New Pharmacotherapy for the Treatment of Heart Failure










- Slides: 27

New Pharmacotherapy for the Treatment of Heart Failure Samer S. Najjar, MD Professor of Medicine, Georgetown University Medical Director, Advanced Heart Failure Program Med. Star Washington Hospital Center

Financial Disclosure Samer Najjar, MD Research Support Medtronic

Outline • FDA approved pharmacotherapy • Experimental pharmacotherapy

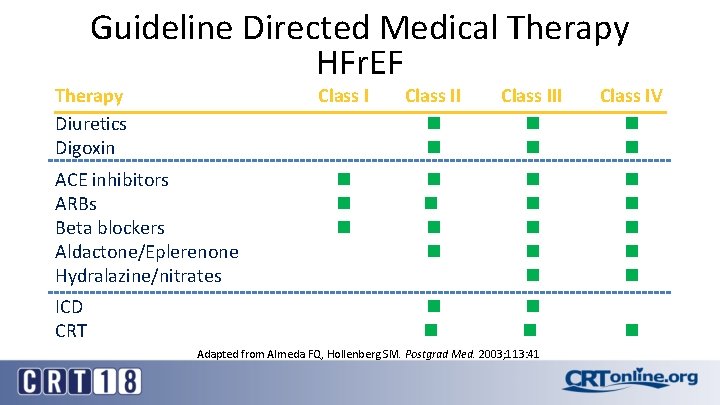
Guideline Directed Medical Therapy HFr. EF Therapy Diuretics Digoxin ACE inhibitors ARBs Beta blockers Aldactone/Eplerenone Hydralazine/nitrates ICD CRT Class III Class IV Adapted from Almeda FQ, Hollenberg SM. Postgrad Med. 2003; 113: 41

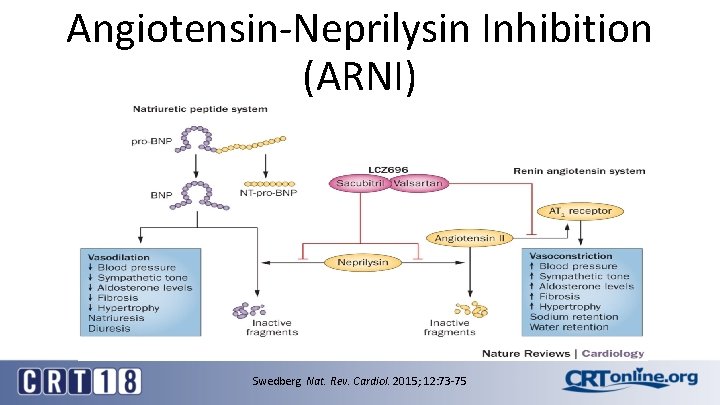
Angiotensin-Neprilysin Inhibition (ARNI) Death from CV causes or Hosp for HF Swedberg Nat. Rev. Cardiol. 2015; 12: 73 -75

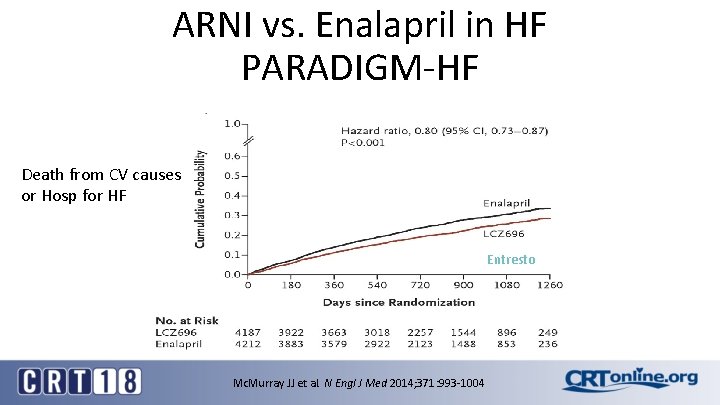
ARNI vs. Enalapril in HF PARADIGM-HF Death from CV causes or Hosp for HF Entresto Mc. Murray JJ et al. N Engl J Med 2014; 371: 993 -1004

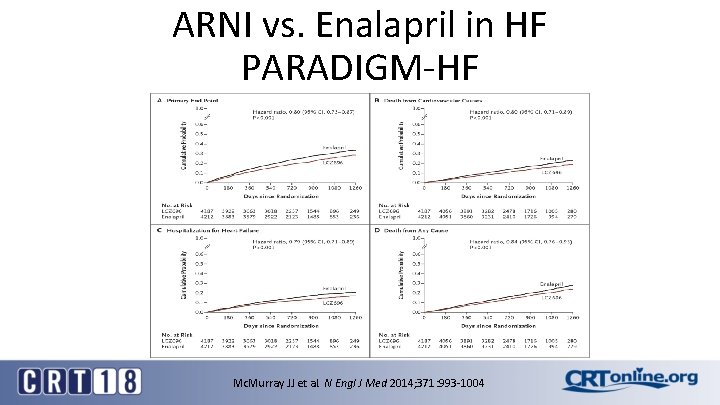
ARNI vs. Enalapril in HF PARADIGM-HF Mc. Murray JJ et al. N Engl J Med 2014; 371: 993 -1004

PARADIGM-HF Mc. Murray JJV et al. N Engl J Med 2014; 371: 993 -1004


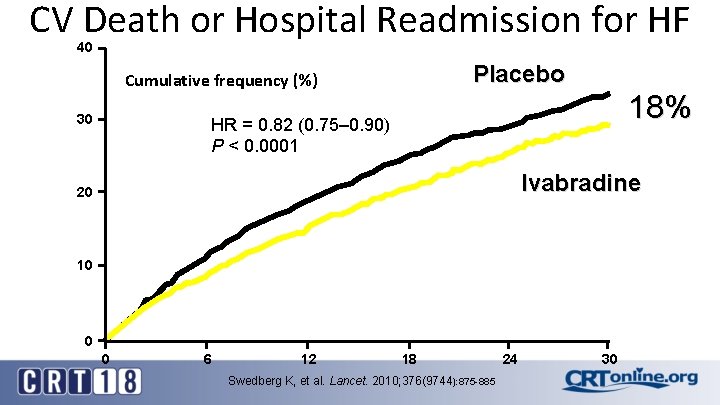
CV Death or Hospital Readmission for HF 40 Placebo Cumulative frequency (%) 30 18% HR = 0. 82 (0. 75– 0. 90) P < 0. 0001 Ivabradine 20 10 0 0 6 12 18 Swedberg K, et al. Lancet. 2010; 376(9744): 875 -885 24 30

Investigational Interventions • • Medications with known mechanisms of action Medications with novel mechanisms of action Anti-inflammatory medications Biological therapeutics – Cell based – Gene based

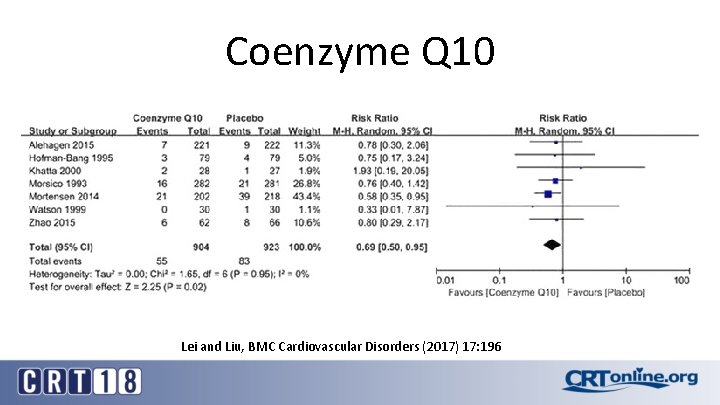
Coenzyme Q 10 Lei and Liu, BMC Cardiovascular Disorders (2017) 17: 196

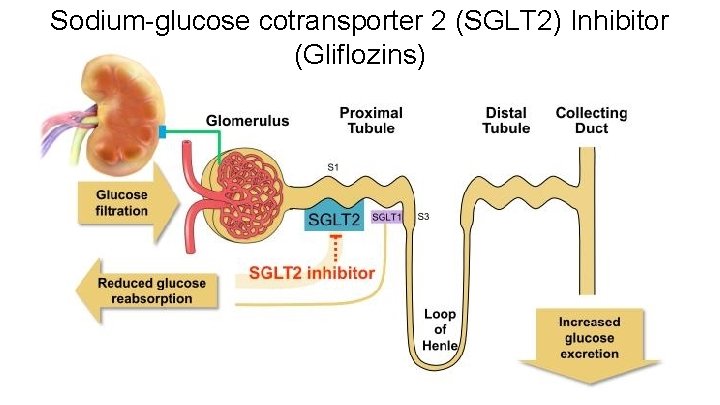
Sodium-glucose cotransporter 2 (SGLT 2) Inhibitor (Gliflozins)

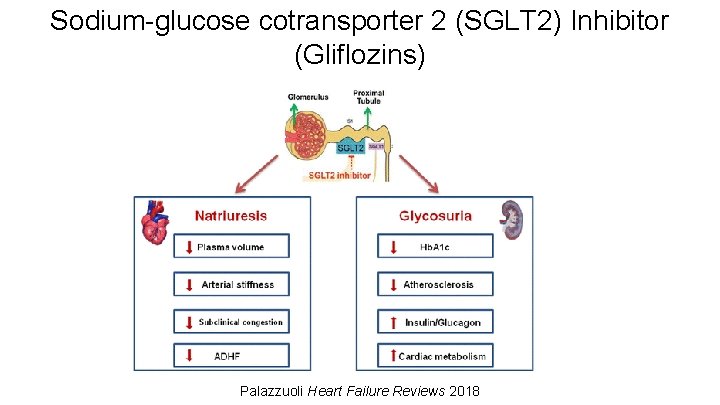
Sodium-glucose cotransporter 2 (SGLT 2) Inhibitor (Gliflozins) Palazzuoli Heart Failure Reviews 2018

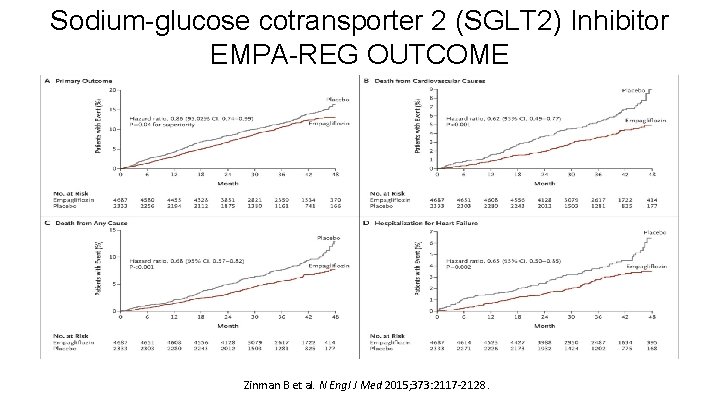
Sodium-glucose cotransporter 2 (SGLT 2) Inhibitor EMPA-REG OUTCOME Zinman B et al. N Engl J Med 2015; 373: 2117 -2128.

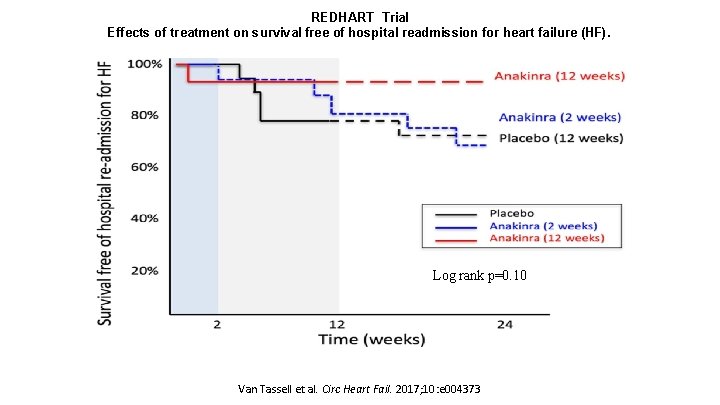
REDHART Trial Effects of treatment on survival free of hospital readmission for heart failure (HF). Log rank p=0. 10 Van Tassell et al. Circ Heart Fail. 2017; 10: e 004373

Heart Failure with preserved Ejection Fraction (HFp. EF) • PARAGON • EMPEROR HF-Preserved • D-HART 2

Biological Therapeutics in Heart Failure STEM CELLS 2017; 35(5): 1131 -1140

Ongoing Clinical Investigations • • • Ularitide Cenderitide Omecamtiv mecarbil Neuregulin-1 Perhexilin • • • Sildenafil Riociguat Sitaxentan Bendavia Finerenone


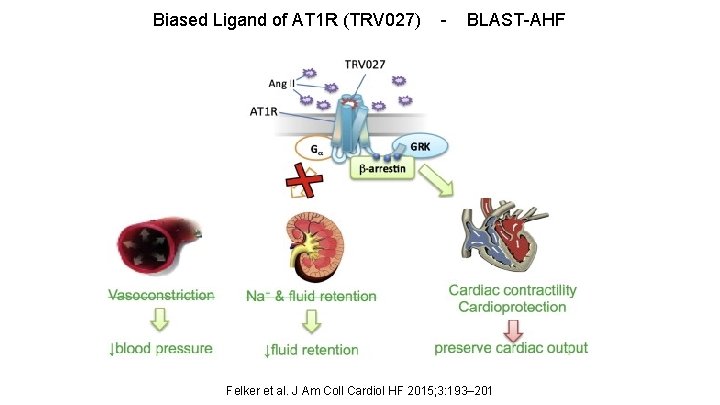
Biased Ligand of AT 1 R (TRV 027) - BLAST-AHF Felker et al. J Am Coll Cardiol HF 2015; 3: 193– 201

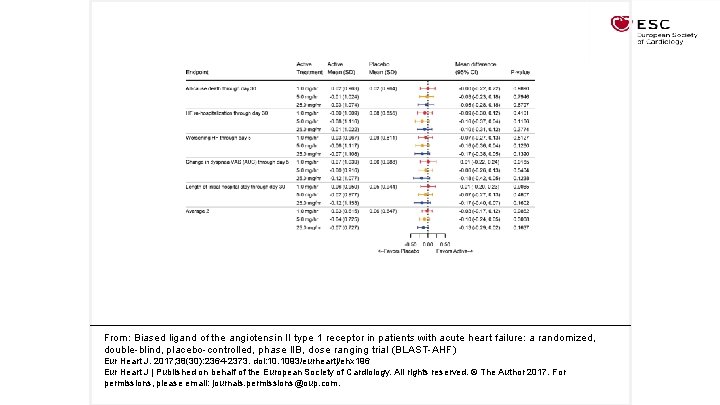
From: Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF) Eur Heart J. 2017; 38(30): 2364 -2373. doi: 10. 1093/eurheartj/ehx 196 Eur Heart J | Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2017. For permissions, please email: journals. permissions@oup. com.

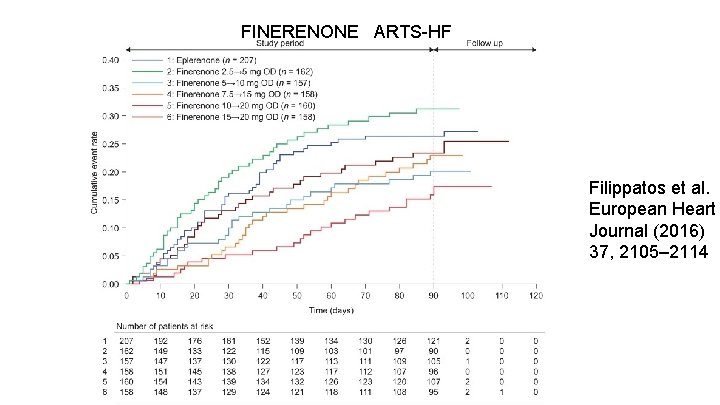
FINERENONE ARTS-HF Filippatos et al. European Heart Journal (2016) 37, 2105– 2114

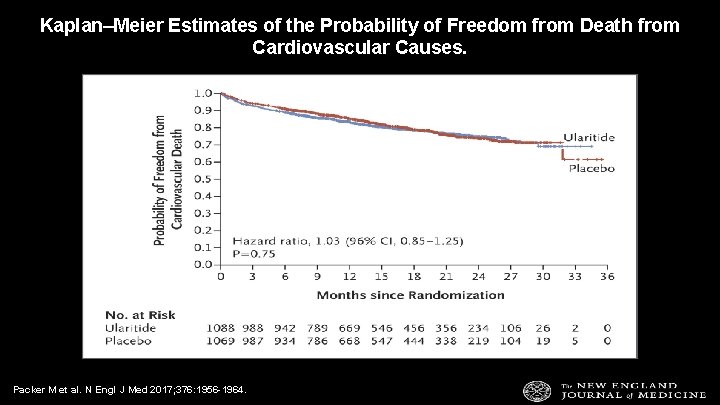
Kaplan–Meier Estimates of the Probability of Freedom from Death from Cardiovascular Causes. Packer M et al. N Engl J Med 2017; 376: 1956 -1964.

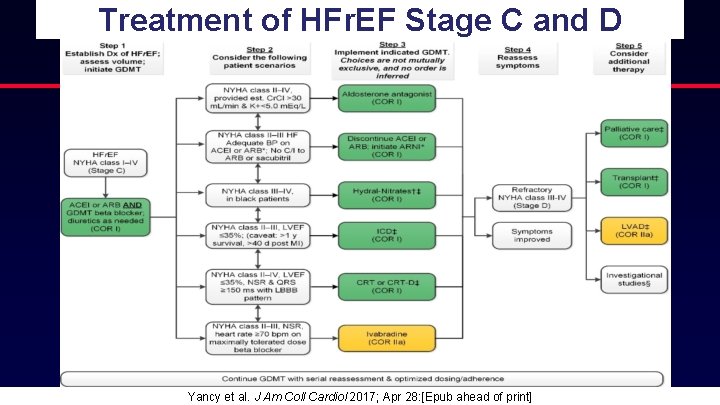
Treatment of HFr. EF Stage C and D Yancy et al. J Am Coll Cardiol 2017; Apr 28: [Epub ahead of print]

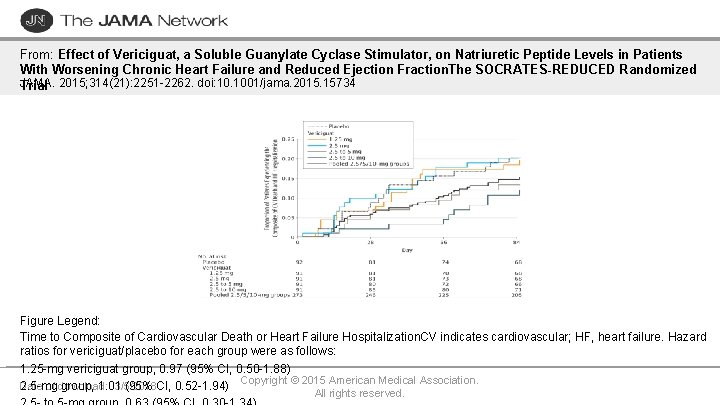
From: Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction. The SOCRATES-REDUCED Randomized JAMA. Trial 2015; 314(21): 2251 -2262. doi: 10. 1001/jama. 2015. 15734 Figure Legend: Time to Composite of Cardiovascular Death or Heart Failure Hospitalization. CV indicates cardiovascular; HF, heart failure. Hazard ratios for vericiguat/placebo for each group were as follows: 1. 25 -mg vericiguat group, 0. 97 (95% CI, 0. 50 -1. 88) Copyright © 2015 American Medical Association. 2. 5 -mg group, 1. 01 (95% CI, 0. 52 -1. 94) Date of download: 3/5/2018 All rights reserved.

 Pharmacotherapy
Pharmacotherapy Pharmacotherapy
Pharmacotherapy Pharmacotherapy workup
Pharmacotherapy workup Causes of cardiomegaly in child
Causes of cardiomegaly in child Failure to sense
Failure to sense Failure to pace
Failure to pace Cup and cone fracture occurs in
Cup and cone fracture occurs in Forrester classification heart failure
Forrester classification heart failure Heart failure definition
Heart failure definition Heart failure defined
Heart failure defined Nursing assessment for congestive heart failure
Nursing assessment for congestive heart failure Nursing assessment for congestive heart failure
Nursing assessment for congestive heart failure Heart failure complications
Heart failure complications Chapter 24 heart failure drugs
Chapter 24 heart failure drugs Heart failure
Heart failure Congestive heart failure zones for management
Congestive heart failure zones for management Lmnop heart failure
Lmnop heart failure Keith rn heart failure case study
Keith rn heart failure case study Donkey analogy heart failure
Donkey analogy heart failure Cor pulmonale
Cor pulmonale Heart failure cells are seen in lungs
Heart failure cells are seen in lungs Pvkov
Pvkov Chlorpromide
Chlorpromide Diabetes and heart failure
Diabetes and heart failure Compensatory mechanisms of heart failure
Compensatory mechanisms of heart failure Electrical conduction system of the heart
Electrical conduction system of the heart Acute vs chronic heart failure
Acute vs chronic heart failure Right vs left-sided heart failure chart
Right vs left-sided heart failure chart