Interactive Antiretroviral Therapy Cases Financial Relationships With Commercial





![Recommended First-line ART for Most Patients With HIV Class INSTI • • DHHS[1] § Recommended First-line ART for Most Patients With HIV Class INSTI • • DHHS[1] §](https://slidetodoc.com/presentation_image/1604c0c2df955e1bb223eeaf371bf7b1/image-9.jpg)











- Slides: 41

Interactive Antiretroviral Therapy Cases

Financial Relationships With Commercial Entities Dr Eron has served as an ad hoc consultant to Janssen, Vii. V Healthcare, Merck & Co, Inc, and Gilead Sciences, Inc. His institution receives contracts for clinical research on which Dr Eron is the local principal investigator from Janssen Therapeutics, Vii. V Healthcare, and Gilead Sciences, Inc. (Updated 07/22/20) Slide 2 of 42

Learning Objectives After attending this presentation, learners will be able to: • List recommended first line antiretroviral regimens for treatment agents • Describe settings in which dolutegravir-containing therapy is a preferred or alternative therapy for women who are pregnant or considering pregnancy • Contrast different antiretroviral agents and their association with weight gain Slide 3 of 42

Initial Antiretroviral Therapy • LR is a 35 yo MSM who was recently diagnosed with HIV infection • His CD 4 cell count is 535 and his HIV RNA is 87, 000 c/m. L • He has no co-morbid conditions, • His ALT is normal, cr = 0. 90 and total cholesterol is 150 • His husband is HIV negative (recently tested) and accompanies him to the visit • His HCV Ab, HBV surface Ab and Ag, HLA B 5701 and HIV RT/pro genotype are pending Slide 4 of 42

ARS Question #1 • Would you start antiretroviral therapy now? 1. Yes 2. No 3. Not Sure Slide 5 of 42

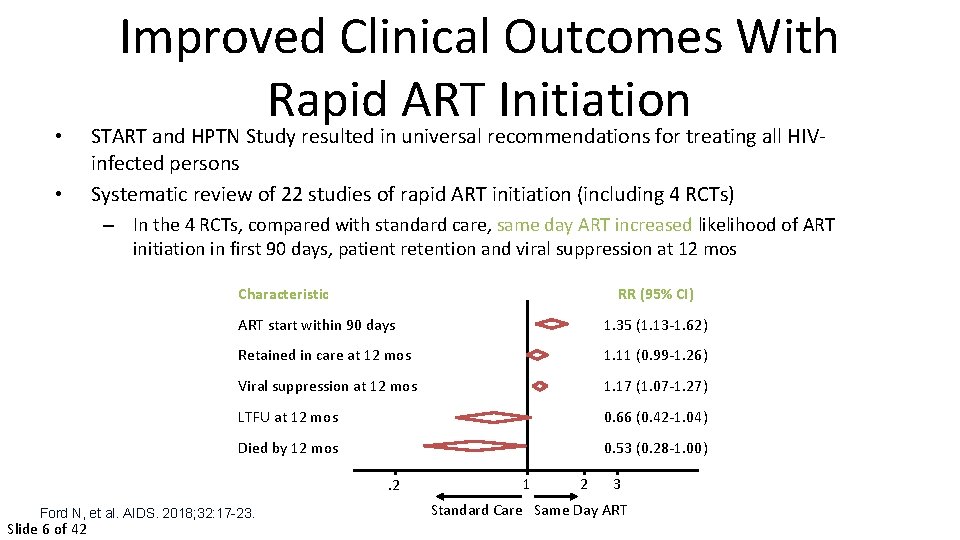
• • Improved Clinical Outcomes With Rapid ART Initiation START and HPTN Study resulted in universal recommendations for treating all HIVinfected persons Systematic review of 22 studies of rapid ART initiation (including 4 RCTs) – In the 4 RCTs, compared with standard care, same day ART increased likelihood of ART initiation in first 90 days, patient retention and viral suppression at 12 mos Characteristic RR (95% CI) ART start within 90 days 1. 35 (1. 13 -1. 62) Retained in care at 12 mos 1. 11 (0. 99 -1. 26) Viral suppression at 12 mos 1. 17 (1. 07 -1. 27) LTFU at 12 mos 0. 66 (0. 42 -1. 04) Died by 12 mos 0. 53 (0. 28 -1. 00). 2 Ford N, et al. AIDS. 2018; 32: 17 -23. Slide 6 of 42 1 2 3 Standard Care Same Day ART

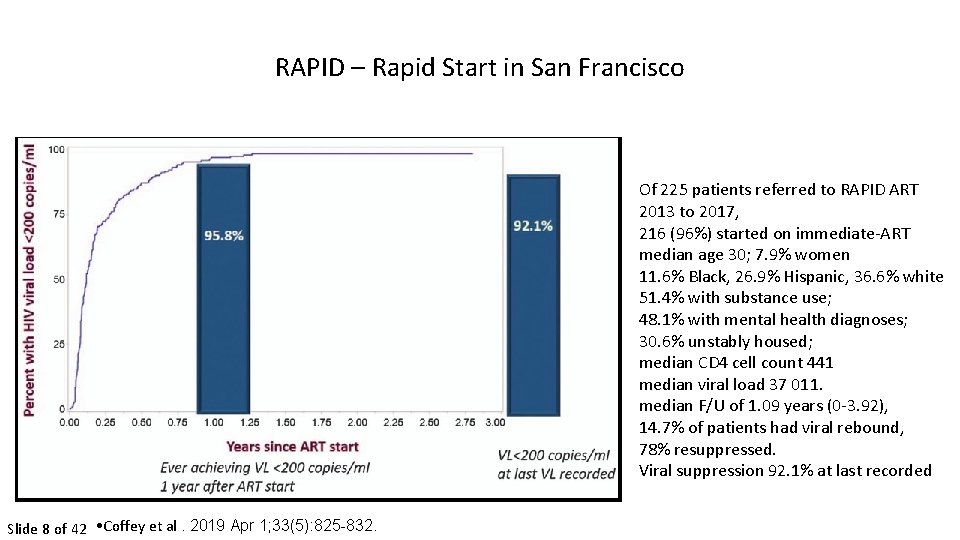
RAPID – Rapid Start in San Francisco Of 225 patients referred to RAPID ART 2013 to 2017, 216 (96%) started on immediate-ART median age 30; 7. 9% women 11. 6% Black, 26. 9% Hispanic, 36. 6% white 51. 4% with substance use; 48. 1% with mental health diagnoses; 30. 6% unstably housed; median CD 4 cell count 441 median viral load 37 011. median F/U of 1. 09 years (0 -3. 92), 14. 7% of patients had viral rebound, 78% resuppressed. Viral suppression 92. 1% at last recorded Slide 8 of 42 • Coffey et al. 2019 Apr 1; 33(5): 825 -832.

ARS Question #2 • What regimen would you start? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Slide 9 of 42 Bictegravir/TAF/FTC* Darunavir/cobi/TAF/FTC* Dolutegravir/abacavir/3 TC* Dolutegravir plus TAF/FTC Dolutegravir/3 TC* Doravirine/TDF/3 TC* Efavirenz (400)/TDF/3 TC (generic)* Raltegravir plus 2 NRTI* Rilpivirine/TAF/FTC* Something else *Single Tablet Regimens 35 yo MSM recently diagnosed with HIV infection CD 4 cell count = 535; HIV RNA = 87, 000 c/m. L Serum Creatinine = 0. 90 Total Cholesterol = 150 Viral hepatitis, HLA-B*5701, resistance testing – all pending
![Recommended Firstline ART for Most Patients With HIV Class INSTI DHHS1 Recommended First-line ART for Most Patients With HIV Class INSTI • • DHHS[1] §](https://slidetodoc.com/presentation_image/1604c0c2df955e1bb223eeaf371bf7b1/image-9.jpg)
Recommended First-line ART for Most Patients With HIV Class INSTI • • DHHS[1] § BIC/FTC/TAF* § DTG/ABC/3 TC* § DTG + (FTC or 3 TC)/(TAF or TDF) § RAL + (FTC or 3 TC)/(TAF or TDF) § DTG/3 TC** IAS-USA[2] § BIC/FTC/TAF* § DTG/ABC/3 TC* § DTG + FTC/TAF Integrase inhibitor-based therapy is recommended for most (all? ) patients Regimens with Boosting agents are not longer recommended Other regimens may be considered in certain clinical situations Is there any reason to use an abacavir containing regimen? *Single-tablet regimens. ** HIV RNA < 500, 000 c/m. L, no active HBV and genotype resistance testing available 1. DHHS ART. Dec 2019. 2. Saag MS, et al. JAMA. 2018; 320: 379. Slide 10 of 42

Initial Antiretroviral Therapy • • LR is a 35 yo MSM who was recently diagnosed with HIV infection His CD 4 cell count is 535 and his HIV RNA is 87, 000 c/m. L He has no co-morbid conditions, His ALT is normal, cr = 0. 90 and total cholesterol is 150 He has multiple partners some of whom are HIV + HCV Ab +, HBV surface Ab +, HLA B 5701 negative HIV RT/pro and integrase resistance testing shows • M 41 L, K 103 N, T 215 D in RT • L 90 M in protease • T 97 A in integrase Slide 11 of 42

ARS Question #3 • Does this change your treatment choice? 1. Yes 2. No 3. Not Sure • If so, How? Slide 12 of 42

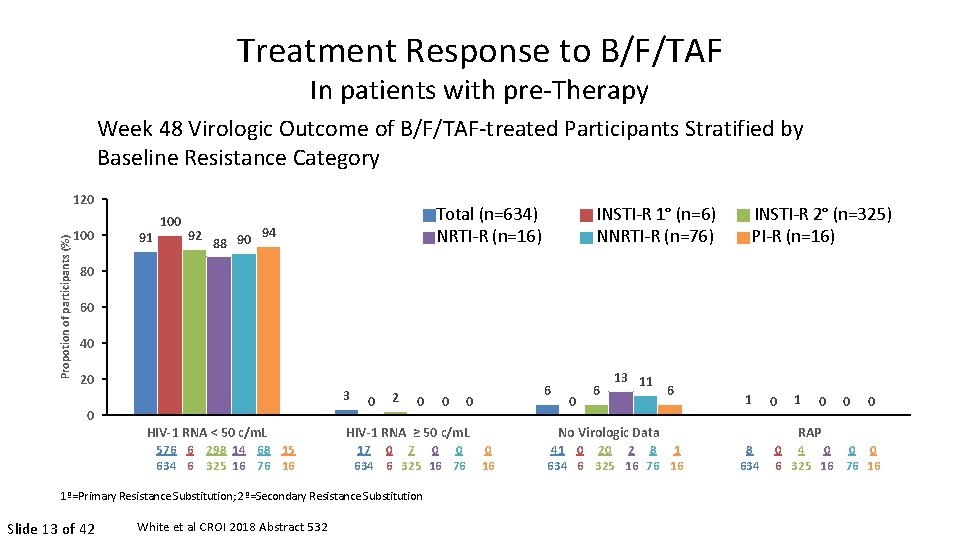
Treatment Response to B/F/TAF In patients with pre-Therapy Week 48 Virologic Outcome of B/F/TAF-treated Participants Stratified by Baseline Resistance Category Propotion of participants (%) 120 100 91 100 92 88 90 Total (n=634) NRTI-R (n=16) 94 INSTI-R 1° (n=6) NNRTI-R (n=76) INSTI-R 2° (n=325) PI-R (n=16) 80 60 40 20 3 0 HIV-1 RNA < 50 c/m. L 576 6 298 14 68 15 634 6 325 16 76 16 0 2 0 White et al CROI 2018 Abstract 532 0 HIV-1 RNA ≥ 50 c/m. L 17 0 0 0 634 6 325 16 76 16 1º=Primary Resistance Substitution; 2º=Secondary Resistance Substitution Slide 13 of 42 0 6 13 11 6 No Virologic Data 41 0 20 2 8 1 634 6 325 16 76 16 1 8 634 0 1 0 0 0 RAP 0 4 0 0 0 6 325 16 76 16

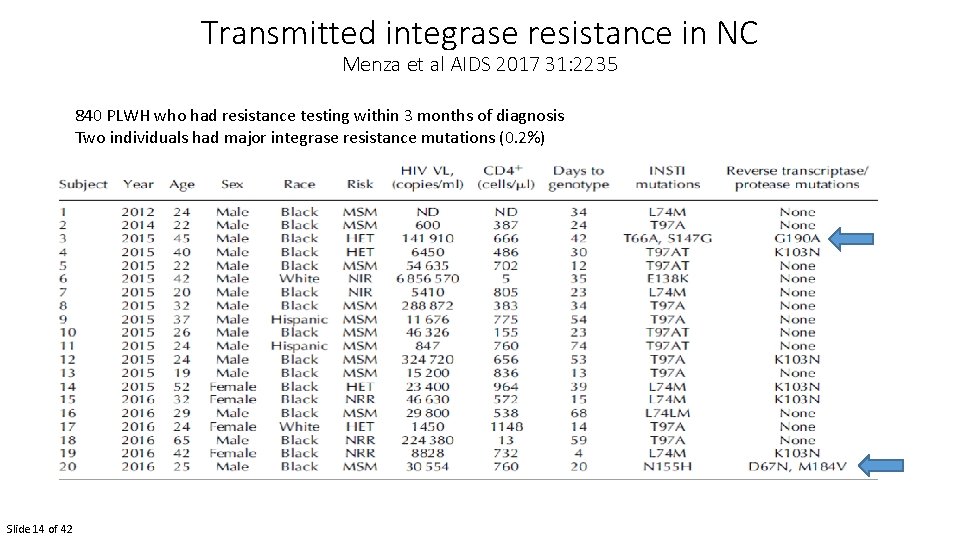
Transmitted integrase resistance in NC Menza et al AIDS 2017 31: 2235 840 PLWH who had resistance testing within 3 months of diagnosis Two individuals had major integrase resistance mutations (0. 2%) Slide 14 of 42

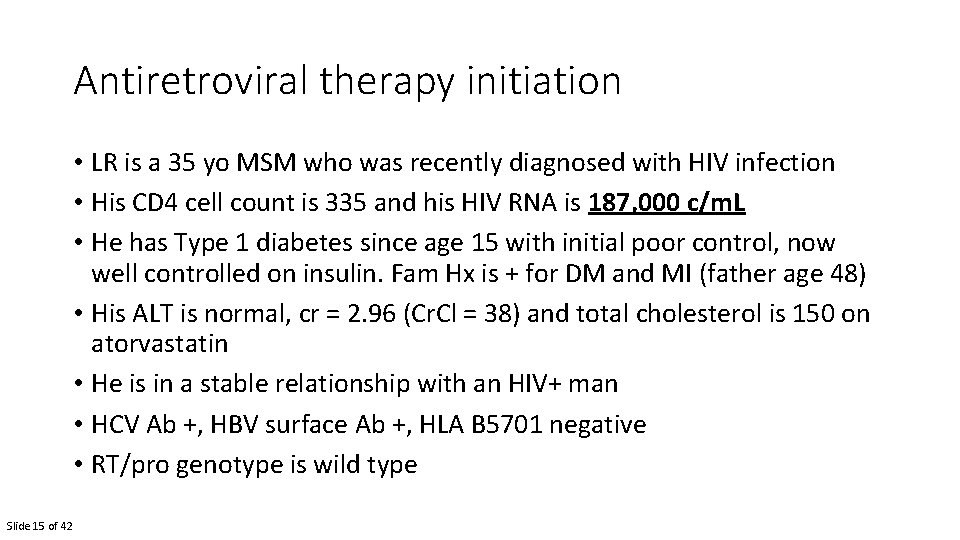
Antiretroviral therapy initiation • LR is a 35 yo MSM who was recently diagnosed with HIV infection • His CD 4 cell count is 335 and his HIV RNA is 187, 000 c/m. L • He has Type 1 diabetes since age 15 with initial poor control, now well controlled on insulin. Fam Hx is + for DM and MI (father age 48) • His ALT is normal, cr = 2. 96 (Cr. Cl = 38) and total cholesterol is 150 on atorvastatin • He is in a stable relationship with an HIV+ man • HCV Ab +, HBV surface Ab +, HLA B 5701 negative • RT/pro genotype is wild type Slide 15 of 42

ARS Question #4 • What regimen would you start? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Slide 16 of 42 Bictegravir or dolutegravir plus TAF/FTC Darunavir/cobi/TAF/FTC Darunavir/boosted plus INSTI Darunavir/boosted plus 3 TC Dolutegravir plus abacavir/3 TC Dolutegravir/3 TC Raltegravir plus TAF/FTC Rilpivirine/dolutegravir Something else

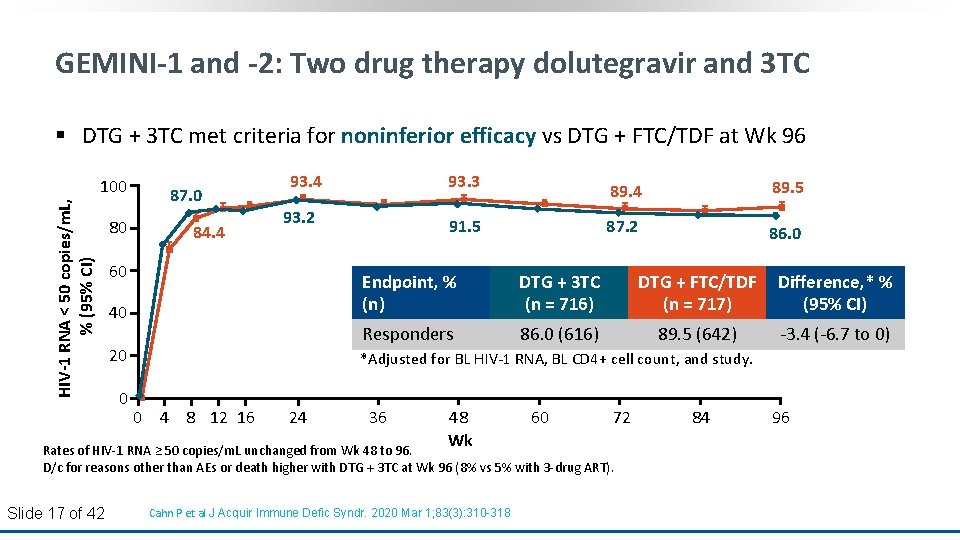
GEMINI-1 and -2: Two drug therapy dolutegravir and 3 TC § DTG + 3 TC met criteria for noninferior efficacy vs DTG + FTC/TDF at Wk 96 HIV-1 RNA < 50 copies/m. L, % (95% CI) 100 80 87. 0 84. 4 93. 3 93. 2 60 91. 5 89. 4 89. 5 87. 2 86. 0 40 Endpoint, % (n) DTG + 3 TC (n = 716) DTG + FTC/TDF (n = 717) Difference, * % (95% CI) 20 Responders 86. 0 (616) 89. 5 (642) -3. 4 (-6. 7 to 0) *Adjusted for BL HIV-1 RNA, BL CD 4+ cell count, and study. 0 0 4 8 12 16 24 36 48 Wk 60 72 Rates of HIV-1 RNA ≥ 50 copies/m. L unchanged from Wk 48 to 96. D/c for reasons other than AEs or death higher with DTG + 3 TC at Wk 96 (8% vs 5% with 3 -drug ART). Slide 17 of 42 Cahn P et al J Acquir Immune Defic Syndr. 2020 Mar 1; 83(3): 310 -318 84 96

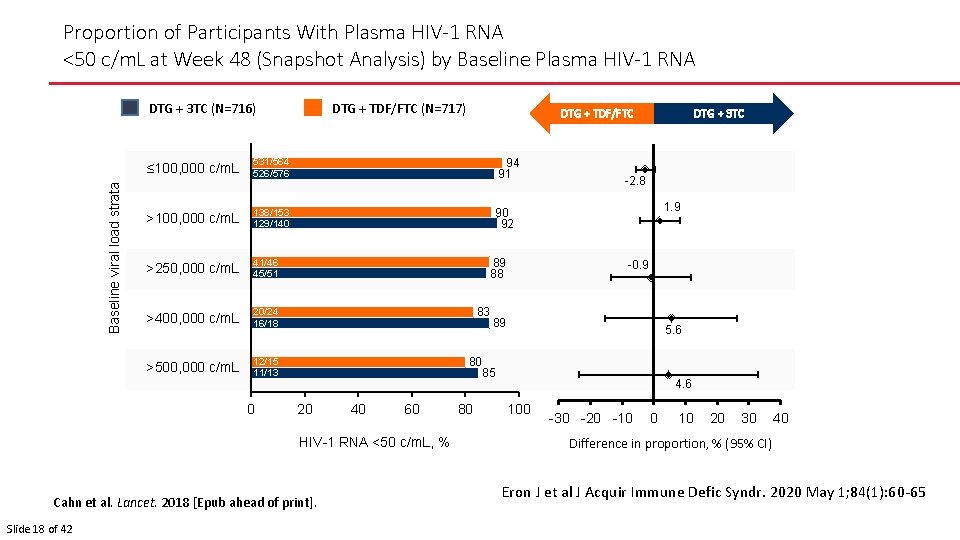
Proportion of Participants With Plasma HIV-1 RNA <50 c/m. L at Week 48 (Snapshot Analysis) by Baseline Plasma HIV-1 RNA DTG + TDF/FTC (N=717) Baseline viral load strata DTG + 3 TC (N=716) ≤ 100, 000 c/m. L 531/564 526/576 94 91 >100, 000 c/m. L 138/153 129/140 90 92 >250, 000 c/m. L 41/46 45/51 >400, 000 c/m. L 20/24 16/18 >500, 000 c/m. L 12/15 11/13 0 89 88 83 80 20 40 60 HIV-1 RNA <50 c/m. L, % Cahn et al. Lancet. 2018 [Epub ahead of print]. Slide 18 of 42 DTG + TDF/FTC 80 DTG + 3 TC -2. 8 1. 9 -0. 9 89 5. 6 85 4. 6 100 -30 -20 -10 0 10 20 30 40 Difference in proportion, % (95% CI) Eron J et al J Acquir Immune Defic Syndr. 2020 May 1; 84(1): 60 -65

Antiretroviral therapy initiation • LR is a 35 yo woman who was recently diagnosed with HIV infection • Her CD 4 cell count is 535 and his HIV RNA is 87, 000 c/m. L • She has no co-morbidities and is only on an oral contraceptive • Her ALT and cr are normal, total cholesterol is 150 • She is in a stable relationship with an HIV negative man. Her husband accompanies her to the visit. She would like to become pregnant as soon as safety possible • HCV Ab negative, HBV surface Ab +, HLA B 5701 negative • RT/pro genotype is wild type Slide 19 of 42

ARS Question #5 • What regimen would you start? 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Slide 20 of 42 Bictegravir/TAF/FTC Darunavir/cobi/TAF/FTC Dolutegravir/abacavir/3 TC Dolutegravir plus TAF/FTC Dolutegravir plus TDF/FTC Efavirenz/TDF/3 TC Raltegravir plus 2 NRTI Rilpivirine/TAF/FTC Ritonavir-boosted PI plus 2 NRTI Something else

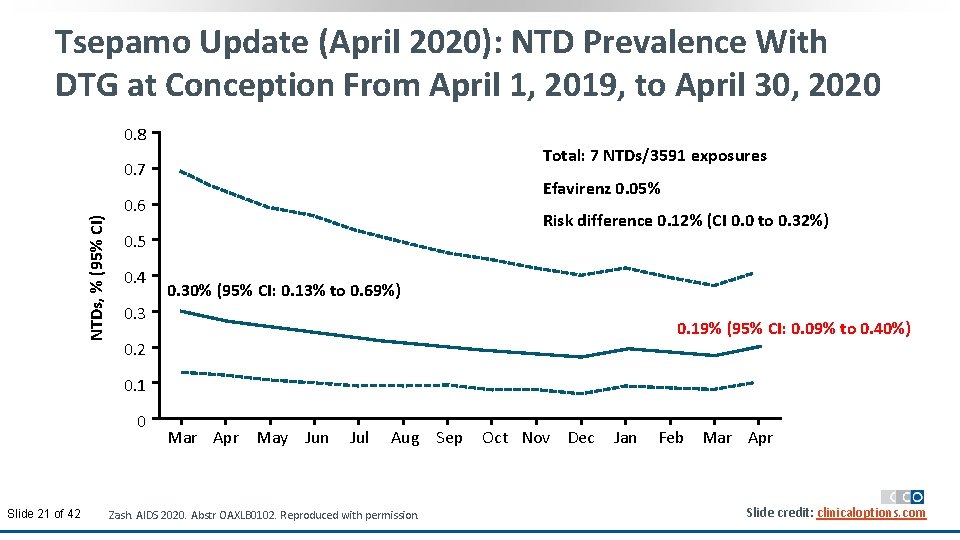
Tsepamo Update (April 2020): NTD Prevalence With DTG at Conception From April 1, 2019, to April 30, 2020 0. 8 Total: 7 NTDs/3591 exposures NTDs, % (95% CI) 0. 7 Efavirenz 0. 05% 0. 6 Risk difference 0. 12% (CI 0. 0 to 0. 32%) 0. 5 0. 4 0. 30% (95% CI: 0. 13% to 0. 69%) 0. 3 0. 19% (95% CI: 0. 09% to to 0. 40%) 0. 2 0. 1 0 Slide 21 of 42 Mar Apr May Jun Jul Aug Sep Zash. AIDS 2020. Abstr OAXLB 0102. Reproduced with permission. Oct Nov Dec Jan Feb Mar Apr Slide credit: clinicaloptions. com

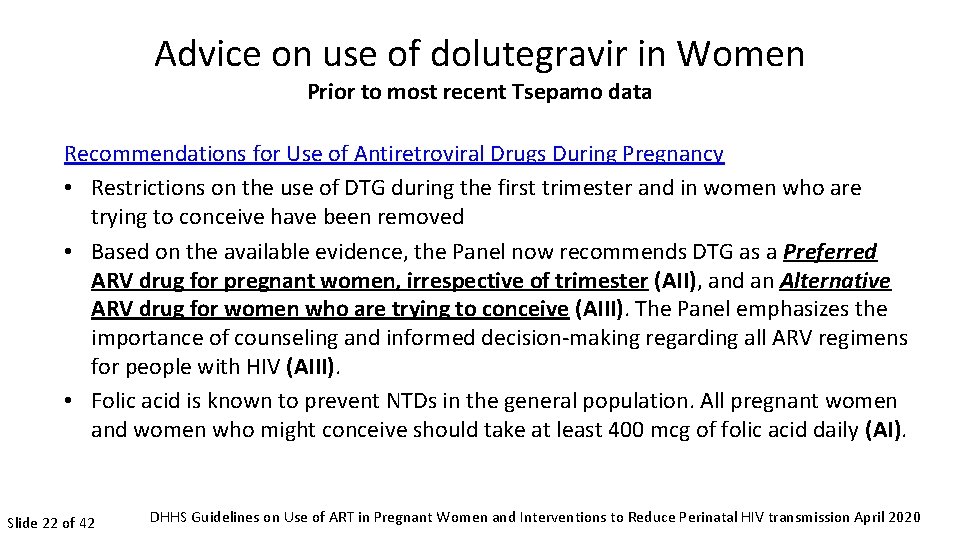
Advice on use of dolutegravir in Women Prior to most recent Tsepamo data Recommendations for Use of Antiretroviral Drugs During Pregnancy • Restrictions on the use of DTG during the first trimester and in women who are trying to conceive have been removed • Based on the available evidence, the Panel now recommends DTG as a Preferred ARV drug for pregnant women, irrespective of trimester (AII), and an Alternative ARV drug for women who are trying to conceive (AIII). The Panel emphasizes the importance of counseling and informed decision-making regarding all ARV regimens for people with HIV (AIII). • Folic acid is known to prevent NTDs in the general population. All pregnant women and women who might conceive should take at least 400 mcg of folic acid daily (AI). Slide 22 of 42 DHHS Guidelines on Use of ART in Pregnant Women and Interventions to Reduce Perinatal HIV transmission April 2020

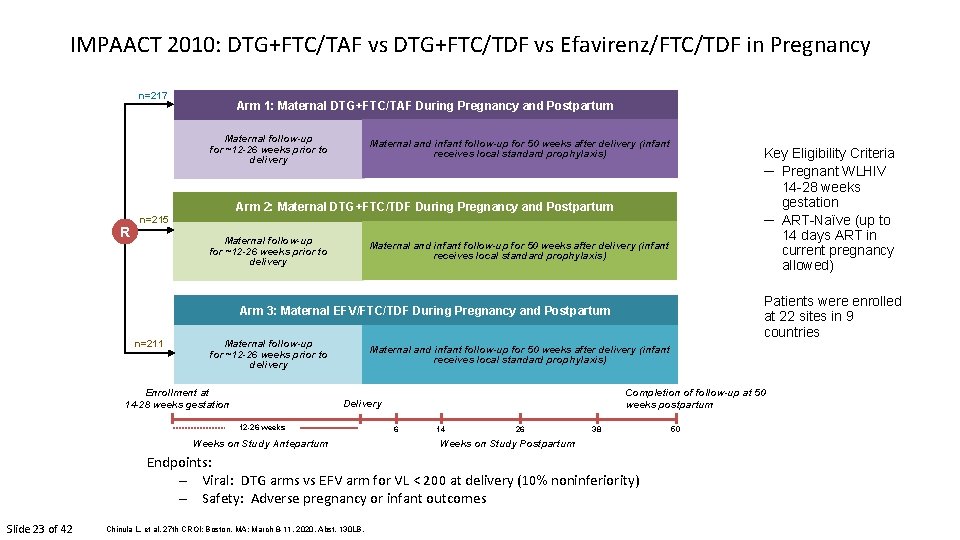
IMPAACT 2010: DTG+FTC/TAF vs DTG+FTC/TDF vs Efavirenz/FTC/TDF in Pregnancy n=217 Arm 1: Maternal DTG+FTC/TAF During Pregnancy and Postpartum Maternal follow-up for ~12 -26 weeks prior to delivery Maternal and infant follow-up for 50 weeks after delivery (infant receives local standard prophylaxis) Key Eligibility Criteria ─ Pregnant WLHIV 14 -28 weeks gestation ─ ART-Naïve (up to 14 days ART in current pregnancy allowed) Arm 2: Maternal DTG+FTC/TDF During Pregnancy and Postpartum R n=215 Maternal follow-up for ~12 -26 weeks prior to delivery Maternal and infant follow-up for 50 weeks after delivery (infant receives local standard prophylaxis) Patients were enrolled at 22 sites in 9 countries Arm 3: Maternal EFV/FTC/TDF During Pregnancy and Postpartum n=211 Maternal follow-up for ~12 -26 weeks prior to delivery Enrollment at 14 -28 weeks gestation Maternal and infant follow-up for 50 weeks after delivery (infant receives local standard prophylaxis) Completion of follow-up at 50 weeks postpartum Delivery 12 -26 weeks Weeks on Study Antepartum 6 14 26 38 Weeks on Study Postpartum Endpoints: – Viral: DTG arms vs EFV arm for VL < 200 at delivery (10% noninferiority) – Safety: Adverse pregnancy or infant outcomes Slide 23 of 42 Chinula L, et al. 27 th CROI; Boston, MA; March 8 -11, 2020. Abst. 130 LB. 50

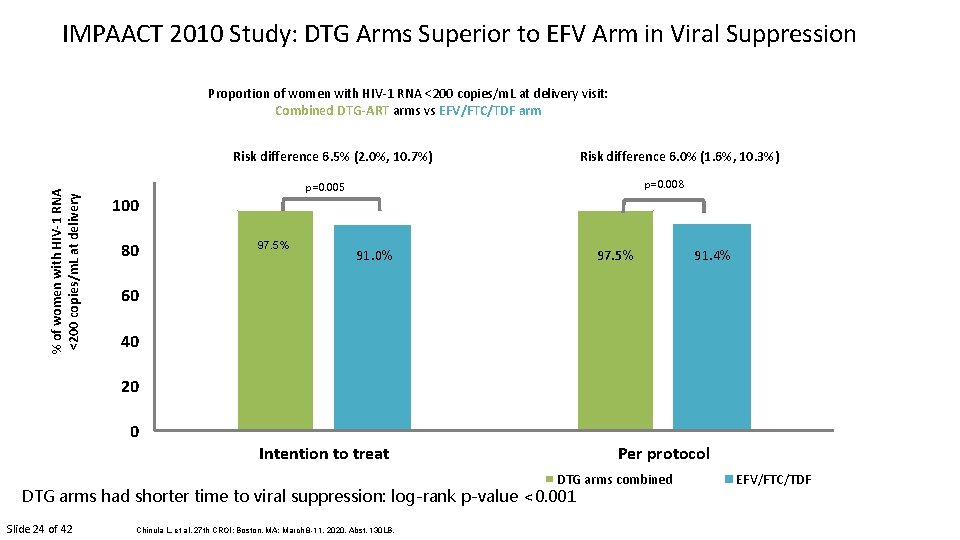
IMPAACT 2010 Study: DTG Arms Superior to EFV Arm in Viral Suppression Proportion of women with HIV-1 RNA <200 copies/m. L at delivery visit: Combined DTG-ART arms vs EFV/FTC/TDF arm % of women with HIV-1 RNA <200 copies/m. L at delivery Risk difference 6. 5% (2. 0%, 10. 7%) Risk difference 6. 0% (1. 6%, 10. 3%) p=0. 008 p=0. 005 100 80 97. 5% 91. 0% 97. 5% 91. 4% 60 40 20 0 Intention to treat Per protocol DTG arms combined DTG arms had shorter time to viral suppression: log-rank p-value <0. 001 Slide 24 of 42 Chinula L, et al. 27 th CROI; Boston, MA; March 8 -11, 2020. Abst. 130 LB. EFV/FTC/TDF

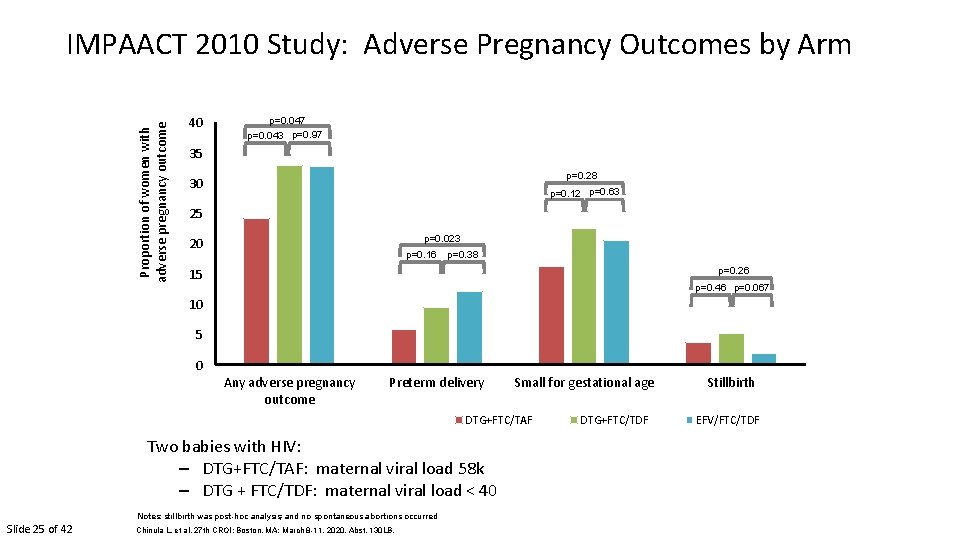
Proportion of women with adverse pregnancy outcome IMPAACT 2010 Study: Adverse Pregnancy Outcomes by Arm 40 p=0. 047 p=0. 043 p=0. 97 35 p=0. 28 30 p=0. 12 p=0. 63 25 p=0. 023 20 p=0. 16 p=0. 38 p=0. 26 15 p=0. 46 p=0. 067 10 5 0 Any adverse pregnancy outcome Preterm delivery Small for gestational age DTG+FTC/TAF Two babies with HIV: – DTG+FTC/TAF: maternal viral load 58 k – DTG + FTC/TDF: maternal viral load < 40 Notes: stillbirth was post-hoc analysis; and no spontaneous abortions occurred Slide 25 of 42 Chinula L, et al. 27 th CROI; Boston, MA; March 8 -11, 2020. Abst. 130 LB. DTG+FTC/TDF Stillbirth EFV/FTC/TDF

Antiretroviral Therapy Intolerance • RJ is a 40 yo Black woman living with HIV who presented with a CD 4 cell count of 350 and an HIV RNA is 37, 000 c/m. L • Diabetes controlled on metformin (500 BID) and BMI of 31 (wt 80 kg) • e. GFR 85 and ALT 38, HBV immune, RT/Pro genotype K 103 N • She was started on bictegravir/FTC/TAF with excellent response • HIV RNA < 40 c/m. L and CD 4 490 after 3 months • She is seeing you after 8 months of therapy • Since starting therapy she has gained 15 kg and her A 1 C has increased from 6. 9 to 7. 7. Her HIV RNA is < 40 (TND) • She is tolerating her BFTAF, she has not changed diet or activity level and the “weight doesn’t bother that much” Slide 26 of 42

ARS Question #6 • What will you do at this point? 1. Continue current antiretroviral therapy – increase metformin to 1000 mg twice daily and refer to nutrition for diet review and advice 2. Consider changing her antiretroviral therapy to address weight gain 3. Something else 4. Not sure Slide 27 of 42

ARS Question #7 • If you are considering changing therapy what regimen would you consider? 1. 2. 3. 4. 5. 6. 7. Slide 28 of 42 Dolutegravir/3 TC Doravirine/TDF/FTC Efavirenz/TDF/3 TC Elvitegravir/cobi/TDF/FTC Rilpivirine/TAF/FTC Rilpivirine/TDF/FTC Something else HIV RNA < 40, CD 4 560, e. GRF normal, diabetes, HBV immune and baseline genotype K 103 N

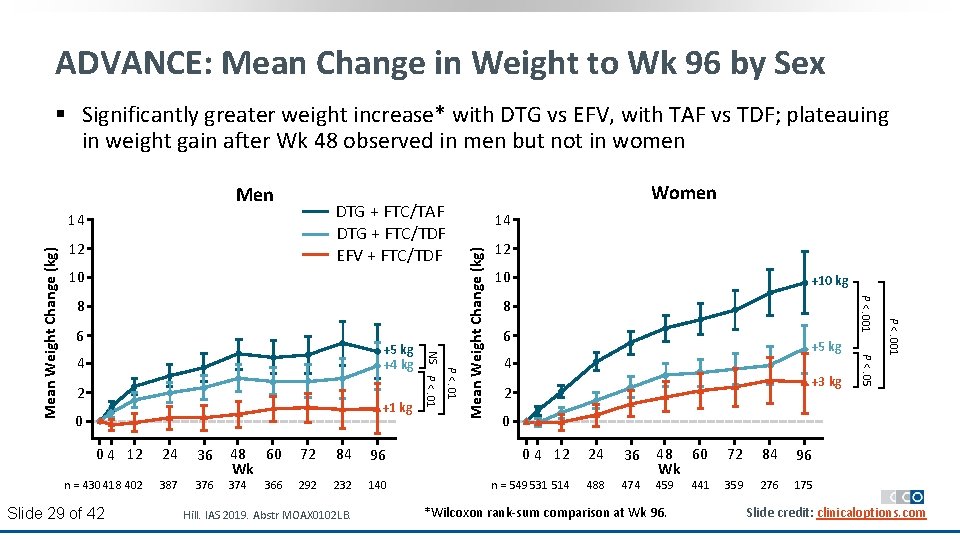
ADVANCE: Mean Change in Weight to Wk 96 by Sex § Significantly greater weight increase* with DTG vs EFV, with TAF vs TDF; plateauing in weight gain after Wk 48 observed in men but not in women DTG + FTC/TAF DTG + FTC/TDF EFV + FTC/TDF 12 10 2 +1 kg 0 0 4 12 24 36 n = 430 418 402 387 376 Slide 29 of 42 48 60 Wk 374 366 10 +10 kg 8 6 +5 kg 4 +3 kg 2 0 72 84 96 0 4 12 24 36 292 232 140 n = 549 531 514 488 474 Hill. IAS 2019. Abstr MOAX 0102 LB. P <. 05 4 P <. 01 +5 kg +4 kg 12 P <. 001 6 14 P <. 001 8 NS P <. 01 Mean Weight Change (kg) 14 Women Mean Weight Change (kg) Men 48 60 Wk 459 *Wilcoxon rank-sum comparison at Wk 96. 441 72 84 96 359 276 175 Slide credit: clinicaloptions. com

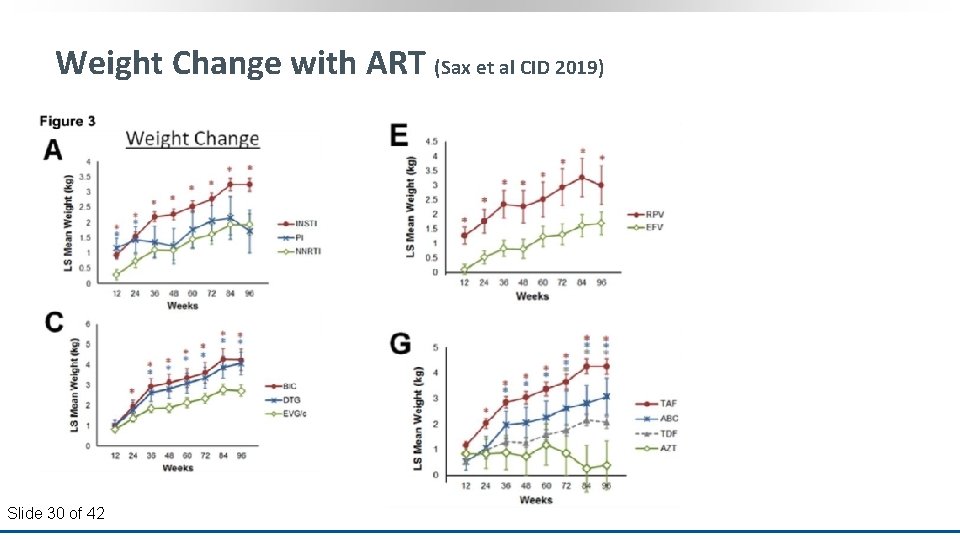
Weight Change with ART (Sax et al CID 2019) Slide 30 of 42

Long Acting Antiretroviral Therapy You live in Montreal, you speak perfect French and there is no COVID-19 Pandemic • You are seeing one of your favorite patients. He is a 48 yo man who you started on raltegravir plus TDF/FTC 10 years ago. Pre-therapy genotype wild type (subtype B), HBV surface Ab + • He has done extremely well. He is now on dolutegravir plus TAF/FTC with CD 4 870 and HIV RNA < 20 c/m. L (TND) and no adverse effects. He travels for work internationally (and brings you back single malt scotch that he gets at the Heathrow airport) • He is tired of taking pills, he is tired of thinking about HIV daily and on his last trip he had to go to a physician in Germany for a weeks supply of dolutegravir and TDF/FTC because he forgot his medication • He has heard about long-acting therapy. He understands it is an IM injection (“Can it be worse than LA-PCN for syphilis? ”). He wants your advice Slide 31 of 42

ARS Question #8 • What do you recommend? 1. Switch to oral cabotegravir and rilpivirine for 4 weeks and then start IM LA CAB + LA RPV every 4 weeks 2. Switch to oral cabotegravir and rilpivirine for 4 weeks and then start IM LA CAB + LA RPV every 8 weeks (as directed) 3. Direct to injection (no 4 week oral therapy) 4. I would not recommend IM LA CAB/RPV 5. Not sure Slide 32 of 42

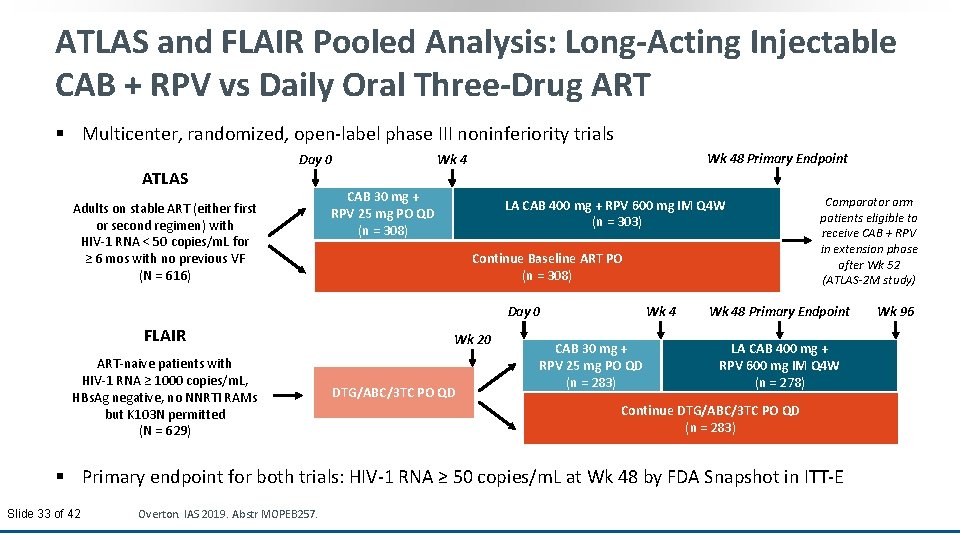
ATLAS and FLAIR Pooled Analysis: Long-Acting Injectable CAB + RPV vs Daily Oral Three-Drug ART § Multicenter, randomized, open-label phase III noninferiority trials ATLAS Day 0 Adults on stable ART (either first or second regimen) with HIV-1 RNA < 50 copies/m. L for ≥ 6 mos with no previous VF (N = 616) Wk 48 Primary Endpoint Wk 4 CAB 30 mg + RPV 25 mg PO QD (n = 308) LA CAB 400 mg + RPV 600 mg IM Q 4 W (n = 303) Continue Baseline ART PO (n = 308) Day 0 FLAIR ART-naive patients with HIV-1 RNA ≥ 1000 copies/m. L, HBs. Ag negative, no NNRTI RAMs but K 103 N permitted (N = 629) Wk 20 DTG/ABC/3 TC PO QD Wk 4 CAB 30 mg + RPV 25 mg PO QD (n = 283) Comparator arm patients eligible to receive CAB + RPV in extension phase after Wk 52 (ATLAS-2 M study) Wk 48 Primary Endpoint LA CAB 400 mg + RPV 600 mg IM Q 4 W (n = 278) Continue DTG/ABC/3 TC PO QD (n = 283) § Primary endpoint for both trials: HIV-1 RNA ≥ 50 copies/m. L at Wk 48 by FDA Snapshot in ITT-E Slide 33 of 42 Overton. IAS 2019. Abstr MOPEB 257. Wk 96

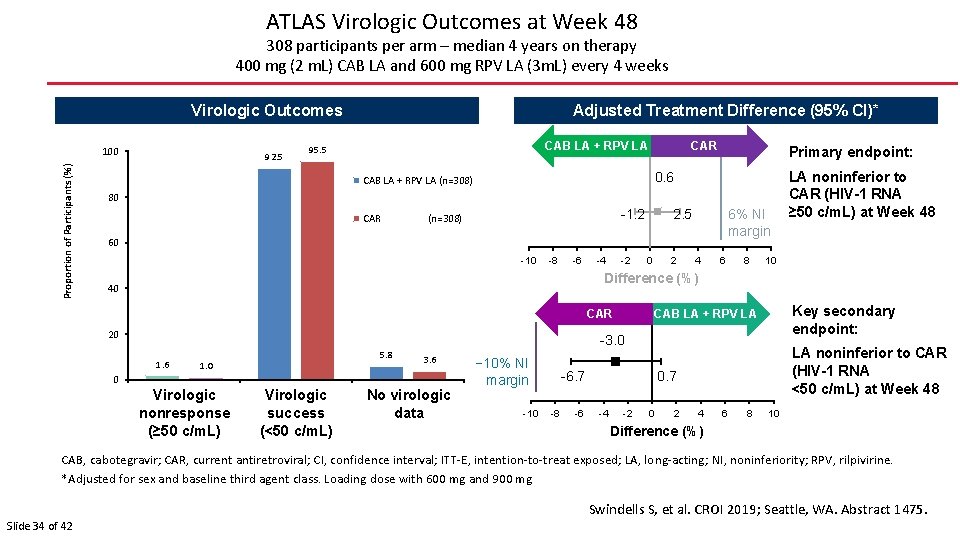
ATLAS Virologic Outcomes at Week 48 308 participants per arm – median 4 years on therapy 400 mg (2 m. L) CAB LA and 600 mg RPV LA (3 m. L) every 4 weeks Virologic Outcomes Proportion of Participants (%) 100 92. 5 Adjusted Treatment Difference (95% CI)* CAR CAB LA + RPV LA 95. 5 Primary endpoint: 0. 6 CAB LA + RPV LA (n=308) 80 CAR -1. 2 (n=308) 6% NI margin 2. 5 60 -10 -8 -6 -4 -2 0 2 4 6 8 LA noninferior to CAR (HIV-1 RNA ≥ 50 c/m. L) at Week 48 10 Difference (%) 40 CAR 20 CAB LA + RPV LA Key secondary endpoint: 0. 7 LA noninferior to CAR (HIV-1 RNA <50 c/m. L) at Week 48 -3. 0 1. 6 5. 8 1. 0 3. 6 0 Virologic nonresponse (≥ 50 c/m. L) Virologic success (<50 c/m. L) No virologic data − 10% NI margin -10 -6. 7 -8 -6 -4 -2 0 2 4 6 8 10 Difference (%) CAB, cabotegravir; CAR, current antiretroviral; CI, confidence interval; ITT-E, intention-to-treat exposed; LA, long-acting; NI, noninferiority; RPV, rilpivirine. *Adjusted for sex and baseline third agent class. Loading dose with 600 mg and 900 mg Swindells S, et al. CROI 2019; Seattle, WA. Abstract 1475. Slide 34 of 42

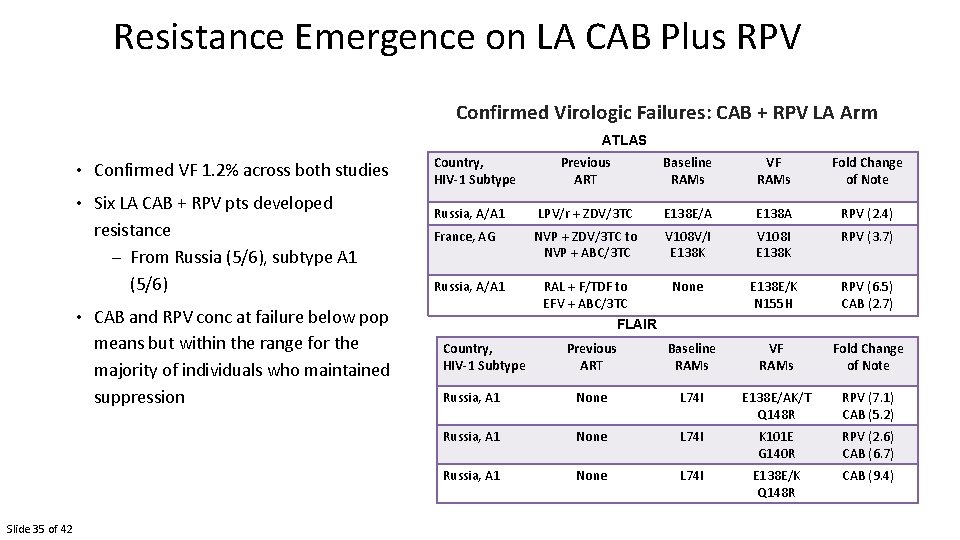
Resistance Emergence on LA CAB Plus RPV Confirmed Virologic Failures: CAB + RPV LA Arm ATLAS • Confirmed VF 1. 2% across both studies • Six LA CAB + RPV pts developed resistance – From Russia (5/6), subtype A 1 (5/6) Country, HIV-1 Subtype Previous ART Baseline RAMs VF RAMs Fold Change of Note Russia, A/A 1 LPV/r + ZDV/3 TC E 138 E/A E 138 A RPV (2. 4) France, AG NVP + ZDV/3 TC to NVP + ABC/3 TC V 108 V/I E 138 K V 108 I E 138 K RPV (3. 7) RAL + F/TDF to EFV + ABC/3 TC None E 138 E/K N 155 H RPV (6. 5) CAB (2. 7) Russia, A/A 1 • CAB and RPV conc at failure below pop means but within the range for the majority of individuals who maintained suppression Slide 35 of 42 FLAIR Country, HIV-1 Subtype Previous ART Baseline RAMs VF RAMs Fold Change of Note Russia, A 1 None L 74 I E 138 E/AK/T Q 148 R RPV (7. 1) CAB (5. 2) Russia, A 1 None L 74 I K 101 E G 140 R RPV (2. 6) CAB (6. 7) Russia, A 1 None L 74 I E 138 E/K Q 148 R CAB (9. 4)

Every-2 -Month Maintenance CAB + RPV Noninferior to Monthly Dosing for 48 Weeks: ATLAS 2 M ET Overton et al Slide 36 of 42

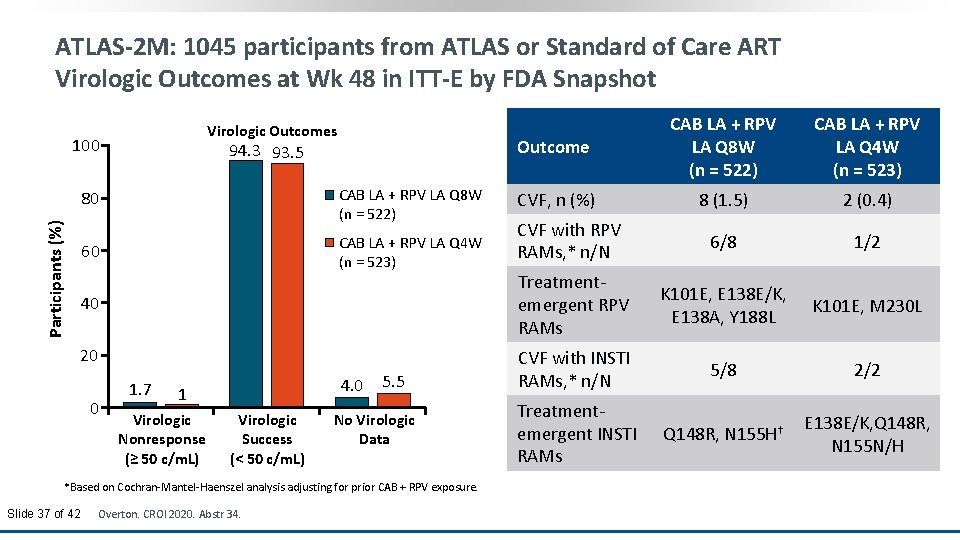
ATLAS-2 M: 1045 participants from ATLAS or Standard of Care ART Virologic Outcomes at Wk 48 in ITT-E by FDA Snapshot Virologic Outcomes 100 94. 3 93. 5 CAB LA + RPV LA Q 8 W (n = 522) Participants (%) 80 CAB LA + RPV LA Q 4 W (n = 523) 60 40 20 0 1. 7 4. 0 1 Virologic Nonresponse (≥ 50 c/m. L) Virologic Success (< 50 c/m. L) 5. 5 No Virologic Data *Based on Cochran-Mantel-Haenszel analysis adjusting for prior CAB + RPV exposure. Slide 37 of 42 Overton. CROI 2020. Abstr 34. Outcome CAB LA + RPV LA Q 8 W (n = 522) CAB LA + RPV LA Q 4 W (n = 523) CVF, n (%) 8 (1. 5) 2 (0. 4) CVF with RPV RAMs, * n/N 6/8 1/2 Treatmentemergent RPV RAMs K 101 E, E 138 E/K, E 138 A, Y 188 L K 101 E, M 230 L CVF with INSTI RAMs, * n/N 5/8 2/2 Treatmentemergent INSTI RAMs Q 148 R, N 155 H† E 138 E/K, Q 148 R, N 155 N/H

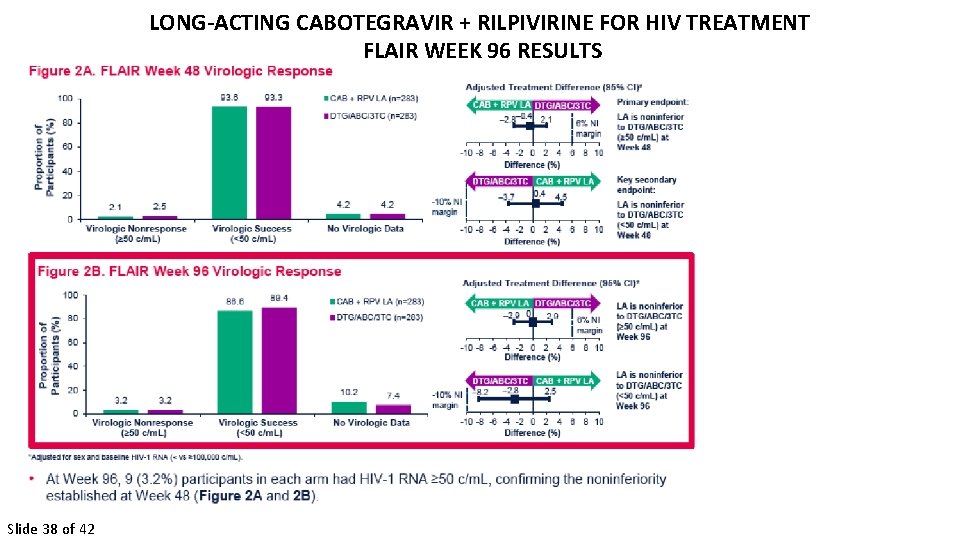
LONG-ACTING CABOTEGRAVIR + RILPIVIRINE FOR HIV TREATMENT FLAIR WEEK 96 RESULTS Orkin et al Slide 38 of 42

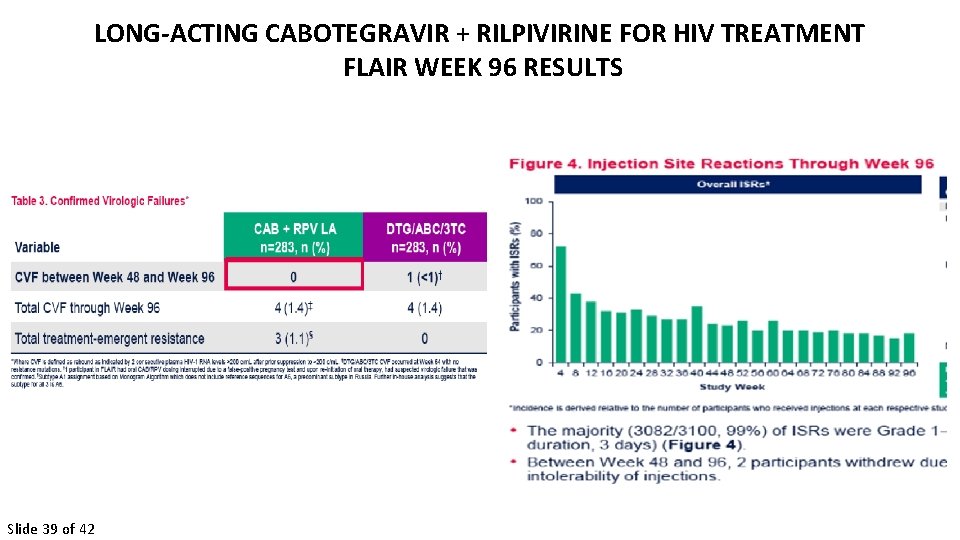
LONG-ACTING CABOTEGRAVIR + RILPIVIRINE FOR HIV TREATMENT FLAIR WEEK 96 RESULTS Slide 39 of 42

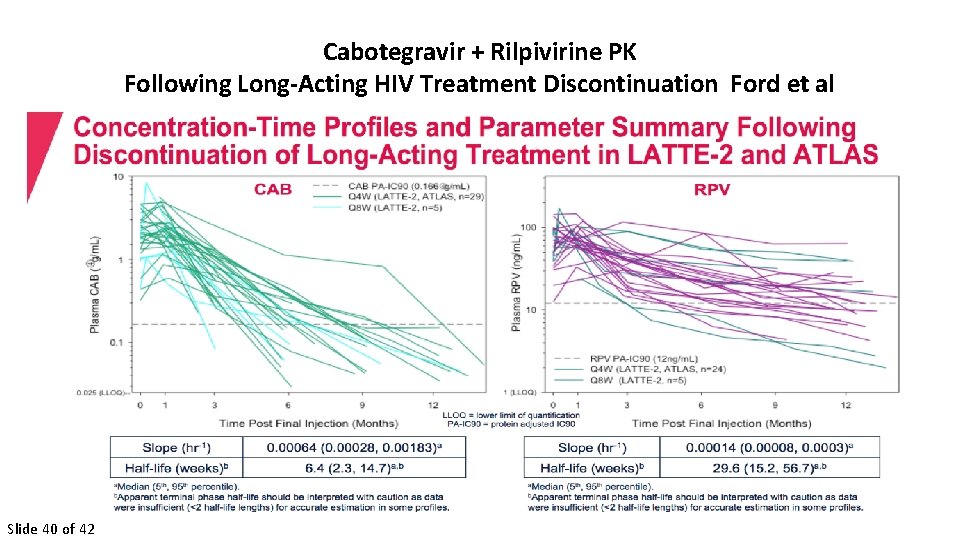
Cabotegravir + Rilpivirine PK Following Long-Acting HIV Treatment Discontinuation Ford et al Slide 40 of 42

Practical Aspects of Using CAB/RPV • Loading dose: CAB LA 600 mg (one 3 -m. L injection) and RPV LA 900 mg (one 3 -m. L injection) • Monthly maintenance: CAB LA 400 mg (one 2 -m. L injection) and RPV LA 600 mg (one 2 -m. L injection) • RPV LA requires cold chain • Injection into gluteus medius (upper outer quadrant of buttock) • Need a private place for injections • What about people with buttock implants? Orkin C, et al. IAS 2019 TUSY 0403; Landovitz, R et al. HIV R 4 P, Madrid, 2018. Abstract #OA 15. 06 LB; Saman R et al, EACS 2019 Slide 41 of 42

Question-and-Answer Session
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Criminal cases vs civil cases
Criminal cases vs civil cases Commercial and noncommercial food service operations
Commercial and noncommercial food service operations Idt therapy
Idt therapy Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Bioness bits cost
Bioness bits cost Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Financial analysis of banks
Financial analysis of banks Financial and non financial methods of motivation
Financial and non financial methods of motivation How to write use cases
How to write use cases Writing effective use cases
Writing effective use cases Clipp cases
Clipp cases Cosmos db use cases
Cosmos db use cases Case description
Case description Couchbase use cases
Couchbase use cases Binary search test cases
Binary search test cases Special cases in simplex method
Special cases in simplex method Service chaining use cases
Service chaining use cases Sdn use case
Sdn use case Republic act 9344
Republic act 9344 Veterolegal definition
Veterolegal definition Sd wan use cases
Sd wan use cases Nspe ethics
Nspe ethics Mastertheorem
Mastertheorem Floating point
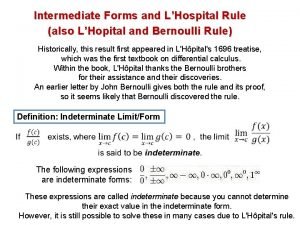
Floating point Indeterminate forms l'hopital
Indeterminate forms l'hopital How to factor special cases trinomials
How to factor special cases trinomials Perfect square trinomial
Perfect square trinomial What is equivalence class partitioning
What is equivalence class partitioning Documenting use cases
Documenting use cases Class iii
Class iii Deloitte trueblood
Deloitte trueblood Chapter 5 defensive driving quiz answers
Chapter 5 defensive driving quiz answers Percentage of criminal cases that go to trial
Percentage of criminal cases that go to trial Test case que es
Test case que es Computer misuse act cases
Computer misuse act cases Precedent cases
Precedent cases Cisco ip telephony cases
Cisco ip telephony cases Cases of deadlock
Cases of deadlock Hyperledger use cases
Hyperledger use cases Test cases for atm system
Test cases for atm system