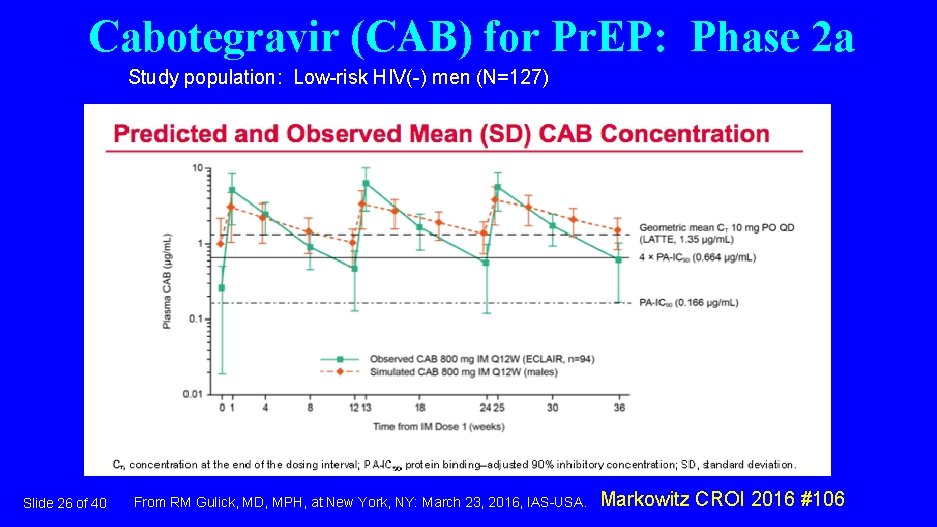
Cabotegravir (CAB) for Pr. EP: Phase 2 a Study population: Low-risk HIV(-) men (N=127) Slide 26 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Markowitz CROI 2016 #106

CD 4 Attachment Inhibitor Needs: • Novel mechanism of action Slide 27 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

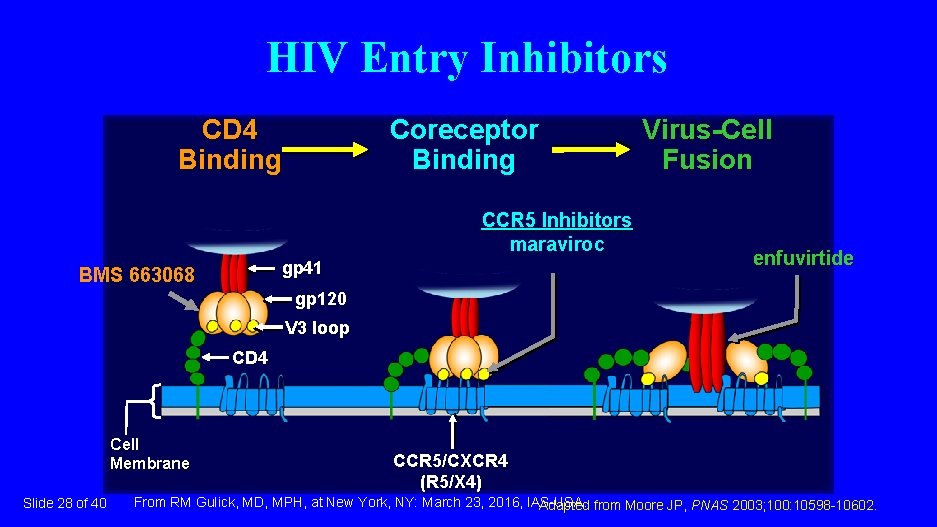
HIV Entry Inhibitors CD 4 Binding Coreceptor Binding CCR 5 Inhibitors maraviroc gp 41 BMS 663068 Virus-Cell Fusion enfuvirtide gp 120 V 3 loop CD 4 Cell Membrane Slide 28 of 40 CCR 5/CXCR 4 (R 5/X 4) From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Adapted from Moore JP, PNAS 2003; 100: 10598 -10602.

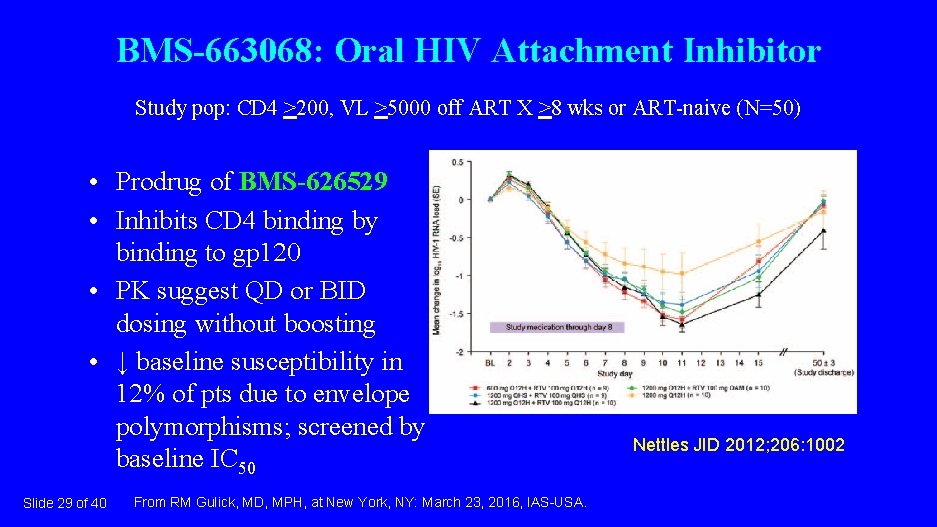
BMS-663068: Oral HIV Attachment Inhibitor Study pop: CD 4 >200, VL >5000 off ART X >8 wks or ART-naive (N=50) • Prodrug of BMS-626529 • Inhibits CD 4 binding by binding to gp 120 • PK suggest QD or BID dosing without boosting • ↓ baseline susceptibility in 12% of pts due to envelope polymorphisms; screened by baseline IC 50 Slide 29 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Nettles JID 2012; 206: 1002

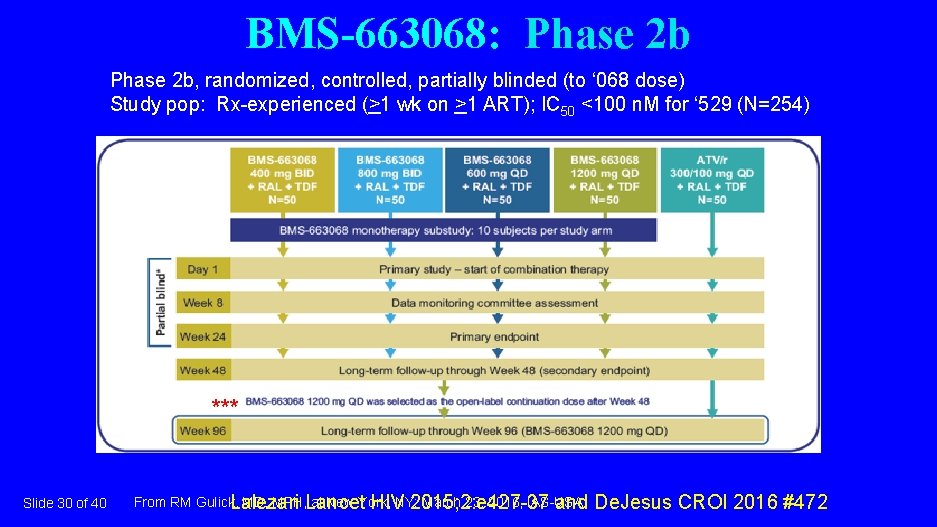
BMS-663068: Phase 2 b, randomized, controlled, partially blinded (to ‘ 068 dose) Study pop: Rx-experienced (>1 wk on >1 ART); IC 50 <100 n. M for ‘ 529 (N=254) *** Slide 30 of 40 From RM Gulick, MD, MPH, Lancet at New York, March 23, 2016, IAS-USA. Lalezari HIVNY: 2015; 2: e 427 -37 and De. Jesus CROI 2016 #472

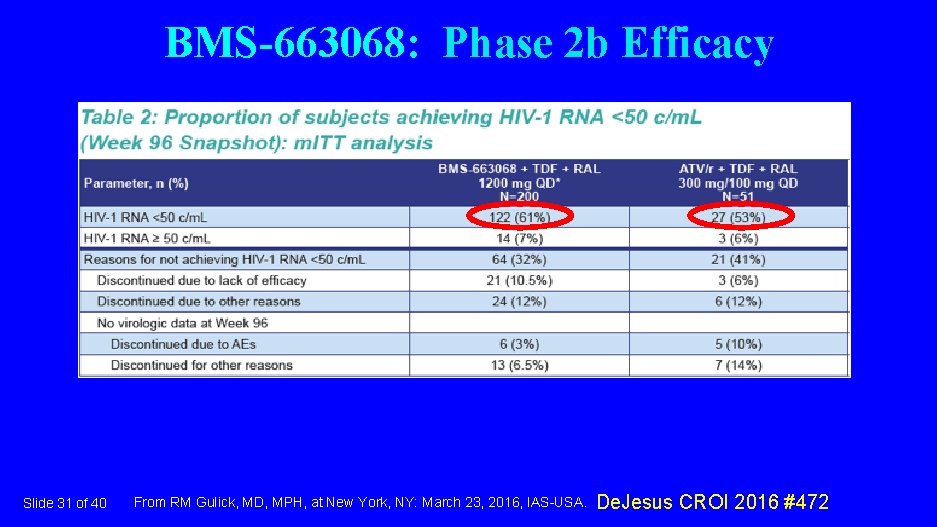
BMS-663068: Phase 2 b Efficacy Slide 31 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. De. Jesus CROI 2016 #472

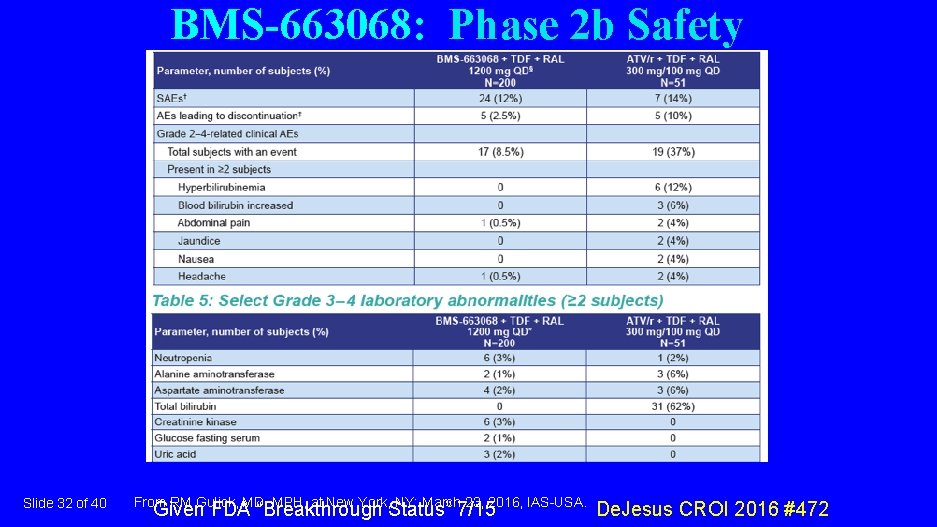
BMS-663068: Phase 2 b Safety Slide 32 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Given FDA “Breakthrough Status” 7/15 De. Jesus CROI 2016 #472

Maturation Inhibitor Needs: • Novel mechanism of action • No baseline polymorphisms that confer resistance Slide 33 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

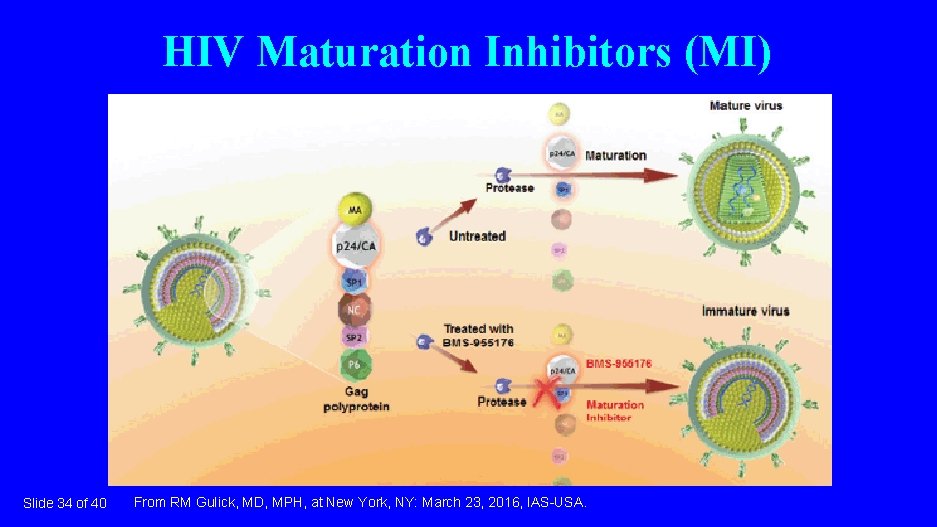
HIV Maturation Inhibitors (MI) Slide 34 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

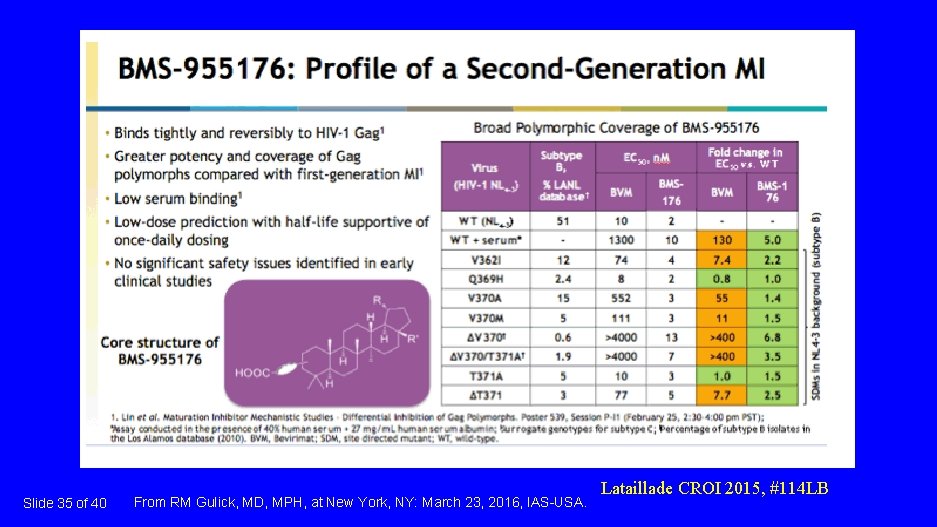
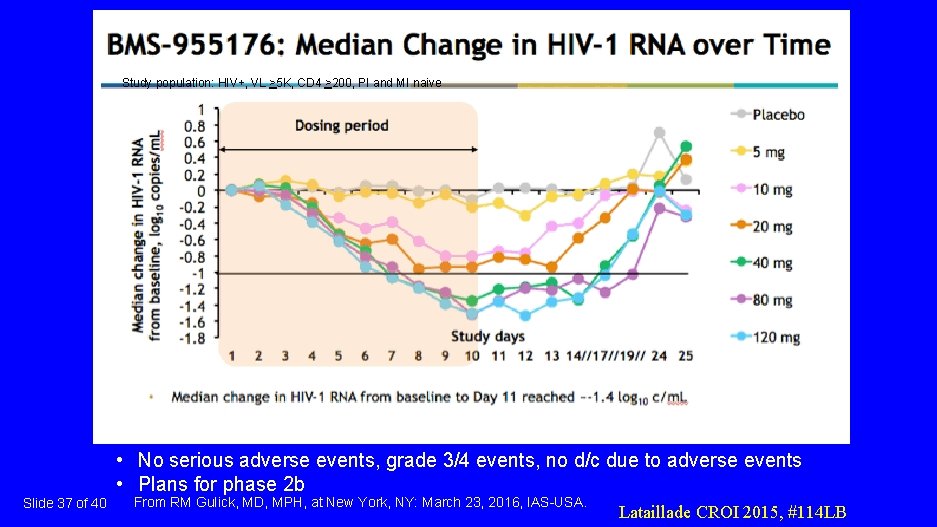
Slide 35 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Lataillade CROI 2015, #114 LB

BMS-955176: PI-resistant viral strains • 21 clinical isolates from 15 patients – median 6 years on PI therapy – all had major PI resistance-associated mutations in protease – 17 of 21 had 2 o changes in GAG associated with PI resistance (at positions 128, 431, 436, 437, 449, 452, 453) • 7 highly PI-resistant virus strains • BMS-955176 retained virologic activity Slide 36 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Ray CROI 2016 #464

Study population: HIV+, VL >5 K, CD 4 >200, PI and MI naive • No serious adverse events, grade 3/4 events, no d/c due to adverse events • Plans for phase 2 b Slide 37 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Lataillade CROI 2015, #114 LB

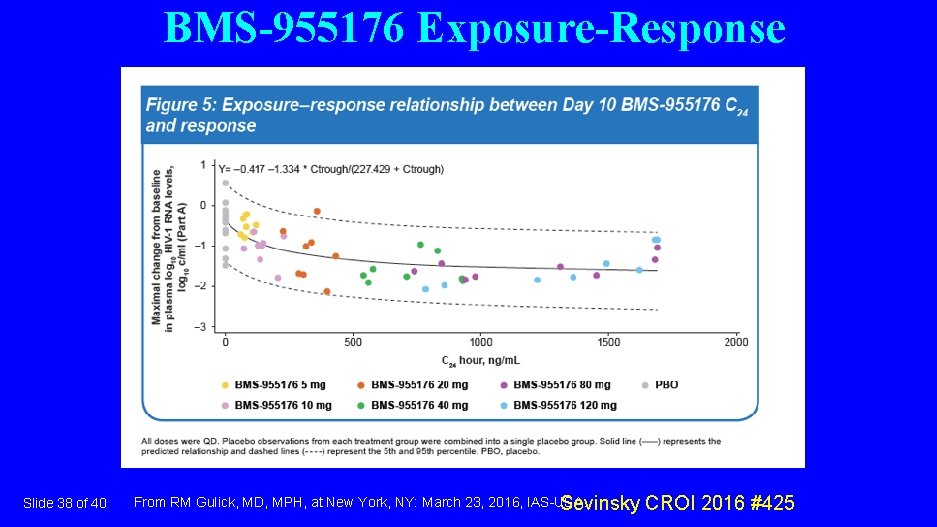
BMS-955176 Exposure-Response Slide 38 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA. Sevinsky CROI 2016 #425

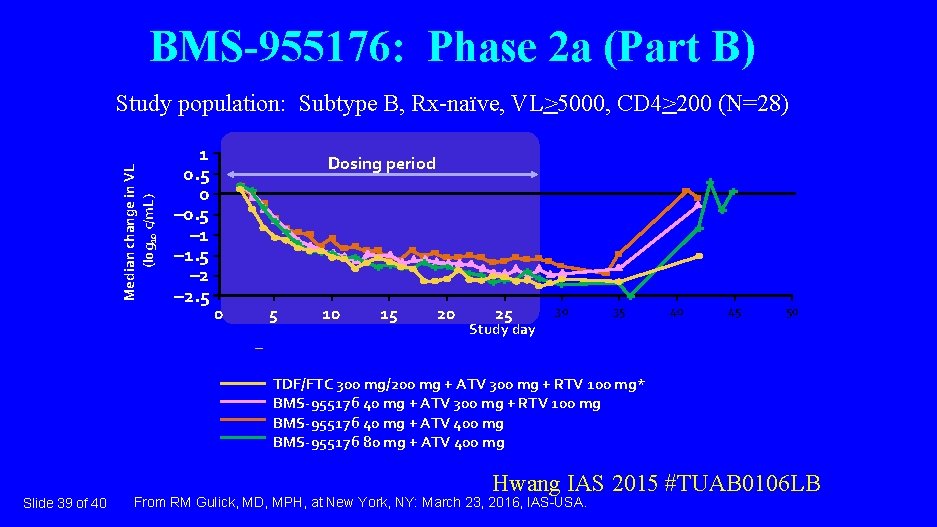
BMS-955176: Phase 2 a (Part B) Median change in VL (log 10 c/m. L) Study population: Subtype B, Rx-naïve, VL>5000, CD 4>200 (N=28) 1 0. 5 0 – 0. 5 – 1. 5 – 2. 5 Dosing period 0 5 10 15 20 25 30 35 40 45 50 Study day TDF/FTC 300 mg/200 mg + ATV 300 mg + RTV 100 mg* BMS-955176 40 mg + ATV 300 mg + RTV 100 mg BMS-955176 40 mg + ATV 400 mg BMS-955176 80 mg + ATV 400 mg Slide 39 of 40 Hwang IAS 2015 #TUAB 0106 LB From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.

Acknowledgments • • • Cornell HIV Clinical Trials Unit (CCTU) Division of Infectious Diseases Weill Cornell Medical College AIDS Clinical Trials Group (ACTG) HIV Prevention Trials Network (HPTN) Division of AIDS, NIAID, NIH • The patient volunteers! Slide 40 of 40 From RM Gulick, MD, MPH, at New York, NY: March 23, 2016, IAS-USA.
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness integrated therapy system occupational therapy
Bioness integrated therapy system occupational therapy What are the major humanistic therapies
What are the major humanistic therapies It's gotta start somewhere it's gotta start sometime
It's gotta start somewhere it's gotta start sometime Triage jump start
Triage jump start Wii hab
Wii hab Elements of poem in literature
Elements of poem in literature Start of a new life
Start of a new life To start a new line in a cell press the ____ keys
To start a new line in a cell press the ____ keys Remains simon armitage context
Remains simon armitage context New york pennsylvania new jersey delaware
New york pennsylvania new jersey delaware New oil and new wineskin
New oil and new wineskin Strengths of articles of confederation
Strengths of articles of confederation Marketing kotler keller
Marketing kotler keller New classical macroeconomics
New classical macroeconomics Chapter 16 toward a new heaven and a new earth
Chapter 16 toward a new heaven and a new earth Neil thisse is a loyalist who fled the colonies
Neil thisse is a loyalist who fled the colonies New classical and new keynesian macroeconomics
New classical and new keynesian macroeconomics Intradermal drug administration
Intradermal drug administration Rate and rhythm control drugs
Rate and rhythm control drugs Neurotransmitters and drugs
Neurotransmitters and drugs Difference between organized and unorganized drugs
Difference between organized and unorganized drugs Section 35-4 the senses
Section 35-4 the senses Transesophageal echocardiogram procedure
Transesophageal echocardiogram procedure Chapter 19 vocabulary glencoe health
Chapter 19 vocabulary glencoe health Chapter 7 alcohol other drugs and driving
Chapter 7 alcohol other drugs and driving Clarke's analysis of drugs and poisons
Clarke's analysis of drugs and poisons Drugs and alcohol toolbox talk
Drugs and alcohol toolbox talk Remains simon
Remains simon Drugs that alter moods thoughts and sense perceptions
Drugs that alter moods thoughts and sense perceptions You must put all chemicals and drugs in locked cupboards
You must put all chemicals and drugs in locked cupboards Psychedelic drugs that distort perceptions and evoke
Psychedelic drugs that distort perceptions and evoke Drugs that alter moods, thoughts, and sense perceptions
Drugs that alter moods, thoughts, and sense perceptions Advantages and disadvantages of drugs
Advantages and disadvantages of drugs Chapter 15 alcohol other drugs and driving
Chapter 15 alcohol other drugs and driving Chemistry food and drugs
Chemistry food and drugs Chapter 44 antiinflammatory and antigout drugs
Chapter 44 antiinflammatory and antigout drugs Chapter 11 medications and drugs
Chapter 11 medications and drugs Storage and maintenance of drugs
Storage and maintenance of drugs