HEAT Chapter Eleven Heat 11 1 Heat 11






















- Slides: 31

HEAT

Chapter Eleven: Heat Ø 11. 1 Heat Ø 11. 2 Heat Transfer

Chapter 11. 1 Learning Goals ØDescribe the relationship between heat, temperature, and thermal energy. ØIdentify and use different units to measure heat. ØExplain how the specific heat of different materials can be used to describe changes in temperature and energy.

Investigation 11 A Temperature and Heat ØKey Question: How are temperature and heat related?

11. 1 What is heat? ØHeat is thermal energy that is moving. ØHeat flows any time there is a difference in temperature. ØBecause your hand has more thermal energy than chocolate, thermal energy flows from your hand to the chocolate and the chocolate begins to melt.

11. 1 What is heat? ØHeat and temperature are related, but are not the same thing. ØThe amount of thermal energy depends on the temperature but it also depends on the amount of matter you have.

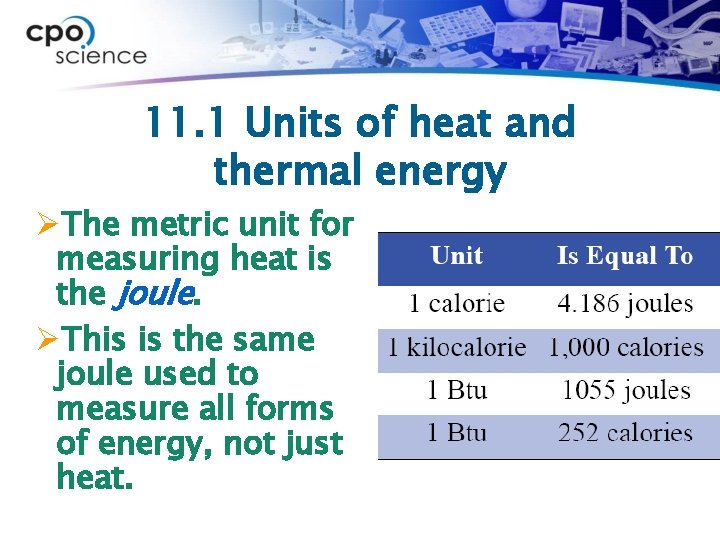
11. 1 Units of heat and thermal energy ØThe metric unit for measuring heat is the joule. ØThis is the same joule used to measure all forms of energy, not just heat.


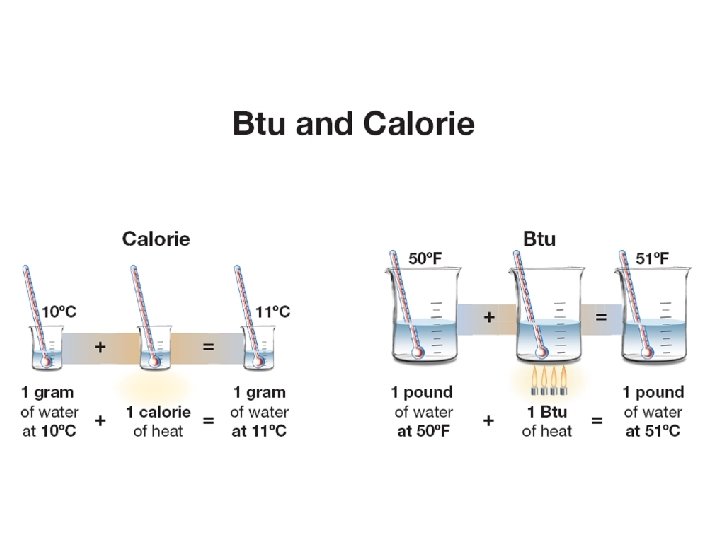
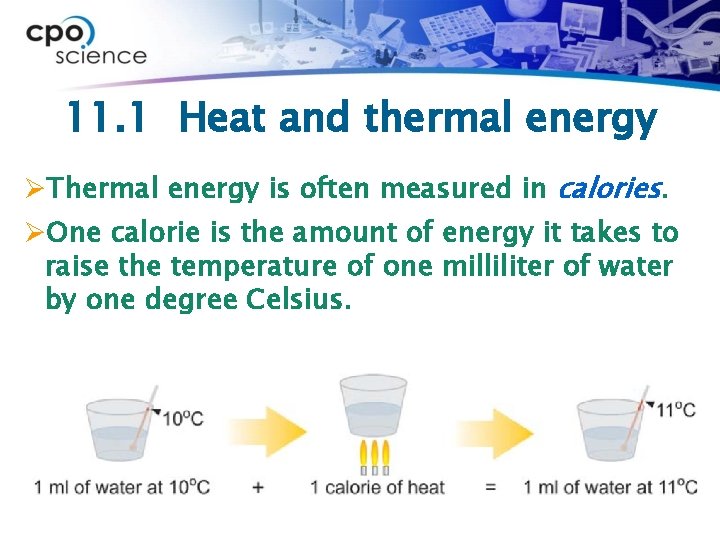
11. 1 Heat and thermal energy ØThermal energy is often measured in calories. ØOne calorie is the amount of energy it takes to raise the temperature of one milliliter of water by one degree Celsius.

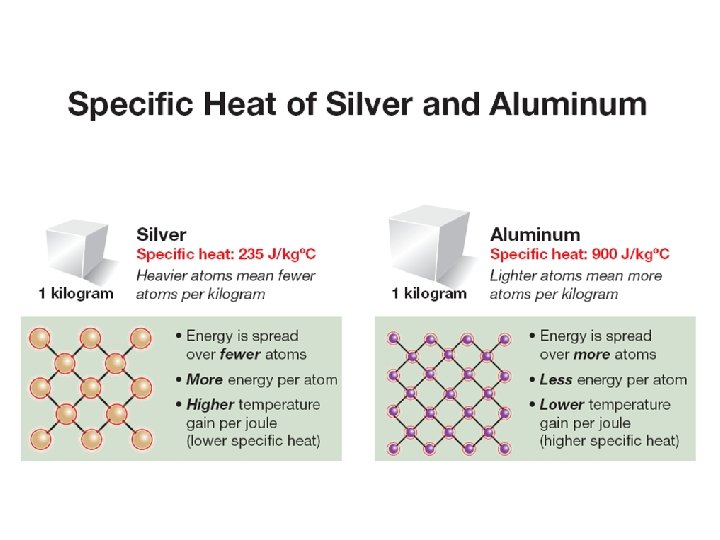
11. 1 Specific heat ØThe specific heat is a property of a substance that tells us how much heat is needed to raise the temperature of one kilogram of a material by one degree Celsius. Knowing the specific heat of a material tells you how quickly the temperature will change as it gains or loses energy.

11. 1 Why is specific heat different for different materials? ØTemperature measures the average kinetic energy per particle. ØEnergy that is divided between fewer particles means more energy per particle, and therefore more temperature change. ØIn general, materials made up of heavy atoms or molecules have low specific heat compared with materials made up of lighter ones.



Solving Problems Ø How much heat is needed to raise the temperature of a 250 -liter hot tub from 20°C to 40°C?

Solving Problems 1. Looking for: Ø …amount of heat in joules 2. Given: Ø V = 250 L, 1 L of water = 1 kg Ø Temp changes from 20°C to 40°C Ø Table specific heat water = 4, 184 J/kg°C 3. Relationships: Ø E = m. Cp(T 2 – T 1) 4. Solution: Ø Sig. E =fig. /Sci. (250 L × not. 1 kg/L) × 4, 184 J/kg°C (40°C - 720°C) 20, 920, 000 J = 2. 1 x 10 J = 20, 920, 000 J

Chapter Eleven: Heat Ø 11. 1 Heat Ø 11. 2 Heat Transfer

Chapter 11. 2 Learning Goals ØCompare and contrast various methods of heat transfer. ØDifferentiate between thermal conductors and thermal insulators. ØExplain what it means when objects are in thermal equilibrium.

Investigation 11 B The Specific Heat of a Metal ØKey Question: What is the identity of an unknown metal sample?

11. 2 Heat transfer Ø Thermal energy flows from higher temperature to lower temperature. This process is called heat transfer. Ø There are three ways heat flows: Ø heat conduction, Ø convection, and Ø thermal radiation.

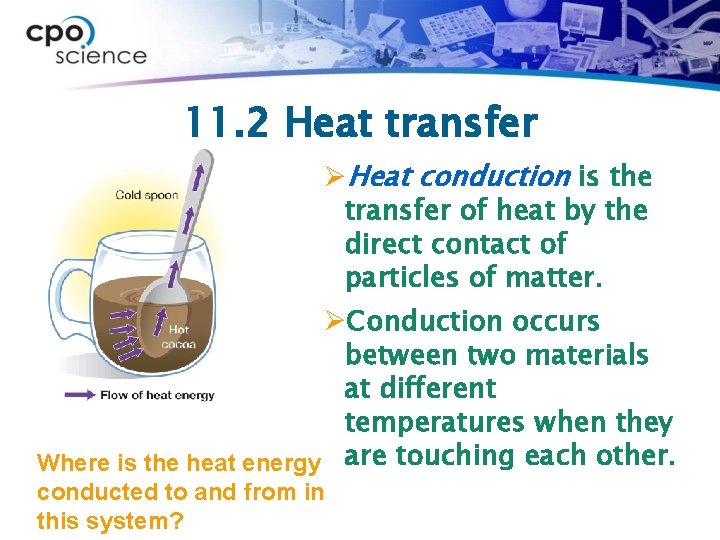
11. 2 Heat transfer ØHeat conduction is the transfer of heat by the direct contact of particles of matter. ØConduction occurs between two materials at different temperatures when they Where is the heat energy are touching each other. conducted to and from in this system?

11. 2 Heat transfer ØThermal equilibrium occurs when two bodies have the same temperature. ØNo heat flows in thermal equilibrium because the temperature is the same in the two materials.

11. 2 Thermal conductors and insulators ØMaterials that conduct heat easily are called thermal conductors and those that conduct heat poorly are called thermal insulators. Is a down coat a conductor or an insulator?


11. 2 Convection ØConvection is the transfer of heat through the motion of matter such as air and water. ØThe hot water at the bottom of the pot rises to the top and replaces the cold water.

11. 2 Convection ØConvection is mainly what distributes heat throughout a room.

11. 2 Thermal radiation ØHeat from the Sun is transferred to Earth by thermal radiation. ØAll the energy the Earth receives from the Sun comes from thermal radiation. ØThe higher the temperature of an object, the more thermal radiation it emits.

11. 2 Thermal radiation ØThermal radiation is also absorbed by objects. ØThe amount of thermal radiation absorbed depends on the surface of a material. ØDark surfaces absorb most of thermal radiation they receive. ØSilver or mirrored surfaces reflect thermal radiation.

11. 2 Heat transfer, winds, and currents ØA thermal is a convection current in the atmosphere. ØWhen a surface, like a road absorbs solar radiation, it emits energy as heat. ØThe warmed air molecules gain kinetic energy and rise. ØColder air is forced aside and sinks.

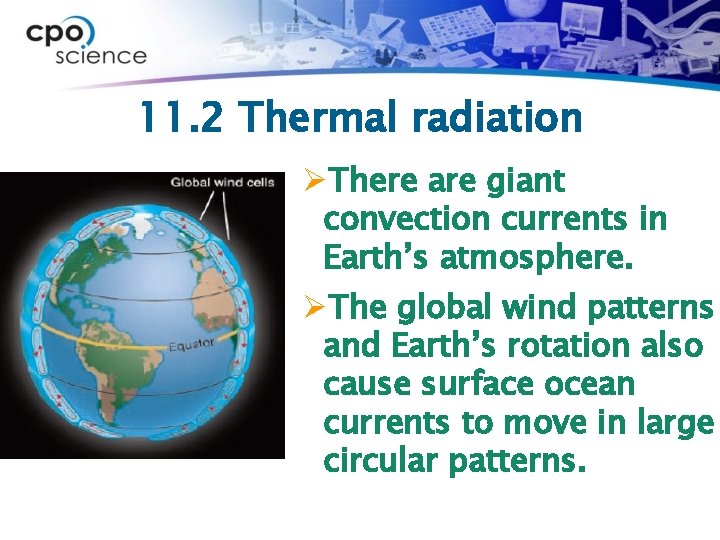
11. 2 Thermal radiation ØThere are giant convection currents in Earth’s atmosphere. ØThe global wind patterns and Earth’s rotation also cause surface ocean currents to move in large circular patterns.

Investigation 11 C Mass Determination ØKey Question: Can the mass of an object be determined without the use of a balance?

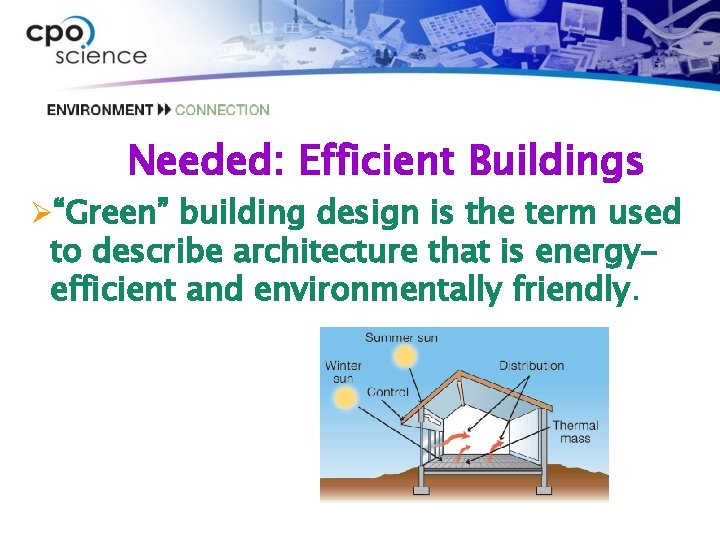
Needed: Efficient Buildings Ø“Green” building design is the term used to describe architecture that is energyefficient and environmentally friendly.
 Eleven writing
Eleven writing Egregious eleven
Egregious eleven Lesson eleven quiz consumer awareness
Lesson eleven quiz consumer awareness Lesson eleven quiz consumer awareness
Lesson eleven quiz consumer awareness One two three four five six to hundred
One two three four five six to hundred Eleven modal
Eleven modal Eleven by sandra cisneros conflict
Eleven by sandra cisneros conflict Egregious eleven
Egregious eleven Nph onset and peak
Nph onset and peak Egregious eleven
Egregious eleven Eleven arguments against moral objectivity
Eleven arguments against moral objectivity Altrusa district eleven
Altrusa district eleven Swot analysis of 7/11
Swot analysis of 7/11 7-eleven
7-eleven 11 sentence paragraph template
11 sentence paragraph template Ten past eleven
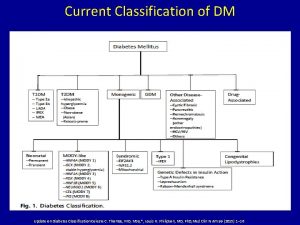
Ten past eleven Egregious eleven diabetes
Egregious eleven diabetes Eleven by sandra cisneros point of view
Eleven by sandra cisneros point of view Eleven to ten
Eleven to ten Five after nine
Five after nine 7-eleven
7-eleven It's twenty five to eleven
It's twenty five to eleven Odysseus travels
Odysseus travels Egregious eleven diabetes
Egregious eleven diabetes Module eleven lesson one self check quiz
Module eleven lesson one self check quiz Hyponozoite
Hyponozoite Asking direction conversation
Asking direction conversation Jargon words examples
Jargon words examples 7-eleven
7-eleven The eleven disciples went to galilee
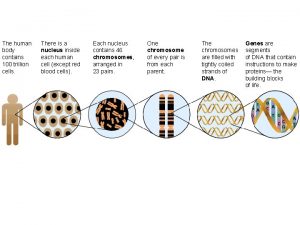
The eleven disciples went to galilee Thirty trillion cells eleven systems
Thirty trillion cells eleven systems Eleven by sandra cisneros audio
Eleven by sandra cisneros audio