Lecture Power Point to accompany Inquiry into Life




















- Slides: 50

Lecture Power. Point to accompany Inquiry into Life Twelfth Edition Sylvia S. Mader Chapter 6 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

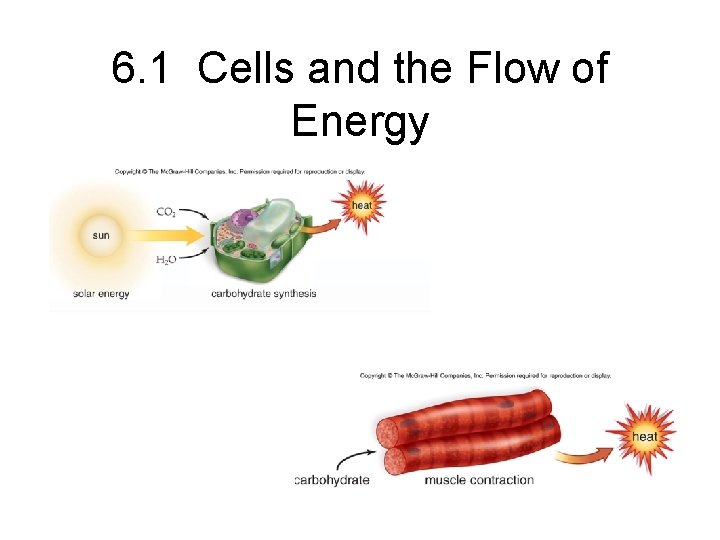
6. 1 Cells and the Flow of Energy

6. 1 Cells and the Flow of Energy • Energy is the ability to do work or bring about change.

6. 1 Cells and the Flow of Energy • Energy is the ability to do work or bring about change. • Forms of Energy

6. 1 Cells and the Flow of Energy • Energy is the ability to do work or bring about change. • Forms of Energy – Kinetic energy is the energy of motion.

6. 1 Cells and the Flow of Energy • Energy is the ability to do work or bring about change. • Forms of Energy – Kinetic energy is the energy of motion. – Potential energy is stored energy.

Flow of Energy

6. 1 Cells and the Flow of Energy • Two Laws of Thermodynamics – Energy cannot be created or destroyed, but it can be changed from one form to another. – Energy cannot be changed from one form to another without a loss of usable energy.

6. 1 Cells and the Flow of Energy

6. 1 Cells and the Flow of Energy • Cells and Entropy – Entropy refers to the relative amount of disorganization.

6. 1 Cells and the Flow of Energy • Cells and Entropy – Entropy refers to the relative amount of disorganization. – Energy transformations in cells increase the amount of entropy.

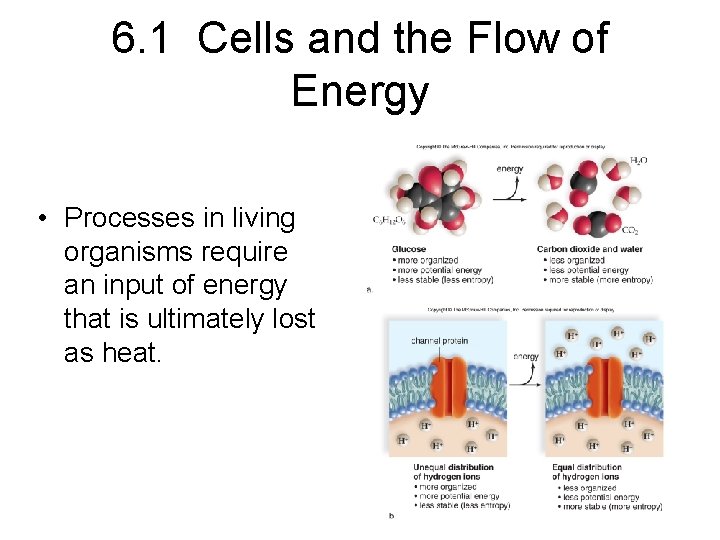
6. 1 Cells and the Flow of Energy • Processes in living organisms require an input of energy that is ultimately lost as heat.
6. 2 Metabolic Reactions and Energy Transformations

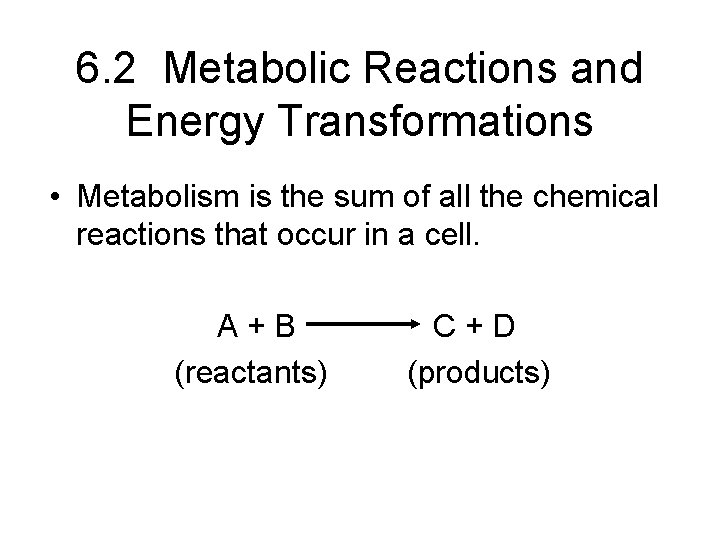
6. 2 Metabolic Reactions and Energy Transformations • Metabolism is the sum of all the chemical reactions that occur in a cell.

6. 2 Metabolic Reactions and Energy Transformations • Metabolism is the sum of all the chemical reactions that occur in a cell. A+B (reactants) C+D (products)

6. 2 Metabolic Reactions and Energy Transformations • Free energy (∆G) is the amount of energy available.

6. 2 Metabolic Reactions and Energy Transformations • Free energy (∆G) is the amount of energy available. – Exergonic reactions are ones where energy is released (∆G is negative)

6. 2 Metabolic Reactions and Energy Transformations • Free energy (∆G) is the amount of energy available. – Exergonic reactions are ones where energy is released (∆G is negative) – Endergonic reactions require an input of energy. (∆G is positive)

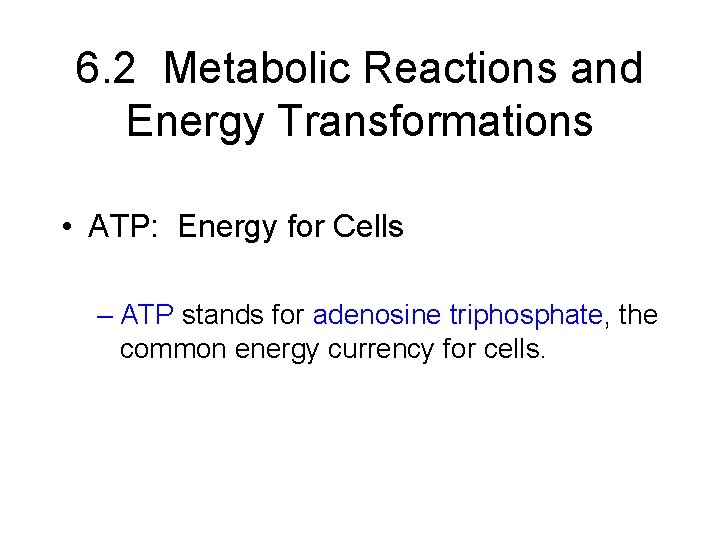
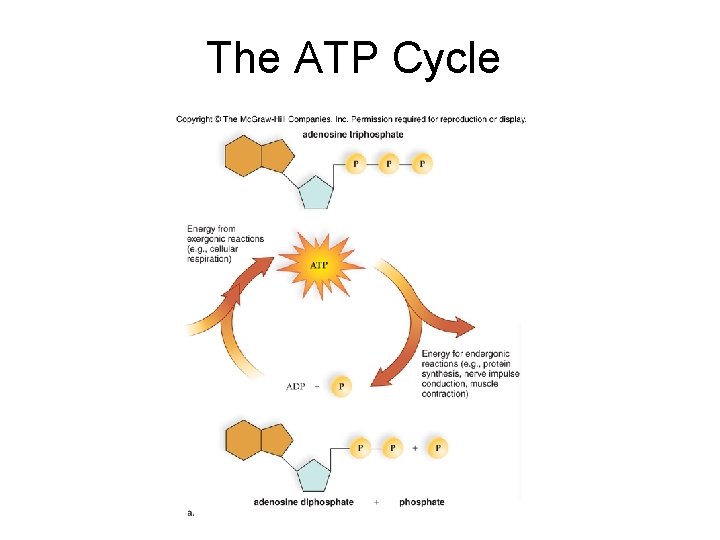
6. 2 Metabolic Reactions and Energy Transformations • ATP: Energy for Cells – ATP stands for adenosine triphosphate, the common energy currency for cells.

6. 2 Metabolic Reactions and Energy Transformations • ATP: Energy for Cells – ATP stands for adenosine triphosphate, the common energy currency for cells. – ATP is generated from ADP (adenosine diphosphate) + an inorganic phosphate molecule ( P )

The ATP Cycle

6. 2 Metabolic Reactions and Energy Transformations • Structure of ATP – ATP is a nucleotide that is composed of: • Adenine (a nitrogen-containing base) • Ribose (a 5 -carbon sugar) • Three phosphate groups

6. 2 Metabolic Reactions and Energy Transformations • Structure of ATP – ATP is a “high energy” compound because a phosphate group can easily be removed.

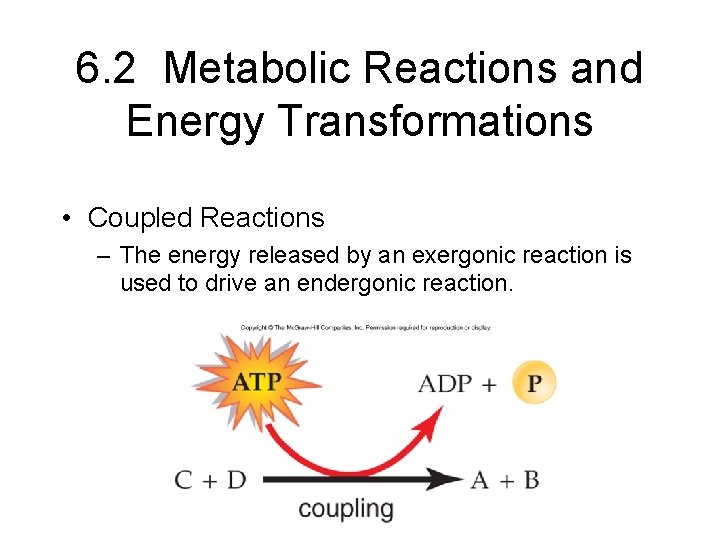
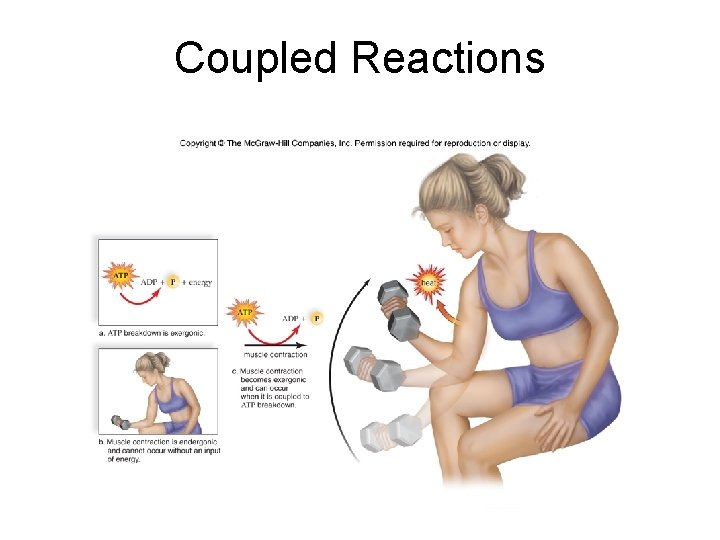
6. 2 Metabolic Reactions and Energy Transformations • Coupled Reactions – The energy released by an exergonic reaction is used to drive an endergonic reaction.

Coupled Reactions

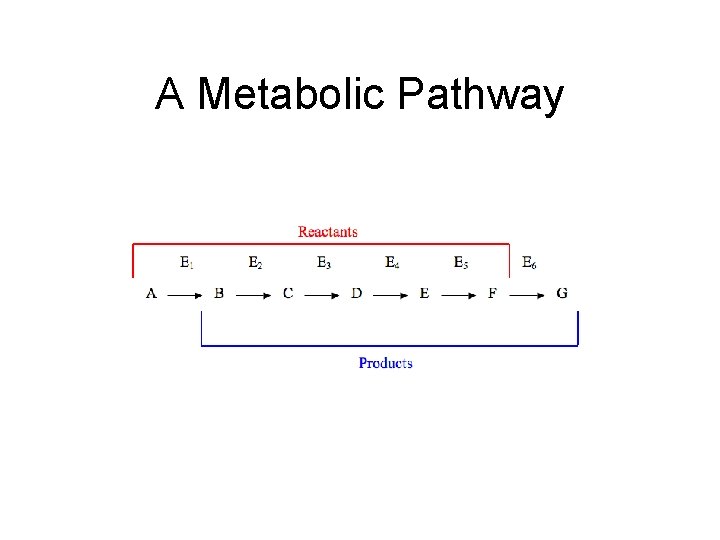
6. 3 Metabolic Pathways and Enzymes • Metabolic pathways are a series of linked reactions. – These begin with a specific reactant and produce an end product

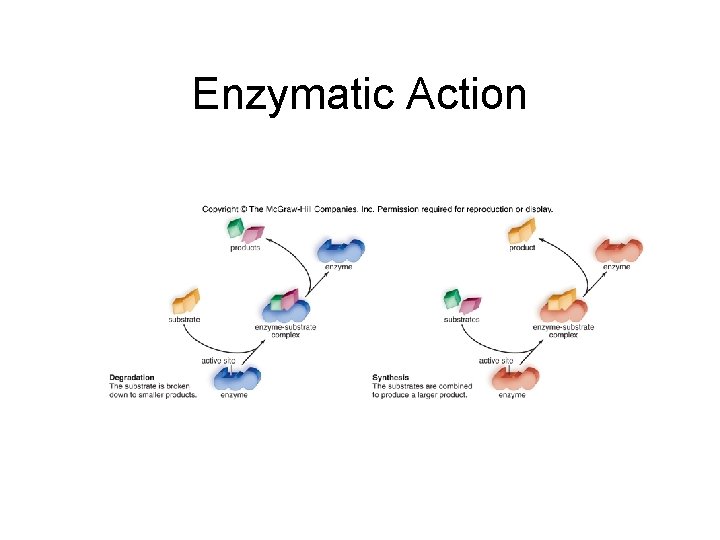
6. 3 Metabolic Pathways and Enzymes • Enzymes are usually proteins that function to speed a chemical reaction. – Enzymes serve as catalysts

A Metabolic Pathway

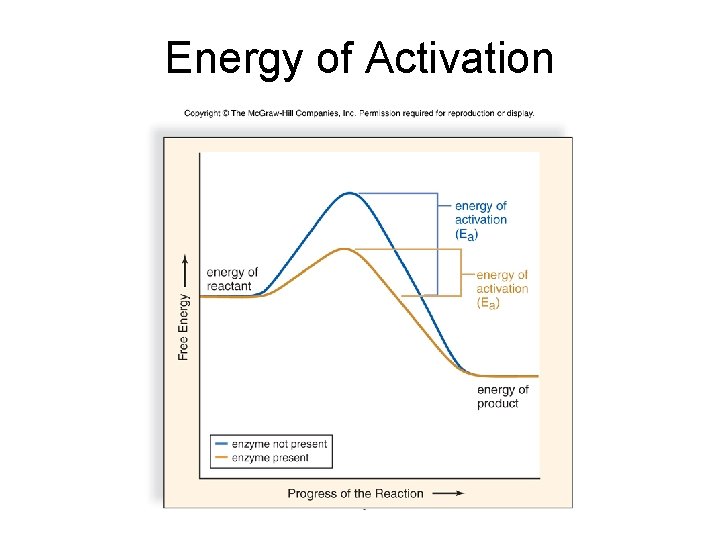
6. 3 Metabolic Pathways and Enzymes • The Energy of Activation (Ea) is the energy that must be added to cause molecules to react with one another.

Energy of Activation

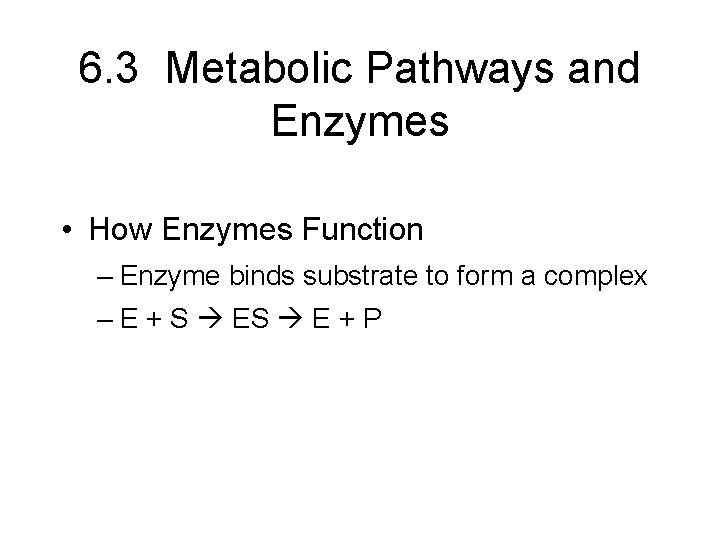
6. 3 Metabolic Pathways and Enzymes • How Enzymes Function – Enzyme binds substrate to form a complex – E + S E + P

Enzymatic Action

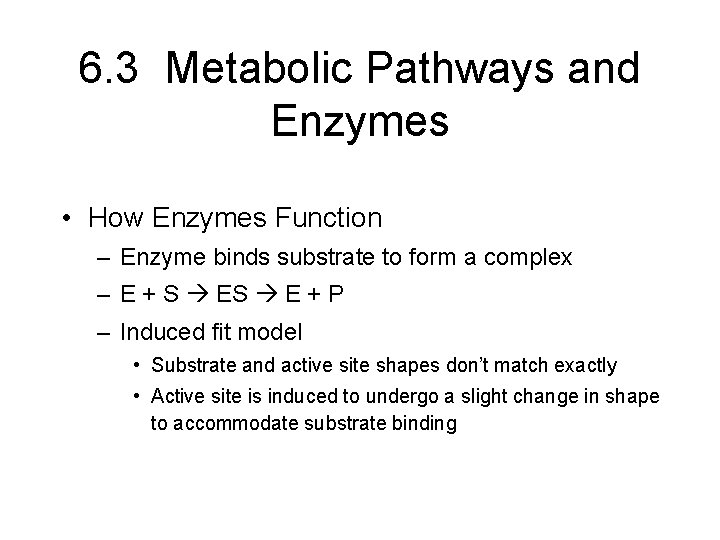
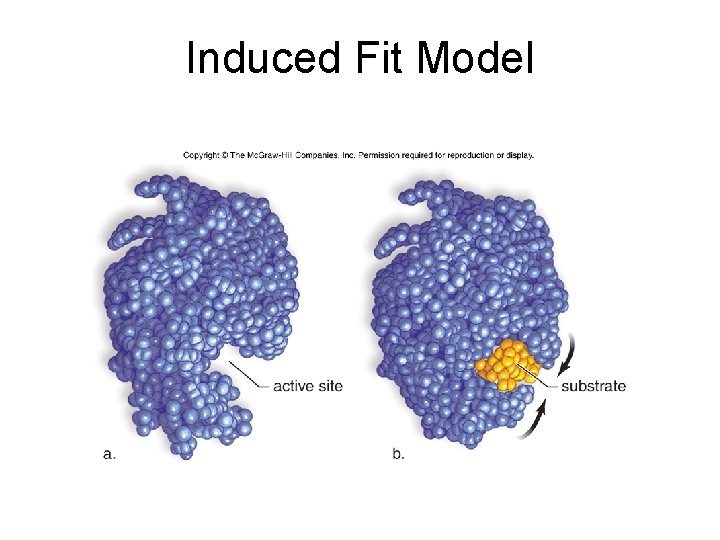
6. 3 Metabolic Pathways and Enzymes • How Enzymes Function – Enzyme binds substrate to form a complex – E + S E + P – Induced fit model • Substrate and active site shapes don’t match exactly • Active site is induced to undergo a slight change in shape to accommodate substrate binding

Induced Fit Model

6. 3 Metabolic Pathways and Enzymes • Factors Affecting Enzymatic Speed – Substrate Concentration – Temperature and p. H – Enzyme Activation – Enzyme Inhibition – Enzyme Cofactors

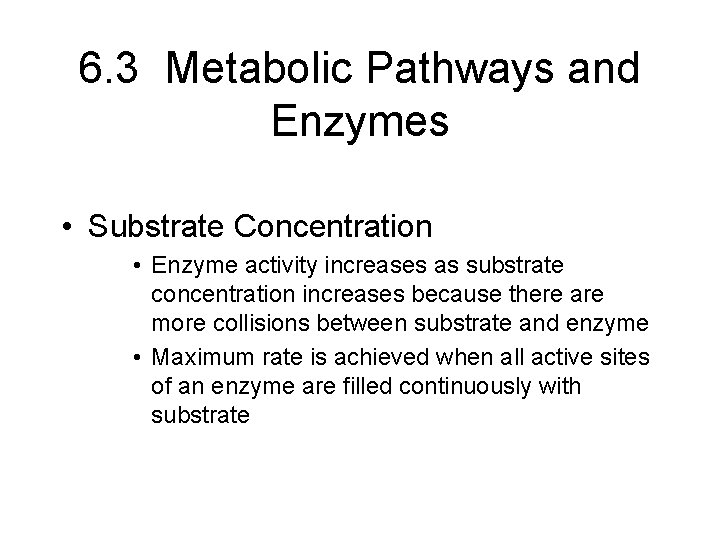
6. 3 Metabolic Pathways and Enzymes • Substrate Concentration • Enzyme activity increases as substrate concentration increases because there are more collisions between substrate and enzyme • Maximum rate is achieved when all active sites of an enzyme are filled continuously with substrate

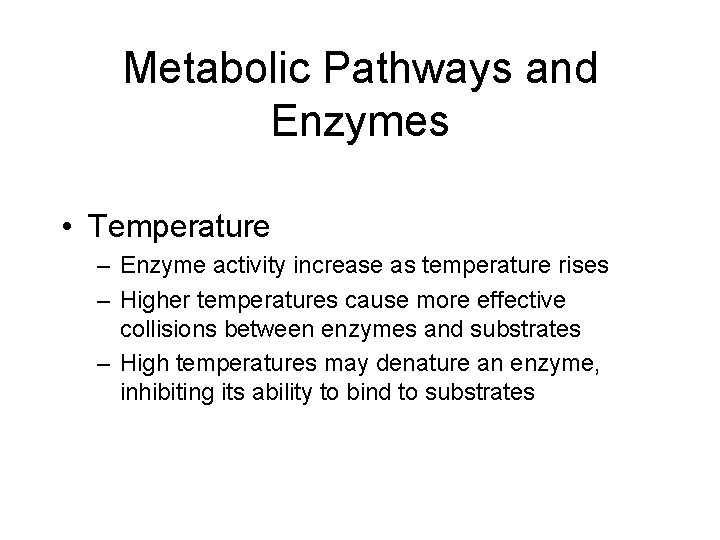
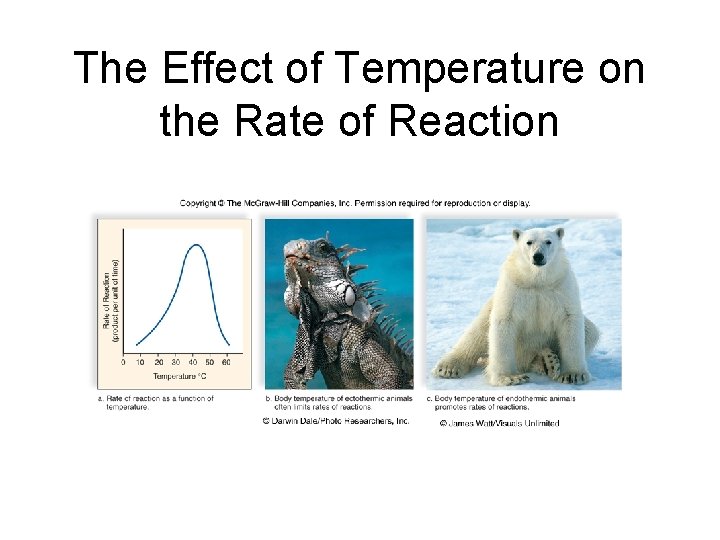
Metabolic Pathways and Enzymes • Temperature – Enzyme activity increase as temperature rises – Higher temperatures cause more effective collisions between enzymes and substrates – High temperatures may denature an enzyme, inhibiting its ability to bind to substrates

The Effect of Temperature on the Rate of Reaction

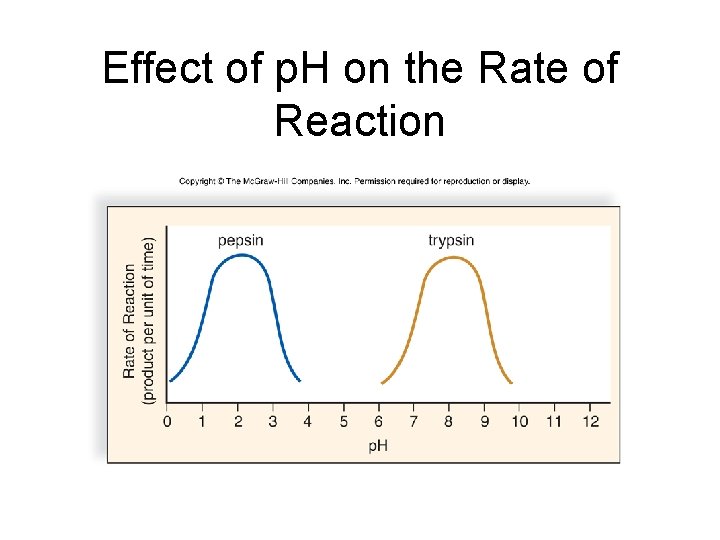
Metabolic Pathways and Enzymes • p. H • Each enzyme has an optimal p. H • Enzyme structure is p. H dependent • Extremes of p. H can denature an enzyme by altering its structure

Effect of p. H on the Rate of Reaction

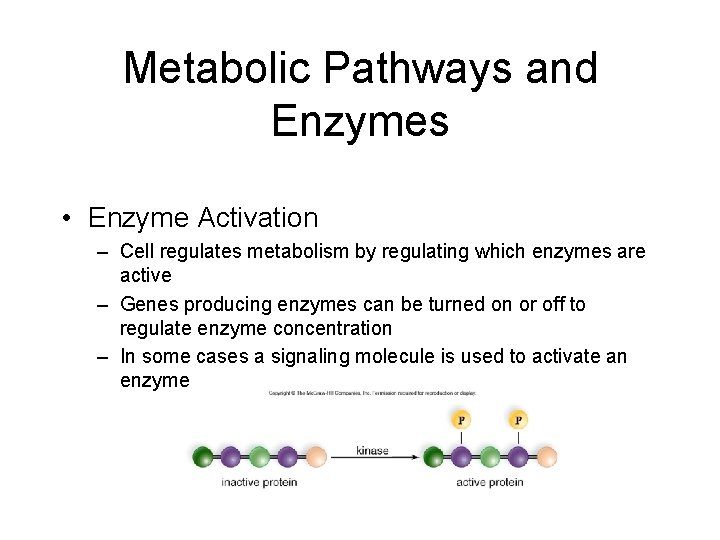
Metabolic Pathways and Enzymes • Enzyme Activation – Cell regulates metabolism by regulating which enzymes are active – Genes producing enzymes can be turned on or off to regulate enzyme concentration – In some cases a signaling molecule is used to activate an enzyme

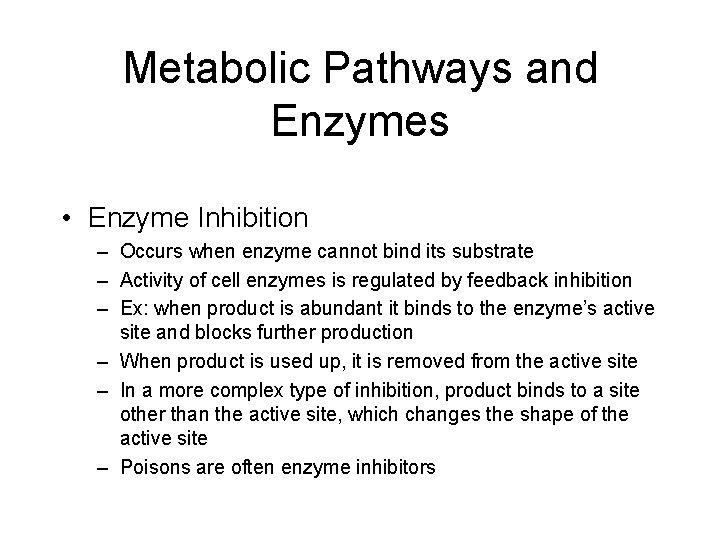
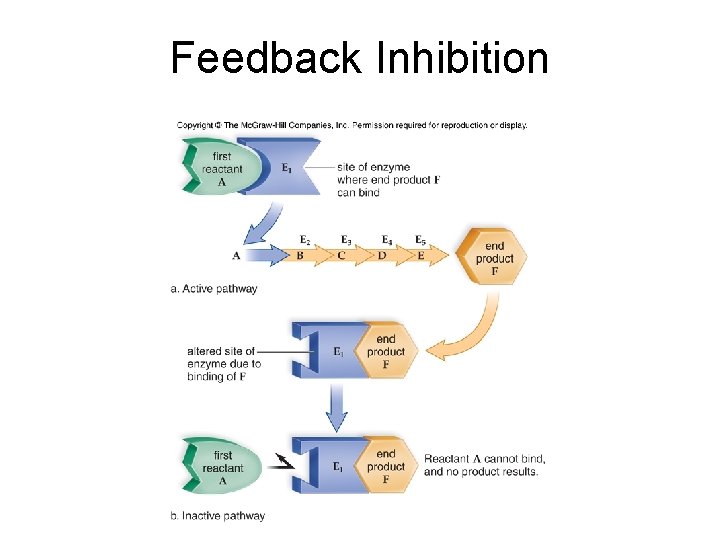
Metabolic Pathways and Enzymes • Enzyme Inhibition – Occurs when enzyme cannot bind its substrate – Activity of cell enzymes is regulated by feedback inhibition – Ex: when product is abundant it binds to the enzyme’s active site and blocks further production – When product is used up, it is removed from the active site – In a more complex type of inhibition, product binds to a site other than the active site, which changes the shape of the active site – Poisons are often enzyme inhibitors

Feedback Inhibition

Metabolic Pathways and Enzymes • Enzyme Cofactors – Molecules which help enzyme function – Copper and zinc are examples of inorganic cofactors – Organic non-protein cofactors are called coenzymes • Vitamins are often components of coenzymes

6. 4 Oxidation-Reduction and the Flow of Energy • Oxidation-Reduction – Oxidation is the loss of electrons – Reduction is the gaining of electrons – Ex: when oxygen combines with a metal like Mg, oxygen receives electrons (becomes negatively charged) and Mg loses electrons (becomes positively charged) • We say Mg has become oxidized, and oxygen is reduced (has a negative charge) when Mg. O forms

6. 4 Oxidation-Reduction and the Flow of Energy • Oxidation-Reduction – The term oxidation is used even when oxygen is not involved • Ex: Na+ + Cl- Na. Cl in which sodium is oxidized and chloride is reduced – This also applies to covalent reactions involving hydrogen atoms – Oxidation is the loss of hydrogen and reduction is the gain of hydrogen atoms

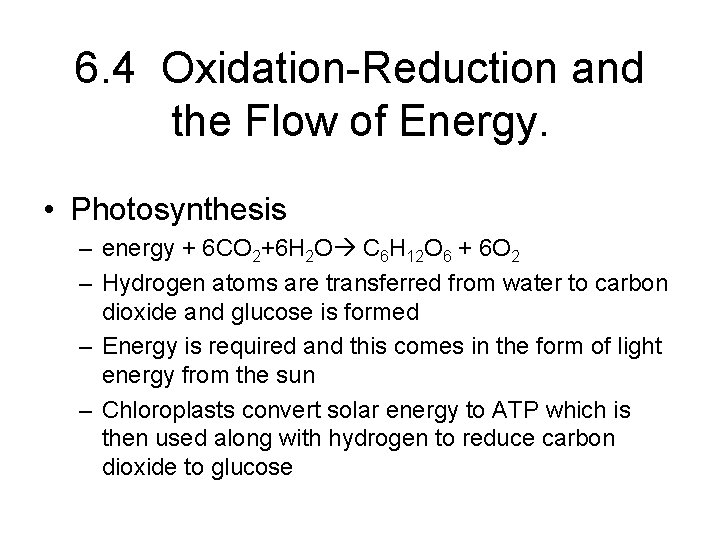
6. 4 Oxidation-Reduction and the Flow of Energy. • Photosynthesis – energy + 6 CO 2+6 H 2 O C 6 H 12 O 6 + 6 O 2 – Hydrogen atoms are transferred from water to carbon dioxide and glucose is formed – Energy is required and this comes in the form of light energy from the sun – Chloroplasts convert solar energy to ATP which is then used along with hydrogen to reduce carbon dioxide to glucose

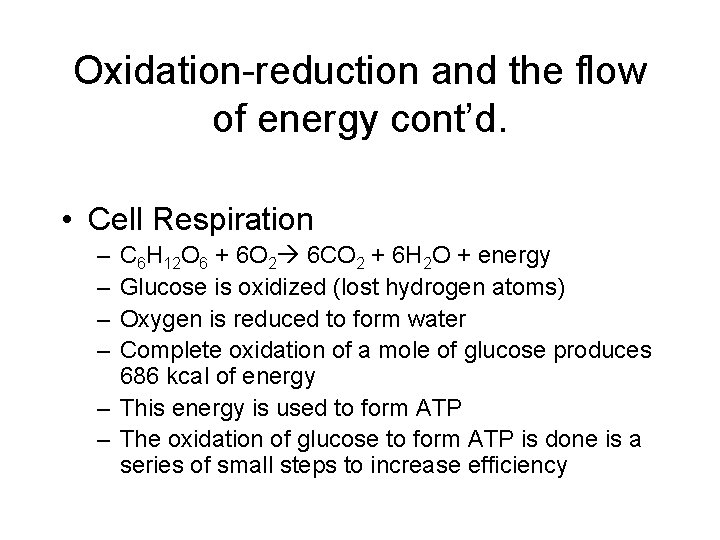
Oxidation-reduction and the flow of energy cont’d. • Cell Respiration – – C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy Glucose is oxidized (lost hydrogen atoms) Oxygen is reduced to form water Complete oxidation of a mole of glucose produces 686 kcal of energy – This energy is used to form ATP – The oxidation of glucose to form ATP is done is a series of small steps to increase efficiency

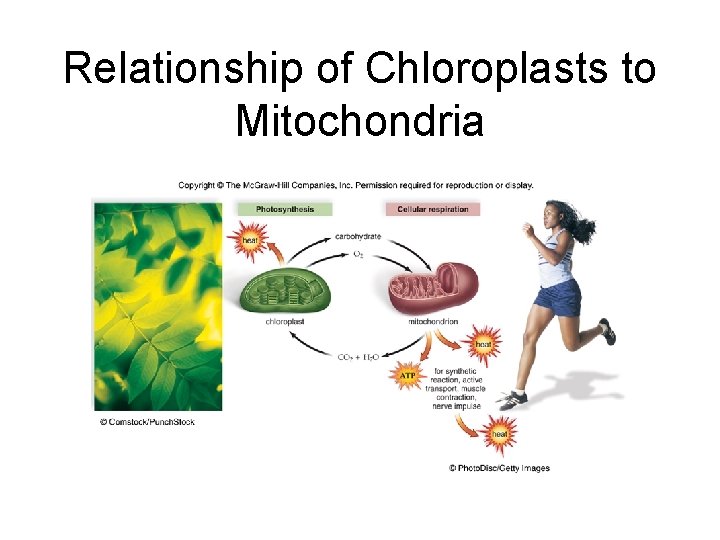
6. 4 Oxidation-Reduction and the Flow of Energy. • Organelles and the flow of energy – Cycling of molecules between chloroplasts and mitochondria allows energy to flow from sun to all living things – Chloroplasts use light energy from the sun to make carbohydrates – Mitochondria break down carbohydrates to form ATP – Cell respiration produces carbon dioxide and water which are used in photosynthesis

Relationship of Chloroplasts to Mitochondria
 Are there lymph nodes in your arm
Are there lymph nodes in your arm Hebrews 6 9
Hebrews 6 9 Accompany chapter 1
Accompany chapter 1 Accompany
Accompany 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Active power reactive power apparent power
Active power reactive power apparent power Informsu
Informsu Point point power
Point point power Inquiry elevates your thinking power
Inquiry elevates your thinking power Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Power system analysis lecture notes
Power system analysis lecture notes Power semiconductor devices lecture notes
Power semiconductor devices lecture notes Switch mode power supply lecture notes
Switch mode power supply lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes What are some traditions in things fall apart
What are some traditions in things fall apart Meaning of this
Meaning of this Drawing the power of jesus christ into our lives
Drawing the power of jesus christ into our lives Invisibility flight the power to split into multiple bodies
Invisibility flight the power to split into multiple bodies Why did japan turn itself into an imperialist power?
Why did japan turn itself into an imperialist power? A high point of land extending into water
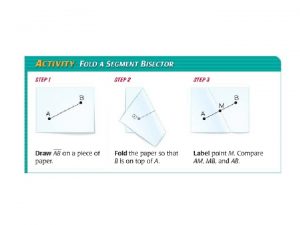
A high point of land extending into water The point that divides a segment into two
The point that divides a segment into two Solar power satellites and microwave power transmission
Solar power satellites and microwave power transmission Potential power
Potential power Flex power power supply
Flex power power supply Dispersive power of a grating is defined as
Dispersive power of a grating is defined as Power of a power property
Power of a power property General power rule vs power rule
General power rule vs power rule Power angle curve in power system stability
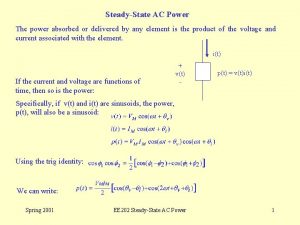
Power angle curve in power system stability Power absorbed or delivered
Power absorbed or delivered People come into your life for 3 reasons
People come into your life for 3 reasons William wordsworth is known as a
William wordsworth is known as a Evangelio del domingo en power point
Evangelio del domingo en power point Ejemplos de ova en power point
Ejemplos de ova en power point Laboutiquedelpowerpointx
Laboutiquedelpowerpointx Power point sul tennis
Power point sul tennis Power point turing complete
Power point turing complete Power point sul riciclo in inglese
Power point sul riciclo in inglese Alat peraga geometri sma
Alat peraga geometri sma Pelajaran sekolah sabat dewasa
Pelajaran sekolah sabat dewasa La boutique del power point x
La boutique del power point x La boutique del power point
La boutique del power point La boutique del power point
La boutique del power point La boutique del powerpoint
La boutique del powerpoint Gizi kuliner adalah
Gizi kuliner adalah Decreto 1330 de 2019 ppt
Decreto 1330 de 2019 ppt Conclusão apresentação power point
Conclusão apresentação power point Portafolio digital estudiantil ejemplo
Portafolio digital estudiantil ejemplo Cara mengoperasikan microsoft powerpoint 2010
Cara mengoperasikan microsoft powerpoint 2010 Potencia electrica formulas
Potencia electrica formulas Advantages and disadvantages of microsoft powerpoint
Advantages and disadvantages of microsoft powerpoint Ventajas y desventajas de powerpoint
Ventajas y desventajas de powerpoint