METALS WARM UP LIST FIVE METALS THAT YOU

































- Slides: 42

METALS

WARM UP LIST FIVE METALS THAT YOU KNOW OF AND A USE FOR EACH! 1. 2. 3. 4. 5.

Properties of Metals • What is a metal? What are some words we can use to describe metals? • Chemists classify an element as a metal based on its properties (what it’s made of). • Look at the periodic table…. All of the Purple Squares to the left of the zig zag line are metals. • There are two properties of metals (1) Physical and (2) Chemical

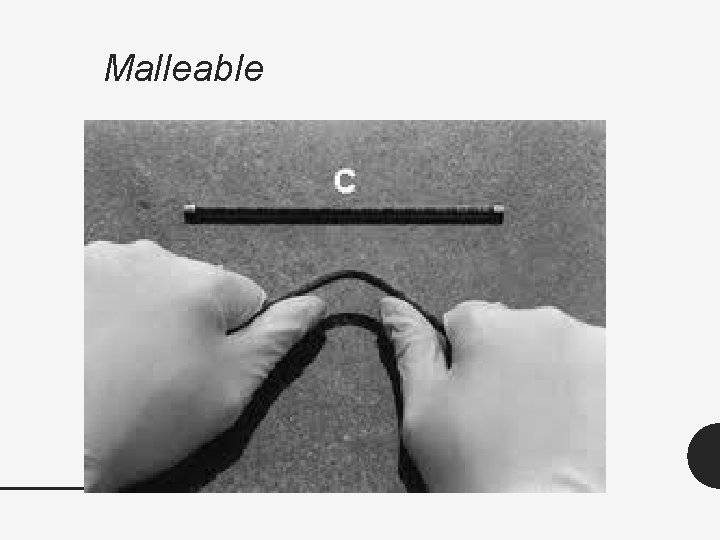
Physical Properties of Metals • The physical properties of metals include shininess, malleability, ductility, and conductivity. • Malleable – material that can be hammered or rolled into flat sheets and other shapes. • Ductile – material that can be pulled out, or drawn into long wire. • Conductivity – ability of an object to transfer heat or electricity to another object. • Physical Properties – physical change done to an object.

Malleable

Conductor?

• Most metals are good conductors. • Iron(Fe), cobalt (Co) and nickel (Ni) are attracted to magnets and can be made into magnets. • Most metals are also solids at room temperature. • Only one metal – Mercury (Hg) – is liquid at room temperature.

Chemical Properties of Metals • The ease and speed with which an element combines, or reacts, with other elements and compounds is called its reactivity. • For example, an iron chain rusts after it has had a chemical reaction with air and water. • Metals usually react by losing electrons to other atoms. • Some metals such as the chain are highly reactive. • Some metals such as gold (Au) or platinum (Pt) are valued for their lack of reactivity because those types of metals are rare.

• The reactivity of most metals fall between the reactivity of gold (not reactive) and sodium(highly reactive). • Iron, reacts slowly with oxygen in the air and form iron oxide (rust). • This destruction of metal through this process is called corrosion.

Rust

















Concept Check Point • What are three physical properties of metals? 1. 2. 3.

Metals in the Periodic Table • The metals in a group, or family, have similar properties, and these family properties change gradually as you move across the table. • The reactivity of metals tends to decrease as you move from left to right across the periodic table.

Alkali Metals • The metals in Group 1 from Lithium (Li) down to Francium (Fr) are called alkali metals. • Alkali metals react with other elements by losing an electron • These metals are so reactive that they are never found as uncombined elements in nature. They are found only in compounds. • The two most important Alkali Metals are Sodium (Na) and Potassium (K). Sodium is found in large amounts in salt water and salt beds. Your diet includes foods that contain compounds of sodium and potassium. • Lithium is an alkali metal used in batteries and some medicines.



Alkali Earth Metals • Group 2 of the periodic table contains the Alkali Earth Metals • Each is a fairly hard, gray-white, and a good conductor of electricity. • Alkaline earth metals react by losing two electrons. • They are not as reactive as elements in Group 1 but they are more reactive than most other metals. • They are never found uncombined in nature like Group 1.

• The two most common are Calcium and Magnesium. Mixing Magnesium and Aluminum makes a lightweight but durable material used on airplanes, ladders, and car wheels. • Calcium is essential for teeth, bones, and making sure muscles work properly. You get Calcium from milk and other dairy products.

Transition Metals • Groups 3 – 12. • The transition metals include most of the familiar metals such as iron, copper, nickel, silver, and gold. Most are hard and shiny and all are good conductors of electricity. • They are less reactive than those in Group 1 and 2. • This lack of reactivity is the reason ancient gold coins and jewelry are as beautiful today as they were thousands of years ago.


Metals in Mixed Groups • Only some elements in Groups 13 -15 of the periodic table are metals. • They are next to the zig zag on the chart. • These are not as reactive as those to the left of the table. • The most familiar ones are aluminum, tin, and lead.

Lanthanides • Two rows of elements are placed below the main part of the periodic table. • The elements in the top row are called lanthanides. • They are soft, malleable, shiny metals with high conductivity. • They are common metals to make alloys (a mixture of a metal with another metal) because they are so soft.

Actinides • The elements below lanthanides are called actinides. • Of all these elements only Actinium (Ac), Thorium (Th), Protactinium (Pa), and Uranium (U) occur naturally on Earth. • Uranium is used to produce energy in nuclear power plants. • The nuclei of these elements are very unstable and break apart quickly into smaller nuclei.

Synthetic Elements • Elements with an atomic number higher than 92 are called Synthetic Elements because they are not found naturally on Earth. • Elements that follow Uranium are made – or Synthesized – when nuclear particles are forced to crash into one another. • To make heavier elements (atomic numbers above 95), scientists use particle accelerators.

Particle Accelerators • Particle accelerators move the atomic nuclei faster and faster until they reach high speeds. • When they reach these high speeds, they crash into one another and combine into a single nucleus. • Curium (Cm) was the first synthetic element made by colliding nuclei. • The difficulty to synthesize new elements increases with atomic number. • New elements are created as newer particle accelerators are built. • In 1996, German scientists synthesized zinc nuclei and lead nuclei and created Element 112. The official names and symbols are agreed upon will be decided by future scientists.

