Long Term Impact of Antiretroviral Therapy in Resource

























- Slides: 43

Long Term Impact of Antiretroviral Therapy in Resource Limited Settings Dr. N. Kumarasamy Chief Medical Officer YRGCARE Medical Centre Chief-Chennai Antiviral Research and Treatment (CART) Clinical Research Site/NIH Voluntary Health Services Chennai, India

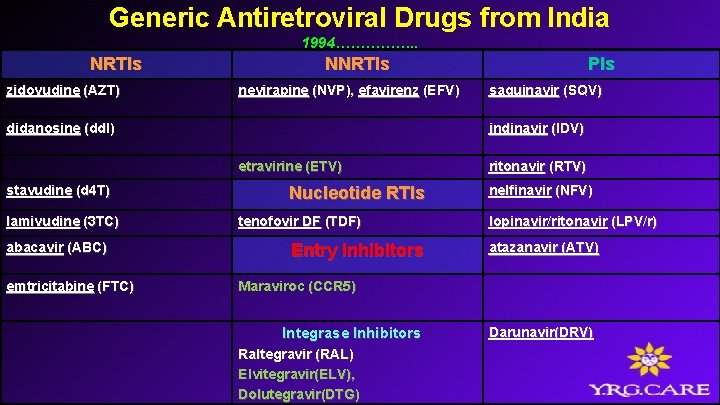
Generic Antiretroviral Drugs from India 1994……………. . NRTIs zidovudine (AZT) NNRTIs nevirapine (NVP), efavirenz (EFV) didanosine (dd. I) lamivudine (3 TC) abacavir (ABC) emtricitabine (FTC) saquinavir (SQV) indinavir (IDV) etravirine (ETV) stavudine (d 4 T) PIs Nucleotide RTIs tenofovir DF (TDF) Entry Inhibitors ritonavir (RTV) nelfinavir (NFV) lopinavir/ritonavir (LPV/r) atazanavir (ATV) Maraviroc (CCR 5) Integrase Inhibitors Raltegravir (RAL) Elvitegravir(ELV), Dolutegravir(DTG) Darunavir(DRV)

Safety, Tolerability and Effectiveness of Generic Antiretroviral Drug Regimens for HIV-infected Patients in South India Generic HAART was safe, well tolerated and effective at increasing CD 4 T-lymphocytes in advanced patients, comparable to the experience with proprietary HAART. Kumarasamy et al. AIDS 2003 Vol 17 No 15: 2267 -9

Percentage of people eligible who are receiving antiretroviral therapy (based on 2010 WHO guidelines) in low- and middle-income countries, by region, 2009– 2012 ( Currently 12. 9 million on ART in LMIC) Source: UNAIDS 2012 estimates.

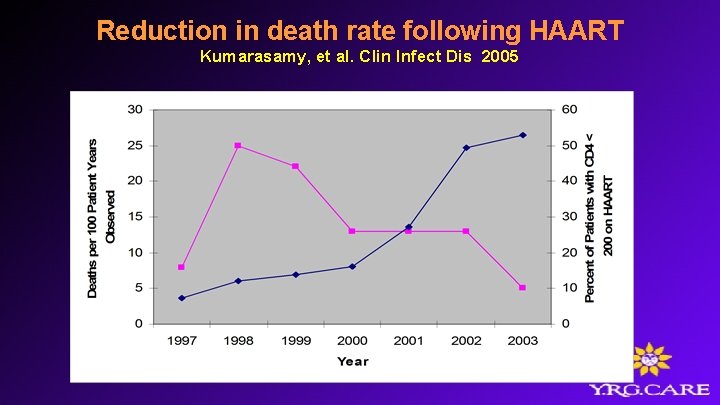
Reduction in death rate following HAART Kumarasamy, et al. Clin Infect Dis 2005

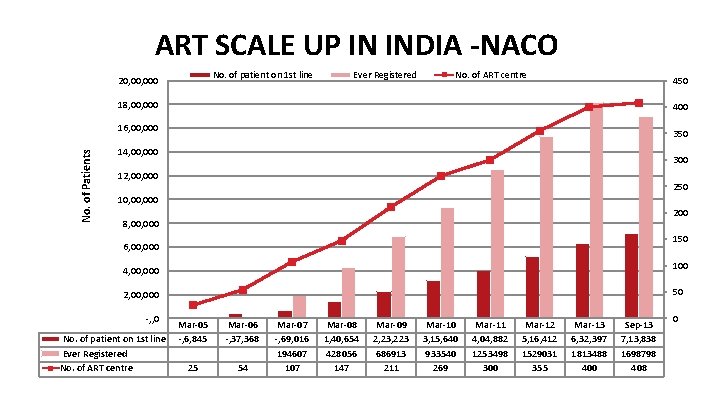
ART SCALE UP IN INDIA -NACO No. of patient on 1 st line 20, 000 Ever Registered No. of ART centre 450 18, 000 400 No. of Patients 16, 000 350 14, 000 300 12, 000 250 10, 000 200 8, 000 150 6, 000 100 4, 000 50 2, 000 -, , 0 No. of patient on 1 st line Ever Registered No. of ART centre Mar-05 -, 6, 845 Mar-06 -, 37, 368 25 54 Mar-07 -, 69, 016 194607 107 Mar-08 1, 40, 654 428056 147 Mar-09 2, 23, 223 686913 211 Mar-10 3, 15, 640 933540 269 Mar-11 4, 04, 882 1253498 300 Mar-12 5, 16, 412 1529031 355 Mar-13 6, 32, 397 1813488 400 Sep-13 7, 13, 838 1698798 408 0

Declining Trends of HIV Epidemic in India Female: 39% of PLHIV; Children: 7% of PLHIV Source: Technical Report India HIV Estimates 2012, NACO & NIMS

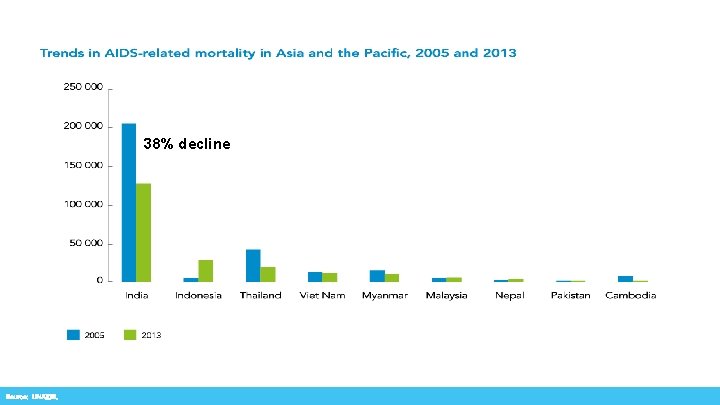
38% decline Source: UNAIDS.

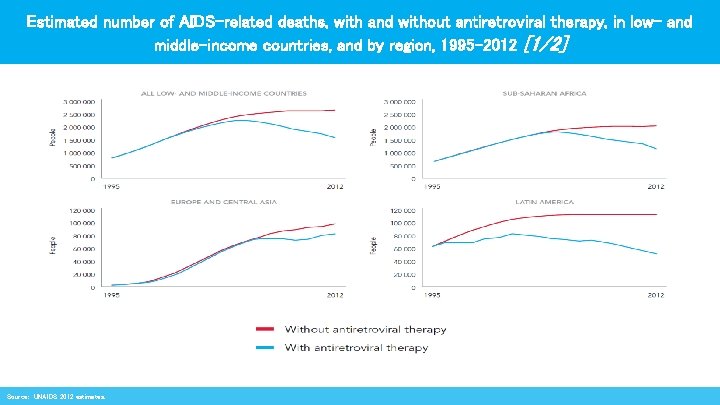
Estimated number of AIDS-related deaths, with and without antiretroviral therapy, in low- and middle-income countries, and by region, 1995– 2012 [1/2] Source: UNAIDS 2012 estimates.

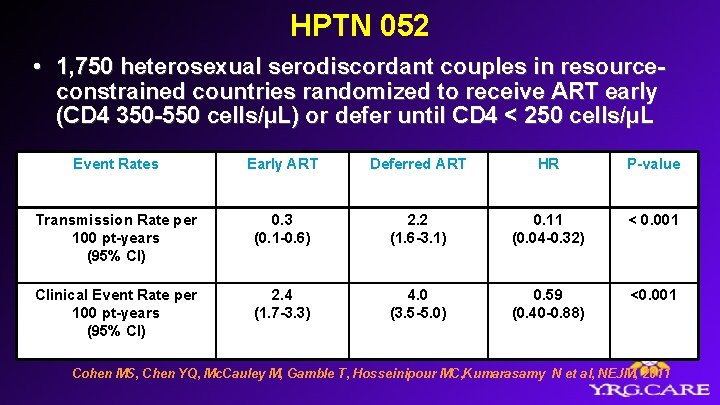
HPTN 052 • 1, 750 heterosexual serodiscordant couples in resourceconstrained countries randomized to receive ART early (CD 4 350 -550 cells/µL) or defer until CD 4 < 250 cells/µL Event Rates Early ART Deferred ART HR P-value Transmission Rate per 100 pt-years (95% CI) 0. 3 (0. 1 -0. 6) 2. 2 (1. 6 -3. 1) 0. 11 (0. 04 -0. 32) < 0. 001 Clinical Event Rate per 100 pt-years (95% CI) 2. 4 (1. 7 -3. 3) 4. 0 (3. 5 -5. 0) 0. 59 (0. 40 -0. 88) <0. 001 Cohen MS, Chen YQ, Mc. Cauley M, Gamble T, Hosseinipour MC, Kumarasamy N et al, NEJM, 2011

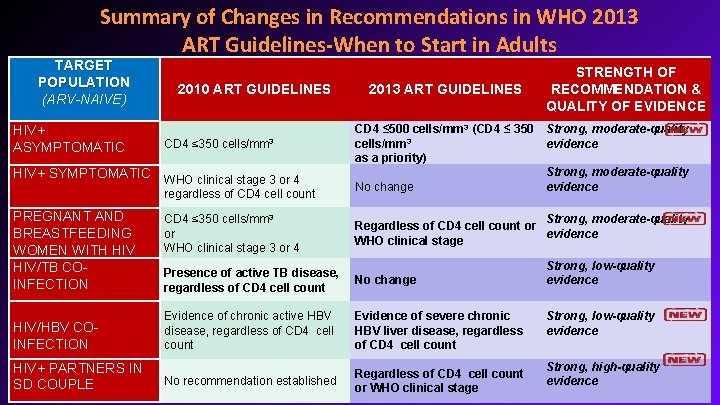
Summary of Changes in Recommendations in WHO 2013 ART Guidelines-When to Start in Adults TARGET POPULATION (ARV-NAIVE) HIV+ ASYMPTOMATIC 2010 ART GUIDELINES CD 4 ≤ 350 cells/mm 3 HIV+ SYMPTOMATIC WHO clinical stage 3 or 4 regardless of CD 4 cell count PREGNANT AND BREASTFEEDING WOMEN WITH HIV/TB COINFECTION HIV/HBV COINFECTION HIV+ PARTNERS IN SD COUPLE CD 4 ≤ 350 cells/mm 3 or WHO clinical stage 3 or 4 2013 ART GUIDELINES STRENGTH OF RECOMMENDATION & QUALITY OF EVIDENCE CD 4 ≤ 500 cells/mm 3 (CD 4 ≤ 350 Strong, moderate-quality cells/mm 3 evidence as a priority) Strong, moderate-quality No change evidence Strong, moderate-quality Regardless of CD 4 cell count or evidence WHO clinical stage Presence of active TB disease, regardless of CD 4 cell count No change Evidence of chronic active HBV disease, regardless of CD 4 cell count Evidence of severe chronic HBV liver disease, regardless of CD 4 cell count No recommendation established Regardless of CD 4 cell count or WHO clinical stage Strong, low-quality evidence Strong, high-quality evidence

• In South Africa, early ART was cost-saving over a 5 -year period. • In both South Africa and India, early ART was projected to be very cost-effective over a lifetime. • With individual, public health, and economic benefits, there is a compelling case for early ART for serodiscordant couples in resource-limited settings.

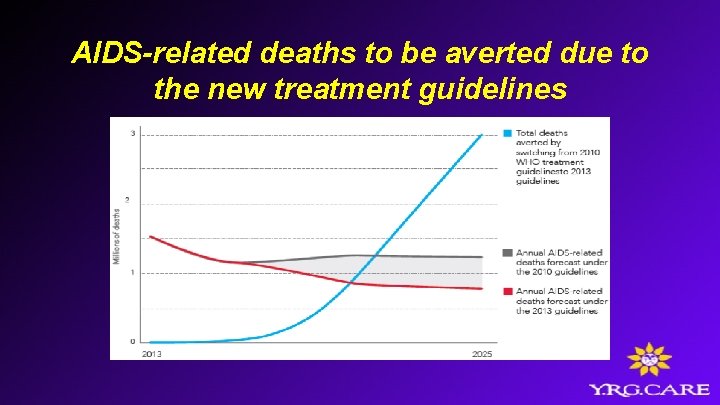
AIDS-related deaths to be averted due to the new treatment guidelines

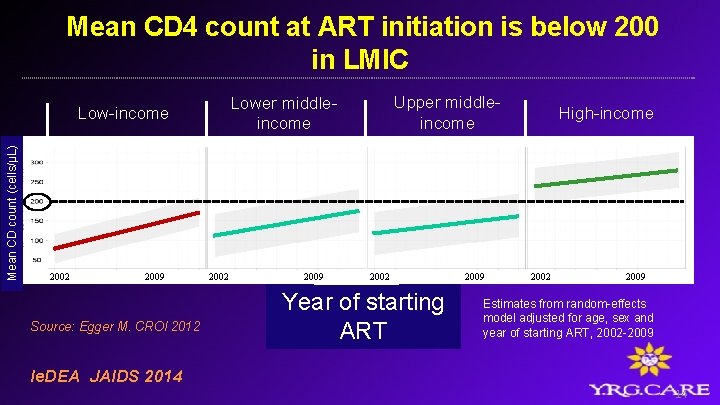
Mean CD 4 count at ART initiation is below 200 in LMIC Mean CD count (cells/µL) 2002 2009 Source: Egger M. CROI 2012 Upper middleincome Low-income 2002 2009 2002 Year of starting ART 2009 High-income 2002 2009 Estimates from random-effects model adjusted for age, sex and year of starting ART, 2002 -2009 Ie. DEA JAIDS 2014 14

Source: UNAIDS.

• Simulation model of HIV testing and treatment - Prevalence and Incidence in different groups - Cost of testing Conclusion: Voluntary HIV screening among National population every 5 yrs offers substantial clinical benefit and cost effective. Annual screening is cost effective among high risk population and in high prevalent districts

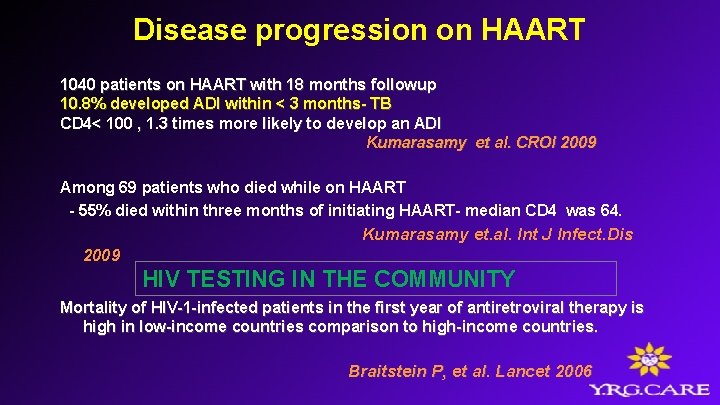
Disease progression on HAART 1040 patients on HAART with 18 months followup 10. 8% developed ADI within < 3 months- TB CD 4< 100 , 1. 3 times more likely to develop an ADI Kumarasamy et al. CROI 2009 Among 69 patients who died while on HAART - 55% died within three months of initiating HAART- median CD 4 was 64. 2009 Kumarasamy et. al. Int J Infect. Dis HIV TESTING IN THE COMMUNITY Mortality of HIV-1 -infected patients in the first year of antiretroviral therapy is high in low-income countries comparison to high-income countries. Braitstein P, et al. Lancet 2006

When to Start ART in TB A 5221/ STRIDE CAMELIA SAPIT N 806 660 429 Sites Africa, Asia, S Am, N Am Cambodia S. Africa Arms Imm vs 8 -12 wk Imm vs 8 wk Early vs 24 wk Endpt Death/AIDS <50 CD 4 Death CD 4 (IQR) 77 (36, 145) 25 (11, 56) 150 (77, 254) Havilr- NEJM 2011, Blanc- NEJM 2011, Abdool Karim, NEJM, 2011

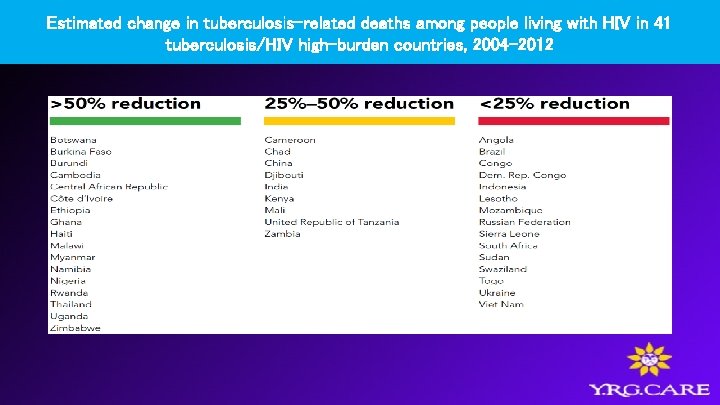
Estimated change in tuberculosis-related deaths among people living with HIV in 41 tuberculosis/HIV high-burden countries, 2004– 2012

d 4 T phased out of WHO guidelines • Severe peripheral neuropathy • Lipoatrophy- mean 20 months • (Saghayam S, Chaguturu S, Kumarasamy N, et al. CID 2004) • Substitute AZT/TDF after 6 -12 months with d 4 T containing HAART in ARV roll outs… • Risk to anemia after AZT substitution is < 1%. ( Kumarasamy et al – IJID 2009)

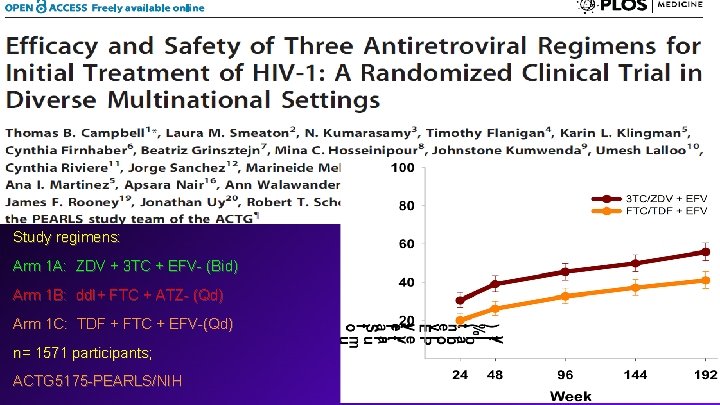
Study regimens: Arm 1 A: ZDV + 3 TC + EFV- (Bid) Arm 1 B: dd. I+ FTC + ATZ- (Qd) Arm 1 C: TDF + FTC + EFV-(Qd) n= 1571 participants; ACTG 5175 -PEARLS/NIH

Clin Infect Dis. 2010 • We evaluated the clinical outcomes and cost-effectiveness of first-line ART using tenofovir in India, compared with current practice using stavudine or zidovudine. • We used a state-transition model of HIV disease to examine strategies using different NRTs, combined with lamivudine and nevirapine, Conclusions: Using tenofovir as part of first-line ART in India will improve survival, is cost-effective by international standards, and should be considered for initial therapy for HIV-infected patients in India.

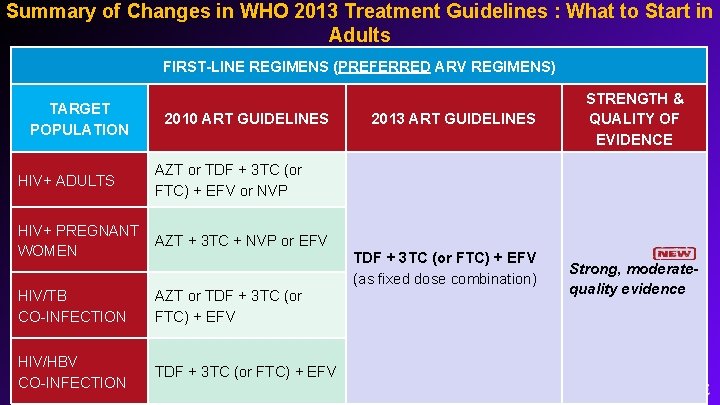
Summary of Changes in WHO 2013 Treatment Guidelines : What to Start in Adults FIRST-LINE REGIMENS (PREFERRED ARV REGIMENS) TARGET POPULATION HIV+ ADULTS 2010 ART GUIDELINES 2013 ART GUIDELINES STRENGTH & QUALITY OF EVIDENCE AZT or TDF + 3 TC (or FTC) + EFV or NVP HIV+ PREGNANT AZT + 3 TC + NVP or EFV WOMEN HIV/TB CO-INFECTION AZT or TDF + 3 TC (or FTC) + EFV HIV/HBV CO-INFECTION TDF + 3 TC (or FTC) + EFV (as fixed dose combination) Strong, moderatequality evidence

Sequencing Therapy in in Resource Limited Setting- 2015 2 NRTIs(TDF+3 TC/FTC) + 1 NNRTI (EFV) 2 NRTIs(AZT+3 TC) + 1 PI/RTV(ATVr or LPVr) 1 PI/RTV(DRVr) + Integrase ± / CCR 5 inhibitor/ 2 nd Gen NNRTI (ETV)

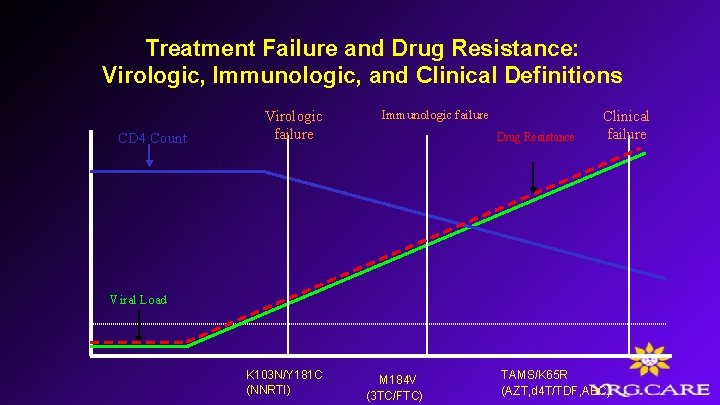
Treatment Failure and Drug Resistance: Virologic, Immunologic, and Clinical Definitions CD 4 Count Viral Load Virologic failure Immunologic failure Drug Resistance Clinical failure

79% of them had M 184 V, 71 % had NNRTI mutations, (K 103 N, Y 181 C, G 190 A) 60% had TAMS, (M 41 L, T 215 Y/F, K 70 R, L 210 W, K 219 E/Q) 11% had Q 151 M 5% had K 65 R and 5% had L 74 V. 26% had 3 or more NNRTI mutations This data clearly warns that patients with immunological failure with standard WHO criteria have severe mutations and which can jeopardize future 2 nd line NRTI options and newer drugs. Urgent need for VIRAL LOAD monitoring

Treatment Failure and Drug Resistance: Virologic, Immunologic, and Clinical Definitions CD 4 Count Virologic failure Immunologic failure Drug Resistance Clinical failure Viral Load K 103 N/Y 181 C (NNRTI) M 184 V (3 TC/FTC) TAMS/K 65 R (AZT, d 4 T/TDF, ABC)

Sequencing Therapy before 2013 without Viral load monitoring 2 NRTIs(AZT or d 4 T) + 1 NNRTI 2 NRTIs(TDF/3 TC) + 1 PI/RTV(ATVr or LPVr) Kumarasamy N, CID 2009; Hosseinipour M, AIDS 2009; Lyagoba F, JAID 2010;

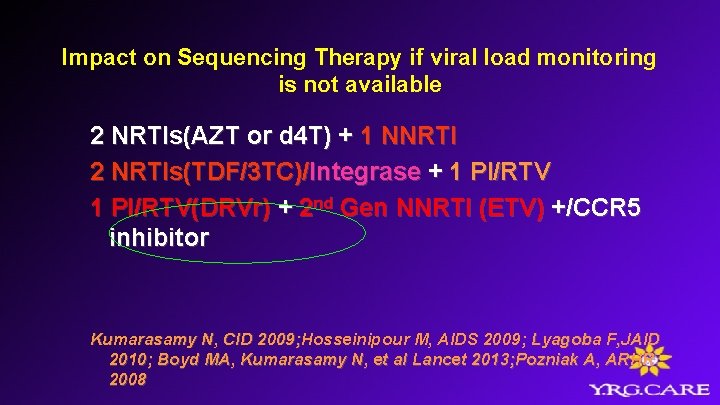
Impact on Sequencing Therapy if viral load monitoring is not available 2 NRTIs(AZT or d 4 T) + 1 NNRTI 2 NRTIs(TDF/3 TC)/Integrase + 1 PI/r (ATVr) Kumarasamy N, CID 2009; Hosseinipour M, AIDS 2009; Lyagoba F, JAID 2009; 2010; Boyd MA, Kumarasamy N, et al Lancet 2013;

Impact on Sequencing Therapy if viral load monitoring is not available 2 NRTIs(AZT or d 4 T) + 1 NNRTI 2 NRTIs(TDF/3 TC)/Integrase + 1 PI/RTV(DRVr) + 2 nd Gen NNRTI (ETV) +/CCR 5 inhibitor Kumarasamy N, CID 2009; Hosseinipour M, AIDS 2009; Lyagoba F, JAID 2009; 2010; Boyd MA, Kumarasamy N, et al Lancet 2013; Pozniak A, ARHR 2008

Sequencing Therapy in in Resource Limited Setting- 2015 2 NRTIs(TDF+3 TC/FTC) + 1 NNRTI (EFV) 2 NRTIs(AZT+3 TC) + 1 PI/RTV(ATVr or LPVr) 1 PI/RTV(DRVr) + Integrase ± / CCR 5 inhibitor/ 2 nd Gen NNRTI (ETV)

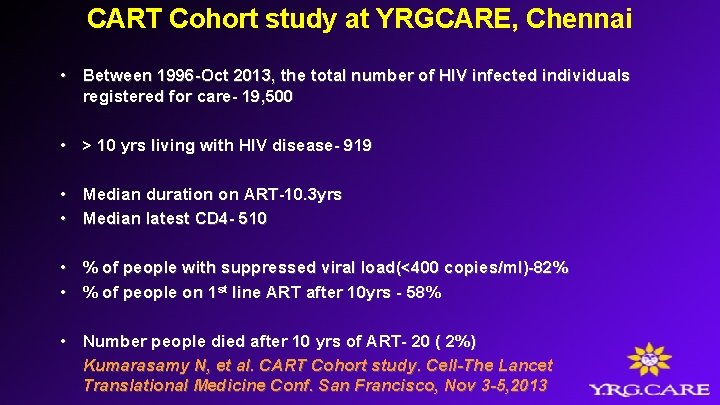
CART Cohort study at YRGCARE, Chennai • Between 1996 -Oct 2013, the total number of HIV infected individuals registered for care- 19, 500 • > 10 yrs living with HIV disease- 919 • Median duration on ART-10. 3 yrs • Median latest CD 4 - 510 • % of people with suppressed viral load(<400 copies/ml)-82% • % of people on 1 st line ART after 10 yrs - 58% • Number people died after 10 yrs of ART- 20 ( 2%) Kumarasamy N, et al. CART Cohort study. Cell-The Lancet Translational Medicine Conf. San Francisco, Nov 3 -5, 2013

Non AIDS causes of morbidity/mortality • Cardiovascular- drugs, inflammation • Renal- drugs, HIV • Diabetes-drugs, HIV • HCV- as co-infection in IDUs &hemophiliacs • Cancers- HPV • Neurocognitive effects- drugs, HIV

Between 1986 and 2007, 3530 patients with a median follow up 3. 9 yrs. 24. 6% died.

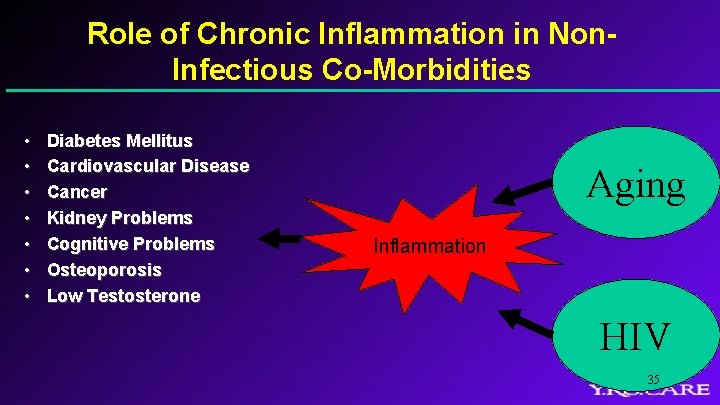
Role of Chronic Inflammation in Non. Infectious Co-Morbidities • • Diabetes Mellitus Cardiovascular Disease Cancer Kidney Problems Cognitive Problems Osteoporosis Low Testosterone Aging Inflammation HIV 35

Integration of HIV in health service delivery Source: GARPR 2013.

Inflammatory markers on ART NWCS 319/A 5175 -Summary • Pre-ART elevations in inflammation and immune activation markers were common – Pre-ART marker levels differed by country and sex – Some pre-ART marker levels were associated with risk of HIV dz progression or death after initiation of ART • Initiation of ART produced only a modest decline in inflammatory and activation markers CROI 2013

Why we need Eradication? • Tissue sanctuaries (reservoirs) -Brain, Lymphnode, Gut • Chronically Infected macrophages - RTIs, PIs • Residual viremia-chronic inflammation -cardiovascular disease -nephropathy -faster evolution of viral hepatitis -cancer

Cure-is it possible? • Sterlizing cure - total eradication of all HIV infected cells including quiescent reservoirs • Functional Cure - < 50 copies Eradication of HIV following transplantation of CCR 5 -deficient haematopoietic stem cells Novel strategies- purging reservoirs followed by aggressive HAART

Current and Upcoming Efforts for HIV cure Study Intervention Reference (clinicaltrials. gov) Status Optiprim ANRS 147 3 vs 5 ARVs during acute infection NCT 01033760 Closed, ongoing Intens. VIH Isentress + Selzentry intensification NCT 00935480 Closed, ongoing NCT 01019551 Closed, ongoing NCT 00976404 Closed, ongoing Eramune 01 Eramune 02 IL-7 + intensifcation Vaccination + intensification Deeks Disulfiram NCT 01286259 Closed, ongoing Margolis Vorinostat (SAHA) NCT 01319383 Recruiting Lewin Vorinostat (SAHA) NCT 01365065 Recruiting Lalezari Zinc finger nuclease (ZFN) NCT 01252641 Closed, ongoing Tebas Zinc finger nuclease (ZFN) NCT 00842634 Closed, ongoing Krishnan Gene therapy/stem cell transplants in HIV lymphoma patients NCT 00569985 Recruiting Moreno Bryostatin N/A Starting soon Hatano Anti-PD 1 antibody N/A Starting soon Woolfrey Autologous HIV-resistant cells N/A Starting soon

Benefits of ART in prevention • Preventing Mother to child transmission • Post exposure prophylaxis (PEP) – Occupational – Sexual (NPEP) • Primary prevention (PREP) • Secondary prevention ( Prevention of sexual transmission of HIV-HPTN 052)

Conclusions • Global progress on scale-up of ART has been extraordinary. Countries show the way! • Decrease in morbidity and mortality • Declining incidence of HIV • Sustainability of ARVs-This will require forward-looking policies, more effective and innovative approaches, together with further investments • Prevention of transmission of resistance strains • Prevention and management of NCDs • ARVs for treatment and prevention are a powerful tool towards ending the HIV epidemic

Collaborators Brown University Charles Carpenter Timothy Flanigan Rami Kantor Susan Cu Uvin Bharat Ramratnam Karen Tashima UCSD & ACTG CTU Constance Benson Robert Schooley Davey Smith Scot Letendre Ajay Bharati Coast Tom Campbell Umesh Lalloo Fred Sattler Susan Swindels Stanford University David Katzenstein Harvard University Kirby Inst-UNSW, Sydney Kenneth Mayer David Cooper Kenneth Freedberg Mathew Law Rochele Walensky Mark Boyd Dan Kuritzkes Sean Emery Fenway Health Center Univ of W. Australia Steve Safren, Marcy Martyn French UCSF Joel Palefsky Mc. Farlane, Melbourne Suzzane Crowe Maria Ekstrand Sharon Lewin Tufts University Griffith University, Gold Christine Wanke Newell Johnson Johns Hopkins Univ Karolinska Inst, Sweden David Celentano Vinod Diwan Shruti Mehtha Rush University Univ. of Miami Alan Landay Savita Pawha Emory Univ
 Short term human resource planning
Short term human resource planning Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Short, medium and long term planning in education
Short, medium and long term planning in education Long term memory vs short term memory
Long term memory vs short term memory Difference between long term and short term liabilities
Difference between long term and short term liabilities Accounting for serial bonds
Accounting for serial bonds Examples of long term goals
Examples of long term goals Short-term financial management
Short-term financial management Short short short long long long short short short
Short short short long long long short short short Once upon a time there lived a fox
Once upon a time there lived a fox Psychodynamic and humanistic therapies have in common
Psychodynamic and humanistic therapies have in common Bioness integrated therapy system price
Bioness integrated therapy system price What are the major humanistic therapies
What are the major humanistic therapies Resource allocation vs resource leveling
Resource allocation vs resource leveling Contoh resource loading
Contoh resource loading Short-term play therapy for children
Short-term play therapy for children In my understanding
In my understanding Term-to-term rule
Term-to-term rule Position-to-term rule
Position-to-term rule Minterm and maxterm expansion
Minterm and maxterm expansion Term to term rule example
Term to term rule example Term to term rule
Term to term rule Wisconsin long term care partnership program
Wisconsin long term care partnership program Long term financing
Long term financing Grade 11 goals
Grade 11 goals Long term scheduler
Long term scheduler Gsa long term lodging
Gsa long term lodging Mts disability
Mts disability Long term impacts of the industrial revolution
Long term impacts of the industrial revolution The long-term future of particle accelerators
The long-term future of particle accelerators Long term causes of ww2
Long term causes of ww2 Long term causes of world war 2
Long term causes of world war 2 Nstu bereavement days
Nstu bereavement days Advantage of retained profit
Advantage of retained profit Long-term finance
Long-term finance Long term memory example
Long term memory example Short term goals exercise
Short term goals exercise Long-term incentive plan examples
Long-term incentive plan examples Prospective memory examples
Prospective memory examples Long term potentiation
Long term potentiation Long term storage and retrieval
Long term storage and retrieval The discount on bonds payable account
The discount on bonds payable account Notes payable journal entry
Notes payable journal entry