Naming and Writing Ionic Compounds Ionic Compounds Occur




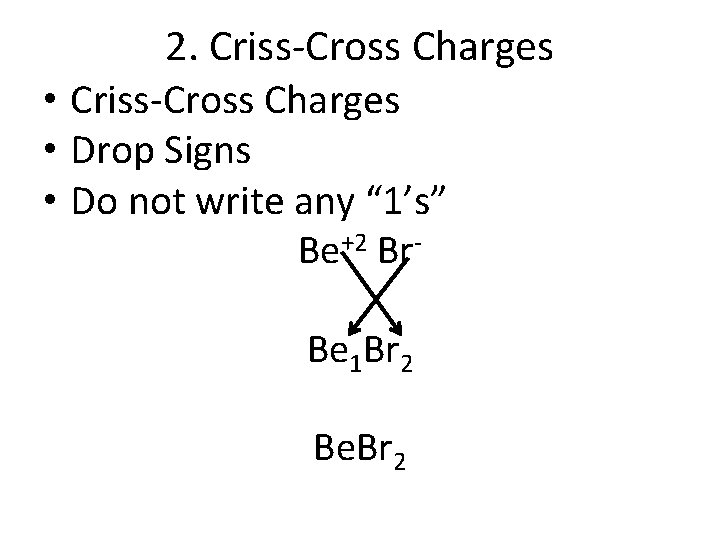







- Slides: 28

Naming and Writing Ionic Compounds

Ionic Compounds • Occur between a metal and non-metal • Occur when electrons are transferred between atoms forming ions • Metals make cations = positive ions • Nonmetals make anions = negative ions • Also use these rules when a polyatomic ion is present

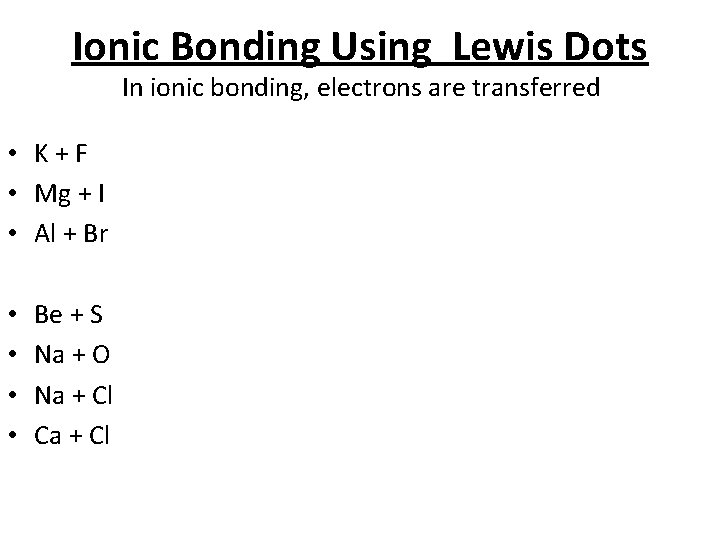
Ionic Bonding Using Lewis Dots In ionic bonding, electrons are transferred • K+F • Mg + I • Al + Br • • Be + S Na + O Na + Cl Ca + Cl

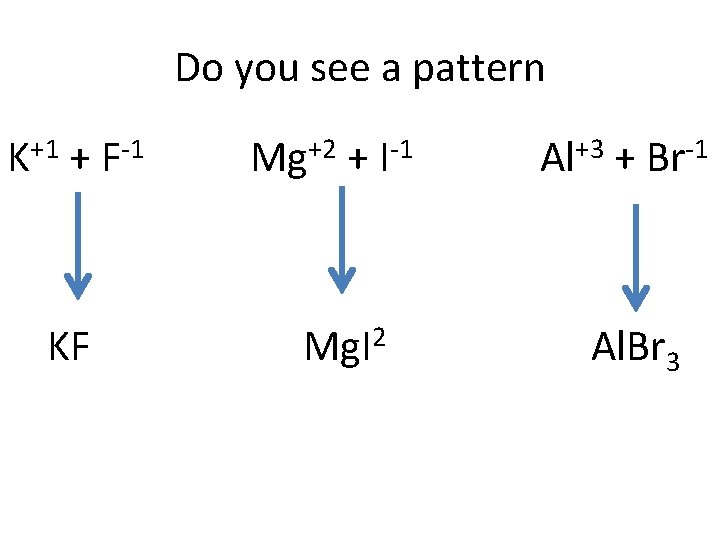
Do you see a pattern K+1 + F-1 Mg+2 + I-1 Al+3 + Br-1 KF Mg. I 2 Al. Br 3

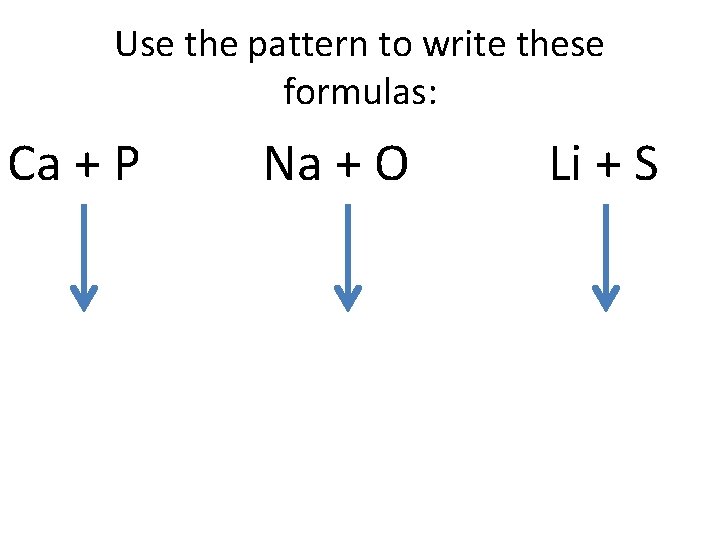
Use the pattern to write these formulas: Ca + P Na + O Li + S

Writing Ionic Compounds

Guide to Writing Formulas from the Name Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 7

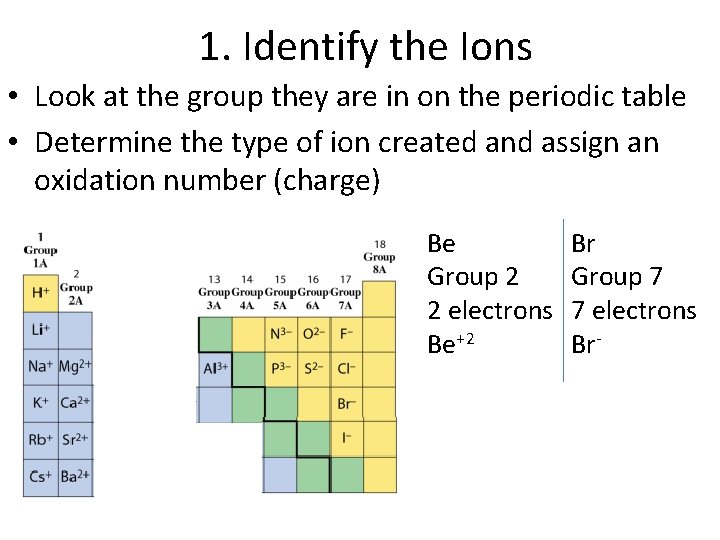
1. Identify the Ions • Look at the group they are in on the periodic table • Determine the type of ion created and assign an oxidation number (charge) Be Group 2 2 electrons Be+2 Br Group 7 7 electrons Br-
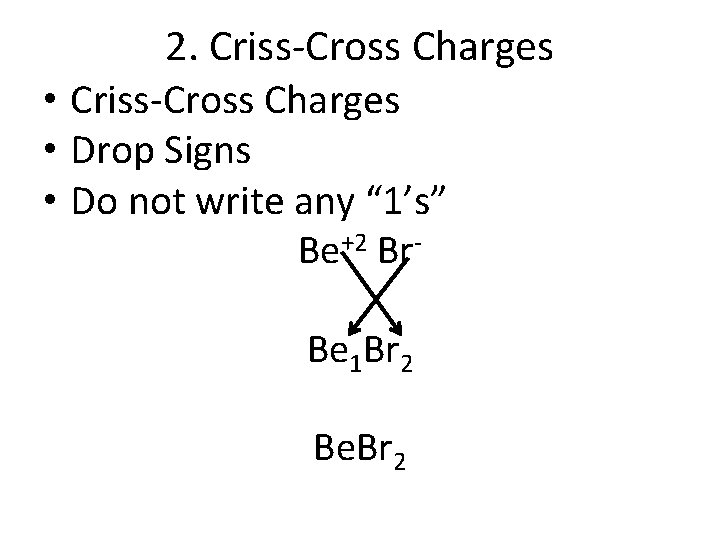
2. Criss-Cross Charges • Drop Signs • Do not write any “ 1’s” Be+2 Br. Be 1 Br 2 Be. Br 2

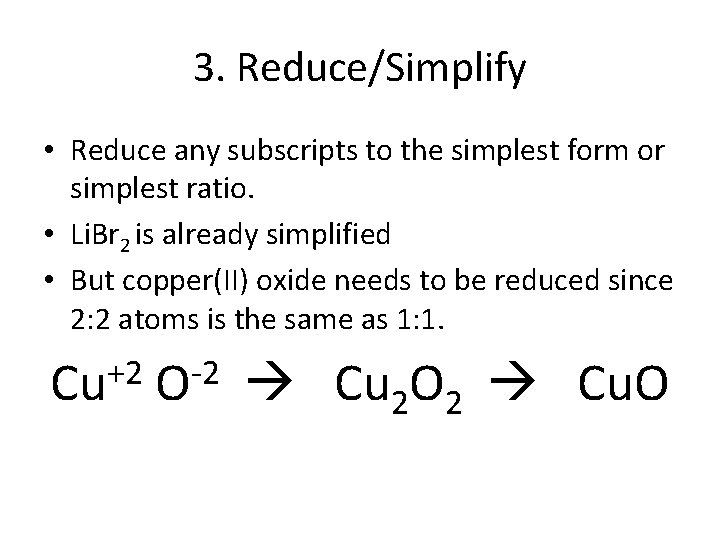
3. Reduce/Simplify • Reduce any subscripts to the simplest form or simplest ratio. • Li. Br 2 is already simplified • But copper(II) oxide needs to be reduced since 2: 2 atoms is the same as 1: 1. +2 Cu -2 O Cu 2 O 2 Cu. O

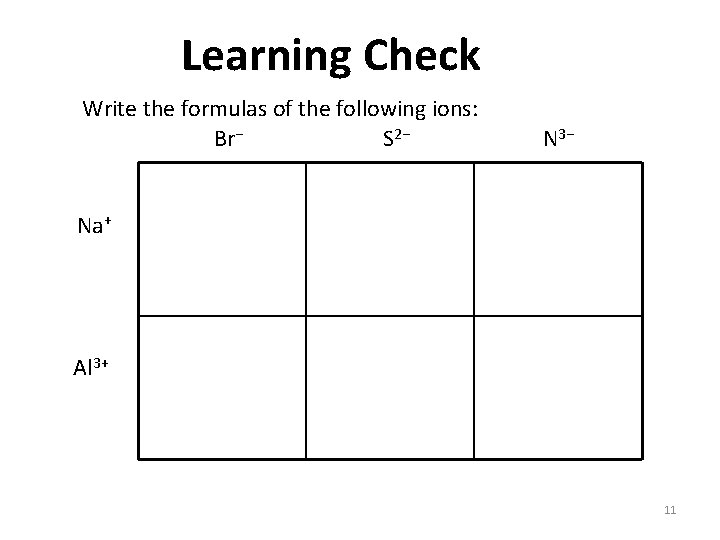
Learning Check Write the formulas of the following ions: Br− S 2− N 3− Na+ Al 3+ 11

Naming Ionic Compounds

Naming Ionic Compounds with Two Elements

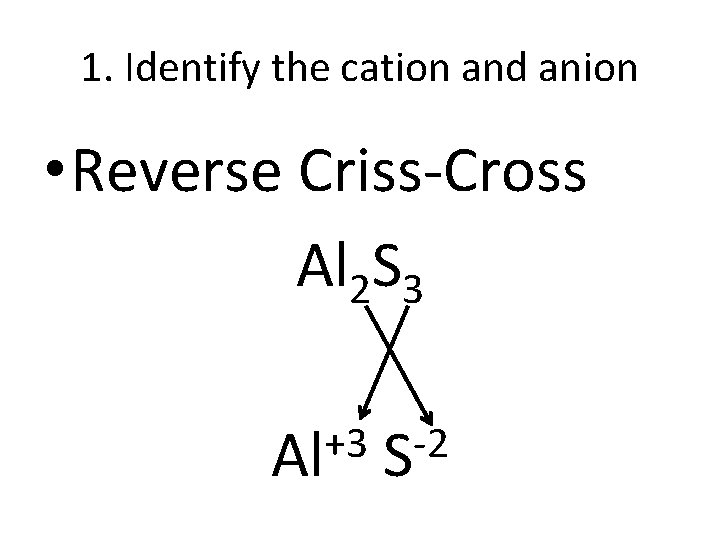
1. Identify the cation and anion • Reverse Criss-Cross Al 2 S 3 +3 Al -2 S

2. Name the Cation Al+3 S-2 • The cation is the positive ion • Use its element name • Al+3 = Aluminum

3. Name the Anion Al+3 S-2 • The anion is the negative ion • Use the element name, but change the last part to -ide -2 • S = Sulfur Sulfide

4. Put it together • Cation name is first • Anion is second • Al 2 S 3 +3 -2 • Al S • Aluminum Sulfide

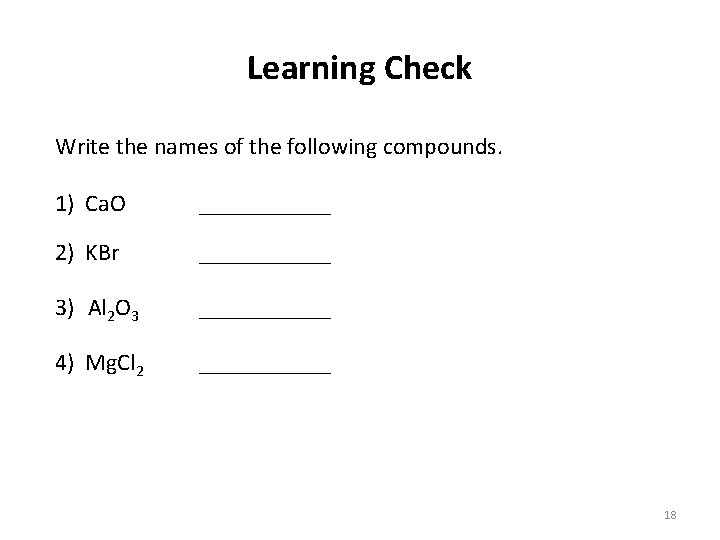
Learning Check Write the names of the following compounds. 1) Ca. O ______ 2) KBr ______ 3) Al 2 O 3 ______ 4) Mg. Cl 2 ______ 18

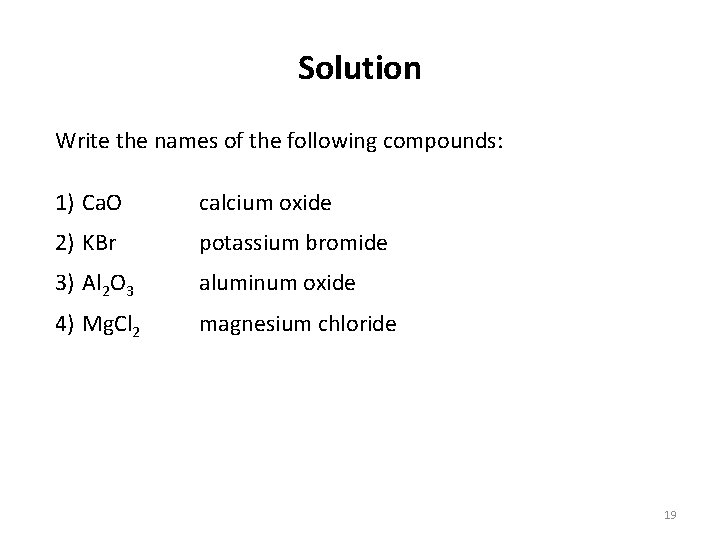
Solution Write the names of the following compounds: 1) Ca. O calcium oxide 2) KBr potassium bromide 3) Al 2 O 3 aluminum oxide 4) Mg. Cl 2 magnesium chloride 19

Other Things to Watch Out for….

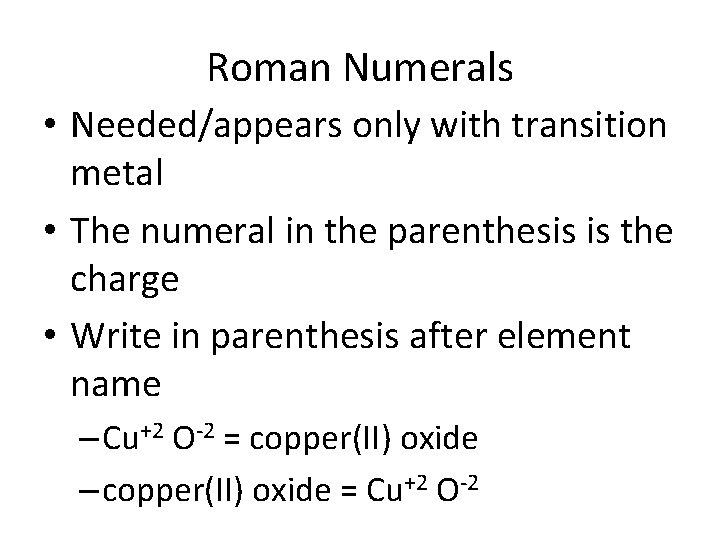
Roman Numerals • Needed/appears only with transition metal • The numeral in the parenthesis is the charge • Write in parenthesis after element name – Cu+2 O-2 = copper(II) oxide – copper(II) oxide = Cu+2 O-2

Polyatomic Ions • When a group of atoms acts like one ion • Use the same rules as other ions when writing: • Place entire polyatomic ion in parenthesis when criss-crossing – Use cheat sheet for their charges instead of a periodic table • Use name on cheat sheet when naming

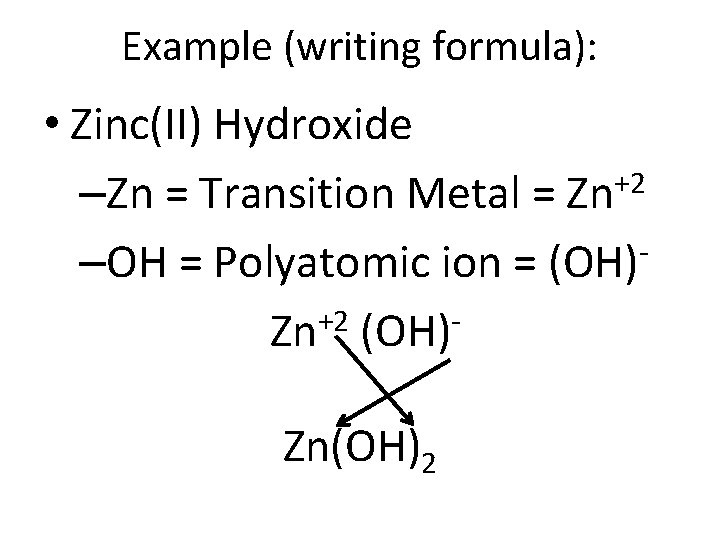
Example (writing formula): • Zinc(II) Hydroxide –Zn = Transition Metal = Zn+2 –OH = Polyatomic ion = (OH) +2 Zn (OH) Zn(OH)2

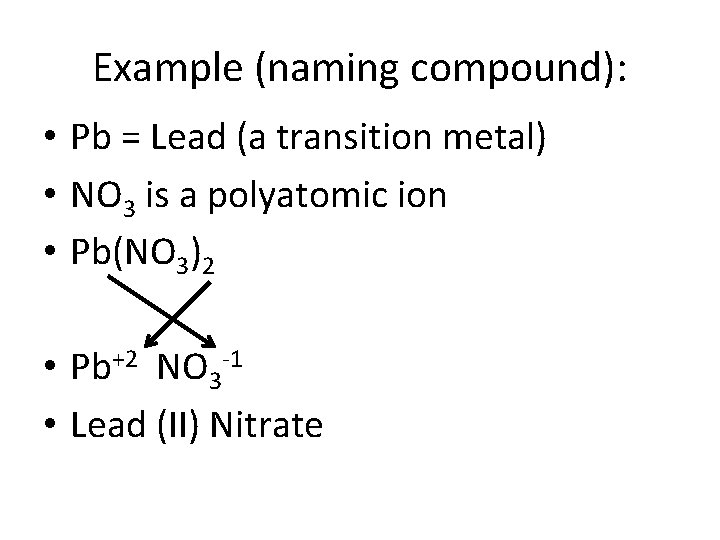
Example (naming compound): • Pb = Lead (a transition metal) • NO 3 is a polyatomic ion • Pb(NO 3)2 • Pb+2 NO 3 -1 • Lead (II) Nitrate

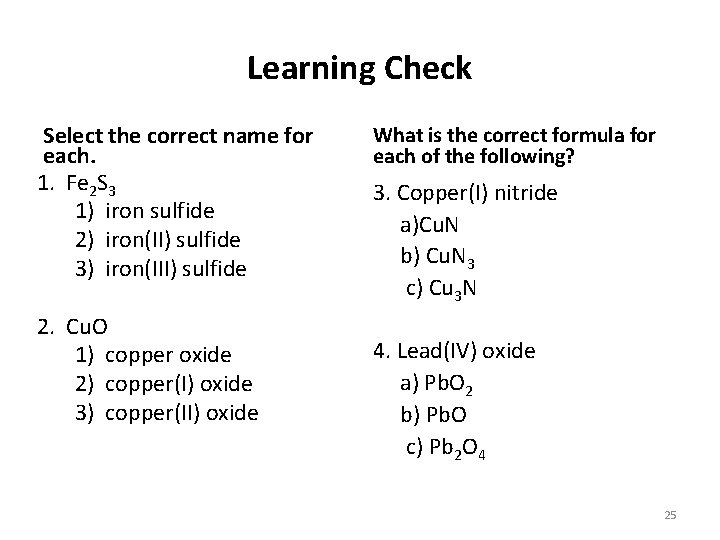
Learning Check Select the correct name for each. 1. Fe 2 S 3 1) iron sulfide 2) iron(II) sulfide 3) iron(III) sulfide 2. Cu. O 1) copper oxide 2) copper(I) oxide 3) copper(II) oxide What is the correct formula for each of the following? 3. Copper(I) nitride a)Cu. N b) Cu. N 3 c) Cu 3 N 4. Lead(IV) oxide a) Pb. O 2 b) Pb. O c) Pb 2 O 4 25

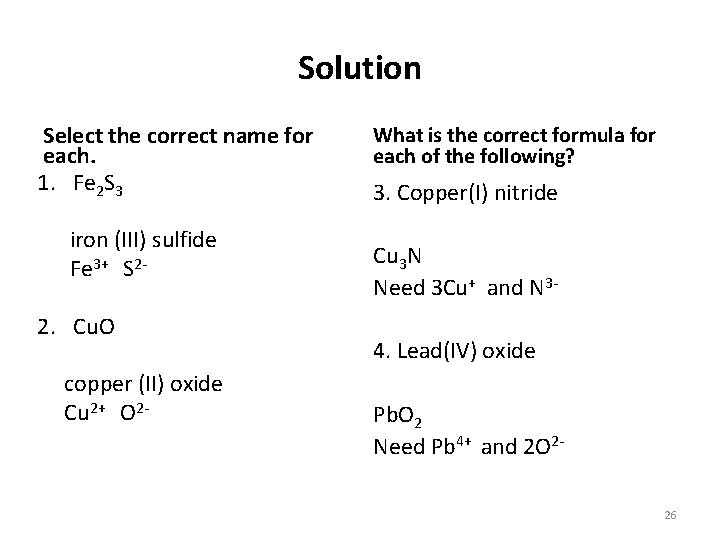
Solution Select the correct name for each. 1. Fe 2 S 3 iron (III) sulfide Fe 3+ S 2 - 2. Cu. O copper (II) oxide Cu 2+ O 2 - What is the correct formula for each of the following? 3. Copper(I) nitride Cu 3 N Need 3 Cu+ and N 34. Lead(IV) oxide Pb. O 2 Need Pb 4+ and 2 O 226

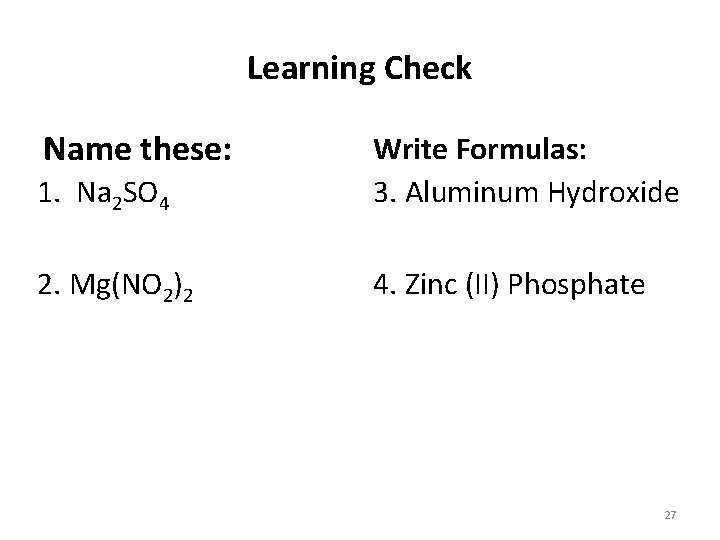
Learning Check Name these: 1. Na 2 SO 4 Write Formulas: 3. Aluminum Hydroxide 2. Mg(NO 2)2 4. Zinc (II) Phosphate 27

Solution Name these: 1. Na 2 SO 4 Sodium Sulfate 2. Mg(NO 2)2 Magnesium Nitrite Write Formulas: 3. Aluminum Hydroxide Al+3 OH-1 Al (OH)3 4. Zinc (II) Phosphate Zn+2 (PO 4)-3 Zn 3(PO 4)2 28