Use of antiretroviral therapy in resourcelimited countries in










- Slides: 14

Use of antiretroviral therapy in resource-limited countries in 2007: up-take of 2 nd-line and pediatric treatment stagnant F. Renaud-Théry 1, B. Dongmo Nguimfack 1, M. Vitoria 1, E. Lee 2, C. Gilks 1, J. Perriëns 1 1 World Health Organization, Department of HIV and AIDS, Geneva, Switzerland, 2 Consultant, Geneva, Switzerland

Background The AIDS Medicines and Diagnostics Service (AMDS) of WHO conducts an annual survey to assess the use of ARVs in low and middle income countries. The objectives are: § to document the use of first and second line adult and pediatric ARVs while treatment programmes are scaling up § to serve as a baseline forecasting ARV demand to inform expansion of ARV production capacity of ARV manufacturers.

Approach and results A questionnaire was sent to 41 countries - 30 responded by August 2007 (73%) The 30 respondent countries covered a total number of 1, 356, 399 patients (54% of total number of patients on treatment at the time of the survey) Adults: 93% Children: 7% Total number of regimens reported: 1 st line: 39 regimens 2 nd line: 149 regimens Countries included Benin, Botswana, Brazil*, Burkina Faso, Burundi, Cambodia, Cameroon, China, Colombia, Cote d'Ivoire, Ethiopia, Guyana, India, Kenya, Lesotho, Malawi, Mali, Mexico*, Mozambique, Namibia, Nigeria, Peru, Russian Federation, Rwanda, Swaziland, United Republic of Tanzania, Thailand, Uganda, Ukraine, Viet Nam, Zambia and Zimbabwe * Brazil and Mexico are not included in this analysis because they present regimen patterns dramatically different from other countries.

Distribution of 1 st and 2 nd line regimens among adults and children:

88% of Adults on 1 st line receive regimens recommended by WHO, with a majority using d 4 T + 3 TC + NVP (51%) 1, 5% use a tenofovir-based regimen

55% of adults on 2 nd-line regimen were receiving regimens recommended by WHO, with a majotity of ABC+dd. I+LPV/r and AZT+dd. I+LPV/r tenofovir-based regimen represented 14% of second-line regimen boosted lopinavir was the large predominant protease inhibitor

91% of children on 1 st line receive regimens recommended by WHO, with a majority using d 4 T + 3 TC + NVP (42%)

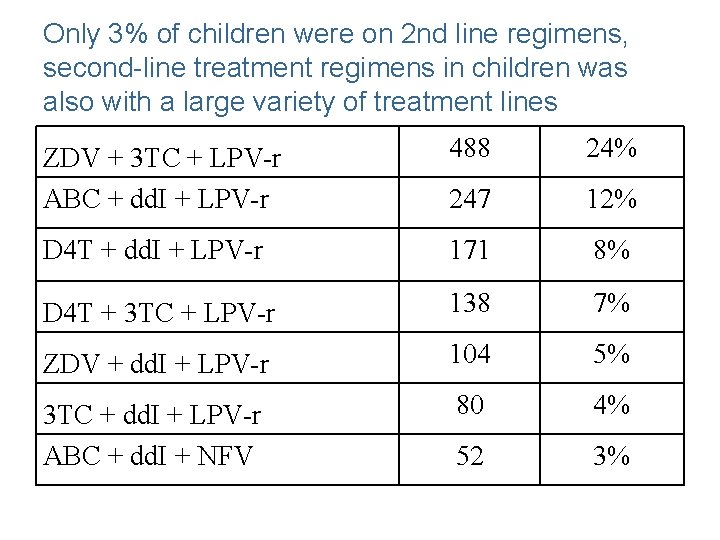
Only 3% of children were on 2 nd line regimens, second-line treatment regimens in children was also with a large variety of treatment lines ZDV + 3 TC + LPV-r ABC + dd. I + LPV-r 488 24% 247 12% D 4 T + dd. I + LPV-r 171 8% D 4 T + 3 TC + LPV-r 138 7% ZDV + dd. I + LPV-r 104 5% 3 TC + dd. I + LPV-r ABC + dd. I + NFV 80 4% 52 3%

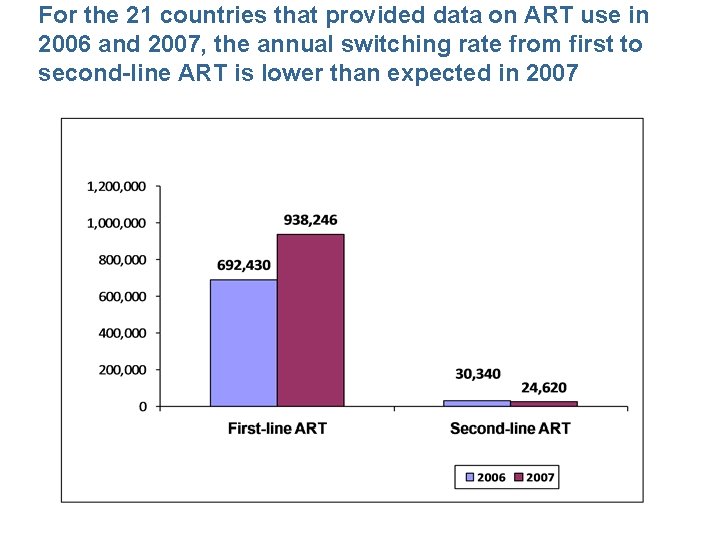
For the 21 countries that provided data on ART use in 2006 and 2007, the annual switching rate from first to second-line ART is lower than expected in 2007

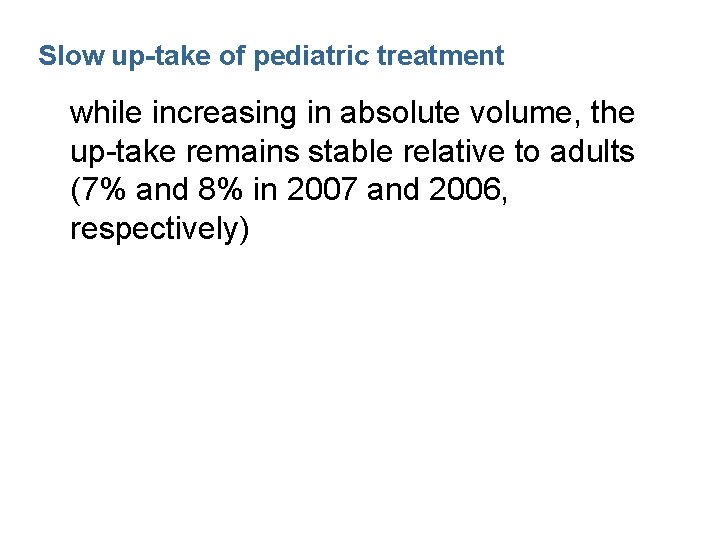
Slow up-take of pediatric treatment while increasing in absolute volume, the up-take remains stable relative to adults (7% and 8% in 2007 and 2006, respectively)

Conclusion This survey documents a good compliance of first-line treatment but still a slower uptake of second-line treatment for adults and children in developing countries. Programmatic difficulties and procurement constraints need to be addressed by countries in order to reach Universal Access:

• Promotion of earlier diagnosis of treatment failure and access to second-line medicines (TDF and/or ABC and heat stable protease inhibitors) • New pediatric formulations with adapted strengths and fixed-dose combinations are being made available. Together with the increased promotion of early diagnosis and treatment in infants by WHO, this might increase the up-take of treatment in children in the near future

The results of this survey will be used to up-date the global forecasts of ARV demand for 2009 to 2012 by WHO and UNAIDS

More information on ARV forecasting is available at: http: //www. who. int/hiv/amds/forecasting/en/i ndex. html amds@who. int
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Humanistic therapies aim to boost
Humanistic therapies aim to boost Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Bioness bits cost
Bioness bits cost Which countries use british english
Which countries use british english Countries that use imperial units
Countries that use imperial units Which countries use imperial system
Which countries use imperial system Metric system
Metric system Yesterday
Yesterday Http://hdr.undp.org/en/countries
Http://hdr.undp.org/en/countries Us organization
Us organization Material well being
Material well being Lactose intolerance by ethnicity
Lactose intolerance by ethnicity Map of middle east countries
Map of middle east countries