Naming Ionic Compounds Ionic Compounds in water l







- Slides: 15


Naming Ionic Compounds

Ionic Compounds in water l Water’s formula is H 2 O. l Take a look at pg 189

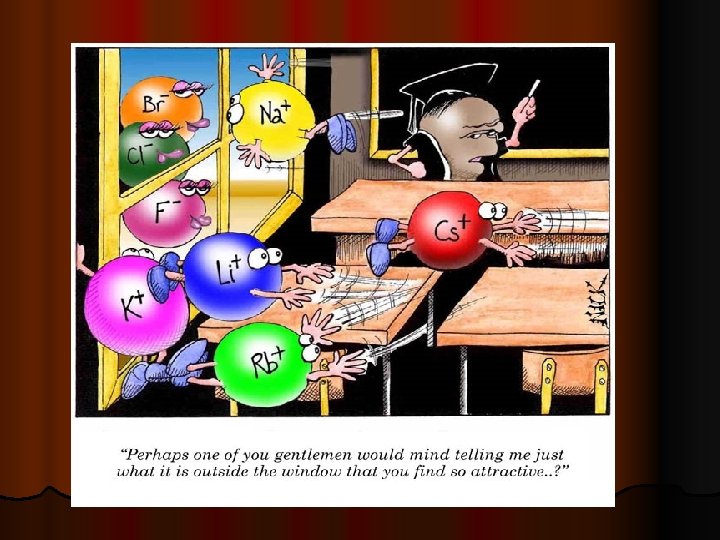
Ionic Compounds l Positive Ion is called a CATION. They are metals. l Negative Ion is called an ANION. They are nonmetals.

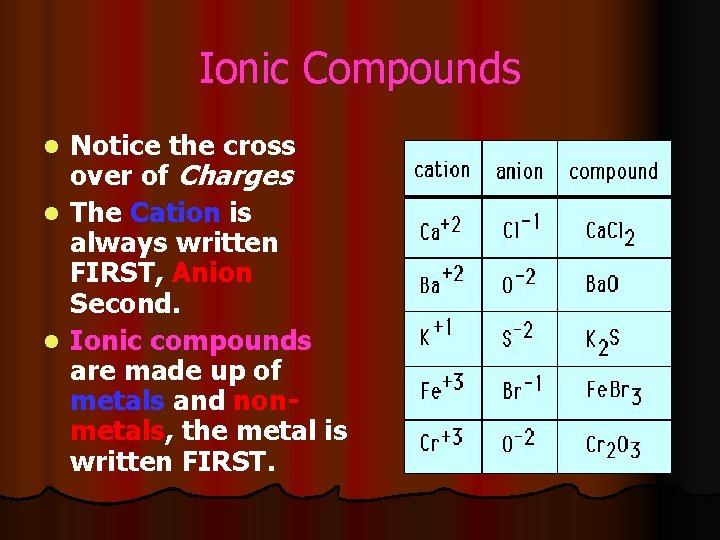
Ionic Compounds Notice the cross over of Charges l The Cation is always written FIRST, Anion Second. l Ionic compounds are made up of metals and nonmetals, the metal is written FIRST. l

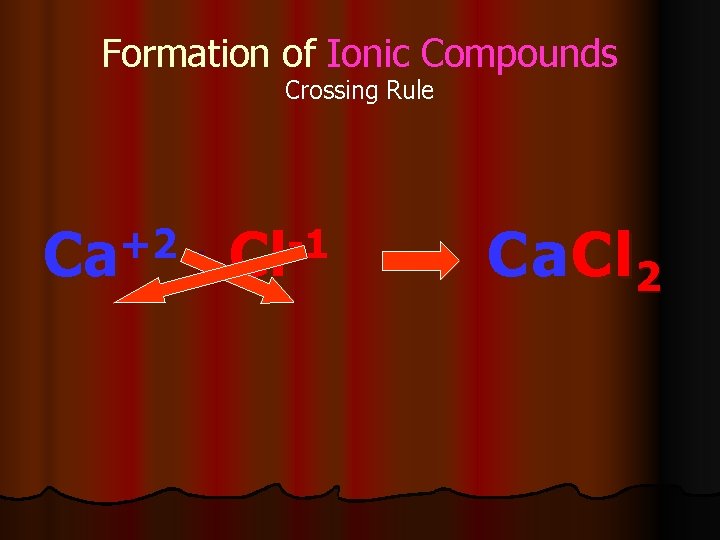
Formation of Ionic Compounds Crossing Rule +2 -1 Ca + Cl Ca. Cl 2

Naming Rules l Step 1: The name of the metal is written first, the same way it is written on the periodic table l Step 2: Write the name of non-metal second, BUT change the ending of the element to –ide. l Step 3: Remember the crossing rule and remember to reduce!

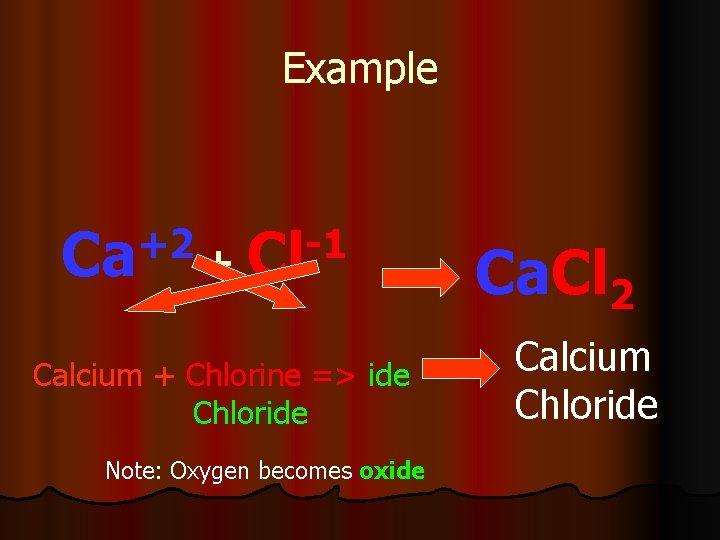
Example +2 -1 Ca + Cl Calcium + Chlorine => ide Chloride Note: Oxygen becomes oxide Ca. Cl 2 Calcium Chloride

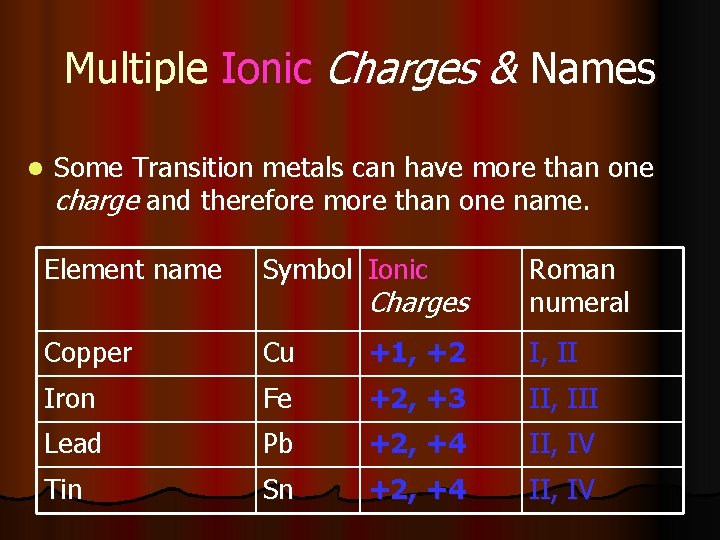
Multiple Ionic Charges & Names l Some Transition metals can have more than one charge and therefore more than one name. Element name Symbol Ionic Charges Roman numeral Copper Cu +1, +2 I, II Iron Fe +2, +3 II, III Lead Pb +2, +4 II, IV Tin Sn +2, +4 II, IV

When writing the names l Write the name of the element followed by the Roman numeral. l EX: Cu 2+ is written Copper(II) l So, what is the name of Fe 3+ ? l How would you write the name of Pb. Cl 4?

Polyatomic Compounds

What is a polyatomic ion? l Polyatomic ions: groups of atoms that tend to stay together and carry an overall ionic charge. See pg 196 -197

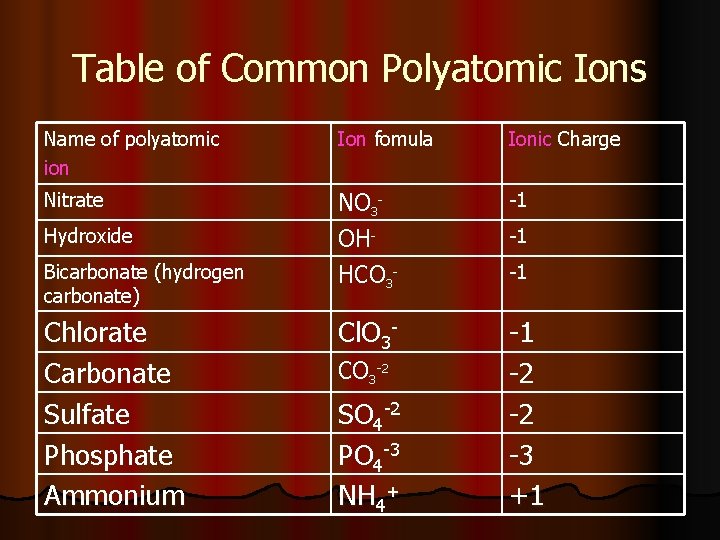
Table of Common Polyatomic Ions Name of polyatomic ion Ion fomula Ionic Charge Nitrate NO 3 OH- -1 Bicarbonate (hydrogen carbonate) HCO 3 - -1 Chlorate Carbonate Sulfate Phosphate Ammonium Cl. O 3 - -1 -2 -2 -3 +1 Hydroxide CO 3 -2 SO 4 -2 PO 4 -3 NH 4+ -1

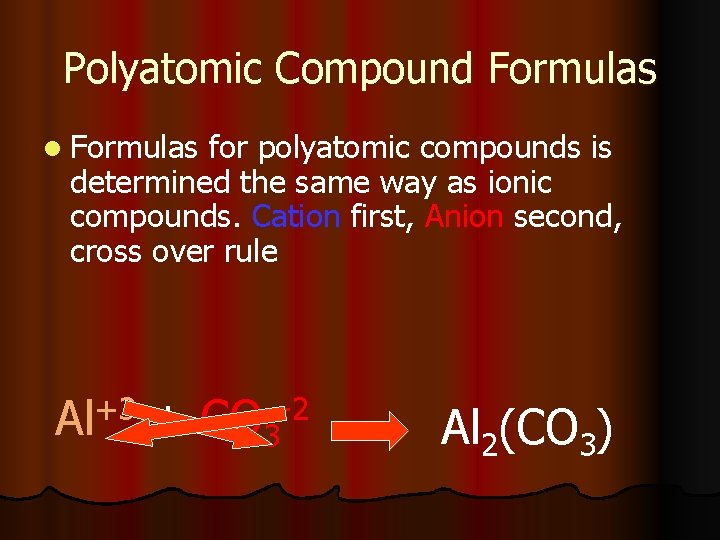
Polyatomic Compound Formulas l Formulas for polyatomic compounds is determined the same way as ionic compounds. Cation first, Anion second, cross over rule Al+3 + CO 3 -2 Al 2(CO 3)

Naming Polyatomic Compounds l Step 1: Write the name of the metal first. l Step 2: Write the name of the polyatomic ion second. l Step 3: Smile, you’re done!