Matter Chapter Eleven Temperature Heat and the Phases










- Slides: 21


Matter

Chapter Eleven: Temperature, Heat and the Phases of Matter • 11. 1 Temperature and the Phases of Matter • 11. 2 Heat

Investigation 11 B The Phases of Matter • How do the mass, volume, and densities of solid, liquid, and gas compare?

11. 2 What is heat? • Heat is thermal energy that is moving. • Heat flows any time there is a difference in temperature. • Because your hand has more thermal energy than the chocolate, thermal energy flows from your hand to the chocolate and the chocolate begins to melt.

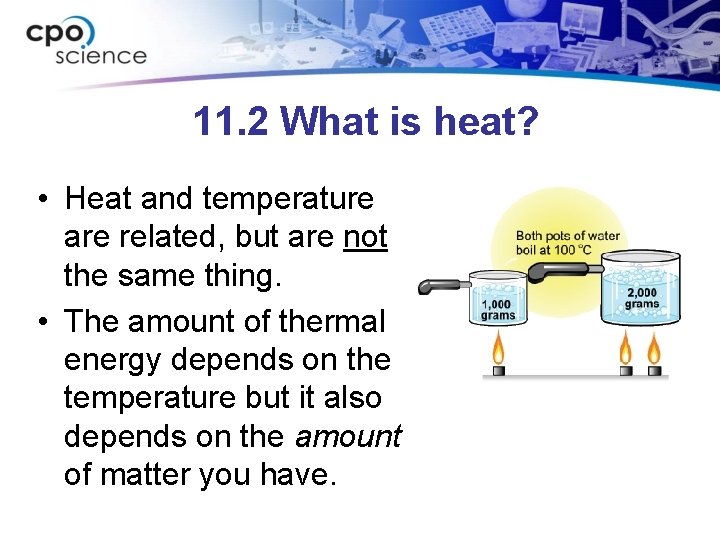
11. 2 What is heat? • Heat and temperature are related, but are not the same thing. • The amount of thermal energy depends on the temperature but it also depends on the amount of matter you have.

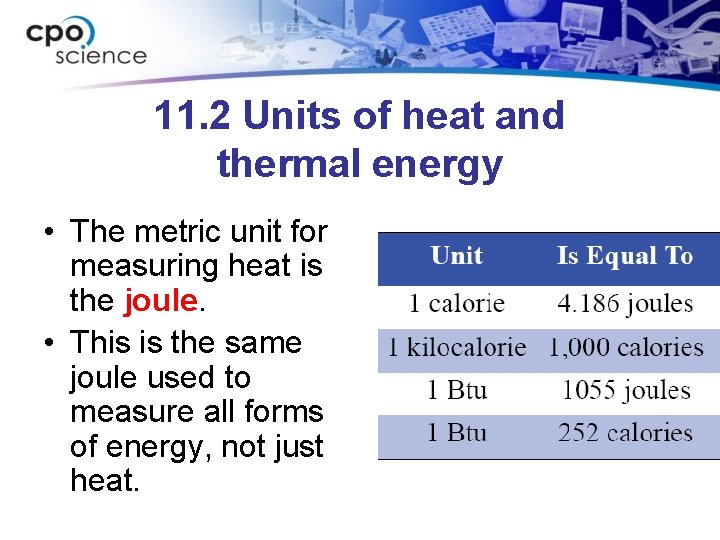
11. 2 Units of heat and thermal energy • The metric unit for measuring heat is the joule. • This is the same joule used to measure all forms of energy, not just heat.

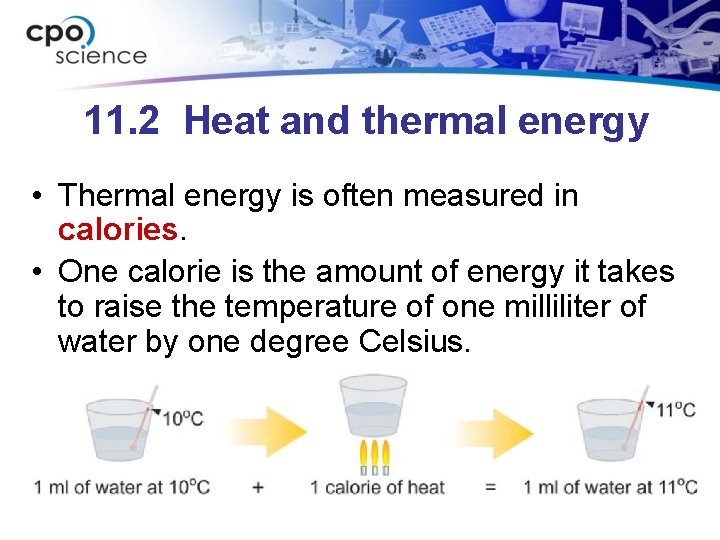
11. 2 Heat and thermal energy • Thermal energy is often measured in calories. • One calorie is the amount of energy it takes to raise the temperature of one milliliter of water by one degree Celsius.

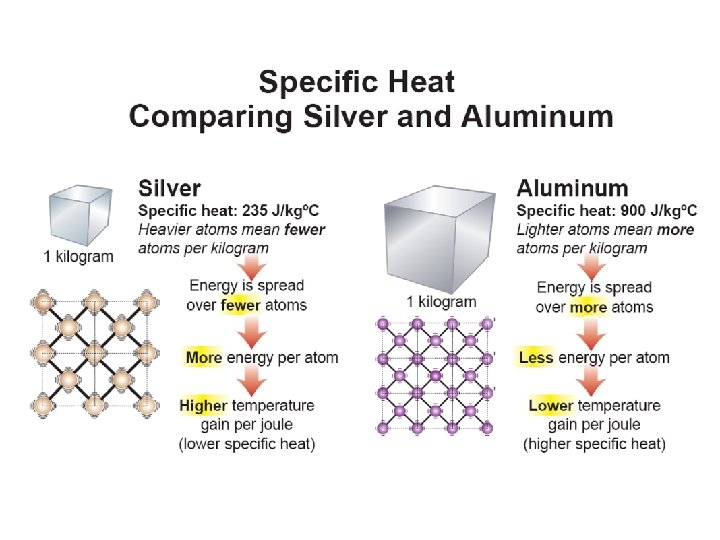
11. 2 Specific heat • The specific heat is a property of a substance that tells us how much heat is needed to raise the temperature of one kilogram of a material by one degree Celsius. Knowing the specific heat of a material tells you how quickly the temperature will change as it gains or loses energy.

11. 2 Why is specific heat different for different materials? • Temperature measures the average kinetic energy per particle. • Energy that is divided between fewer particles means more energy per particle, and therefore more temperature change. • In general, materials made up of heavy atoms or molecules have low specific heat compared with materials made up of lighter ones.


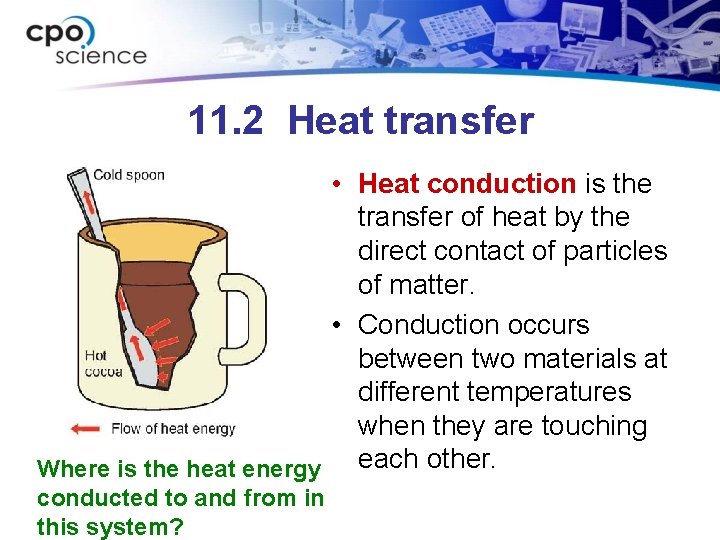
11. 2 Heat transfer • Heat conduction is the transfer of heat by the direct contact of particles of matter. • Conduction occurs between two materials at different temperatures when they are touching each other. Where is the heat energy conducted to and from in this system?

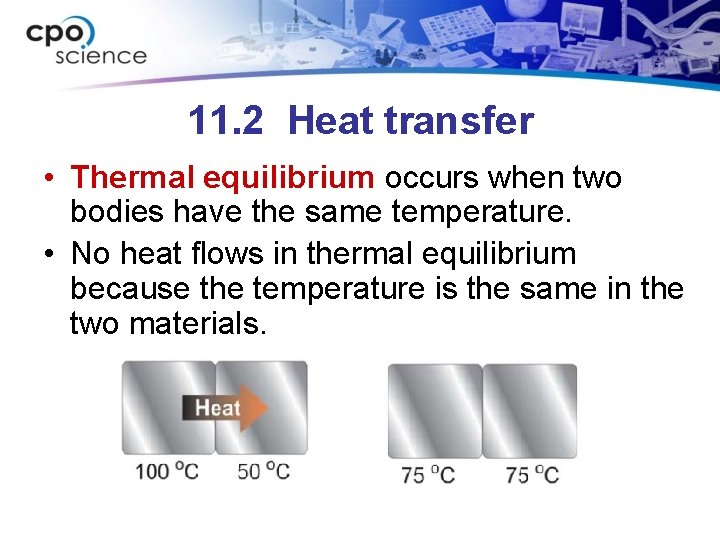
11. 2 Heat transfer • Thermal equilibrium occurs when two bodies have the same temperature. • No heat flows in thermal equilibrium because the temperature is the same in the two materials.

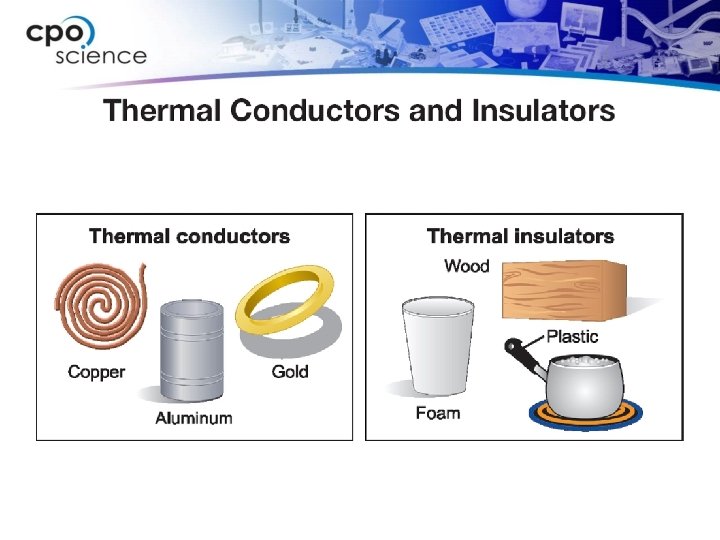
11. 2 Thermal conductors and insulators • Materials that conduct heat easily are called thermal conductors and those that conduct heat poorly are called thermal insulators. Is a down coat a conductor or an insulator?


11. 2 Convection • Convection is the transfer of heat through the motion of matter such as air and water. • In a container, warmer fluid rises to the top and cooler fluid sinks to the bottom. • This is called natural convection.

11. 2 Convection • Convection is mainly what distributes heat throughout a room.

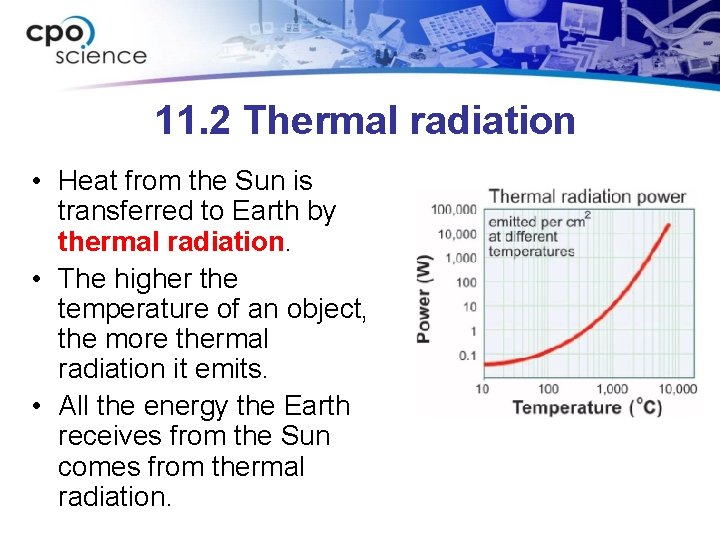
11. 2 Thermal radiation • Heat from the Sun is transferred to Earth by thermal radiation. • The higher the temperature of an object, the more thermal radiation it emits. • All the energy the Earth receives from the Sun comes from thermal radiation.

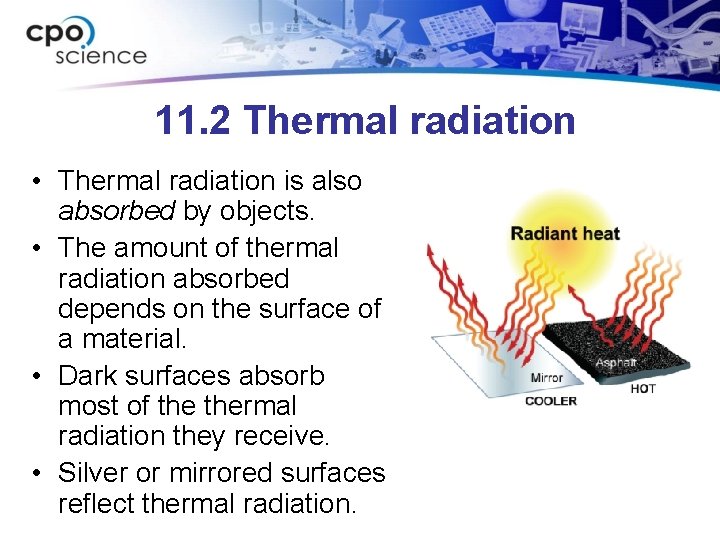
11. 2 Thermal radiation • Thermal radiation is also absorbed by objects. • The amount of thermal radiation absorbed depends on the surface of a material. • Dark surfaces absorb most of thermal radiation they receive. • Silver or mirrored surfaces reflect thermal radiation.

Technology Connection Extraordinary Materials • Many materials you are familiar with were discovered accidentally. • Other materials were created in a laboratory with a particular use in mind.

Activity Magical Ice Cream Topping • Have you ever put a “shell” topping on a frozen dessert like ice cream? • It is a liquid in the plastic bottle, but when you put it on ice cream, it hardens very quickly.