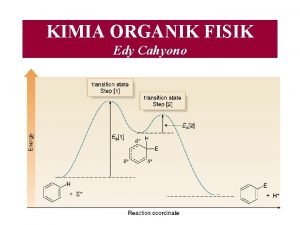
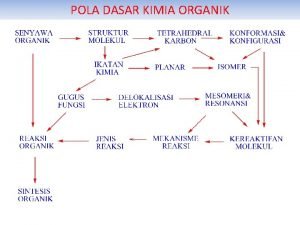
KIMIA DASAR I APAKAH KIMIA ORGANIK Ilmu yang










- Slides: 31

KIMIA DASAR I

APAKAH KIMIA ORGANIK ? Ilmu yang mempelajari senyawa -senyawa hidrokarbon dan derivatnya Perbandingan : 7 million senyawa organik 1. 5 million senyawa anorganik

SENYAWA ORGANIK n Materi tanaman / hewan n Makanan n Bahan farmasi/ kosmetik n Plastik n Komponen minyak bumi n Pakaian

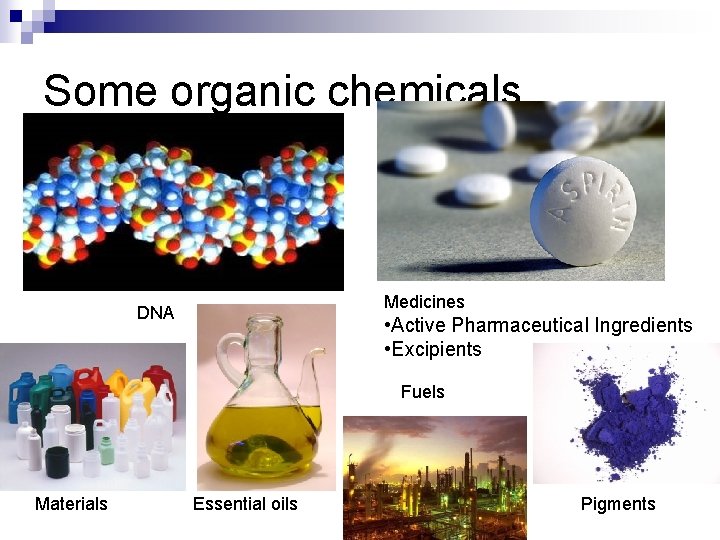
Some organic chemicals Medicines DNA • Active Pharmaceutical Ingredients • Excipients Fuels Materials Essential oils Pigments

HSenyawa I D R Oyang K Aterdiri RBO N dari karbon dan hidrogen Berdasarkan ikatannya Hidrokarbon jenuh Hidrokarbon tak jenuh alkana, alkena, alkuna, benzena

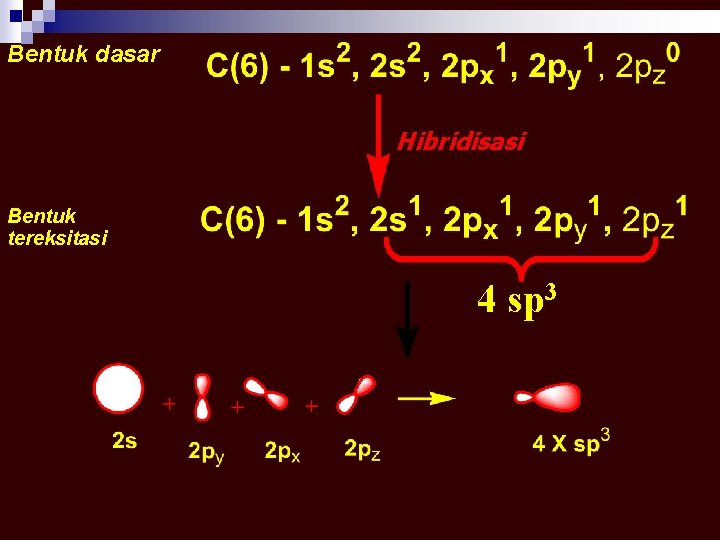
Carbon (C) Unsur kehidupan di atas bumi adalah C MENGAPA ? ? ? memiliki 4 buah elektron bonding yang dapat membentuk ikatan kovalen yang kuat, dapat berupa ikatan tunggal dan rangkap (2 atau 3)

Molekul organik paling sederhana: Susunan OKTET yang stabil metana Covalent Bonding – Atoms Share Electrons

ALKANA

Bentuk dasar Hibridisasi Bentuk tereksitasi 4 sp 3

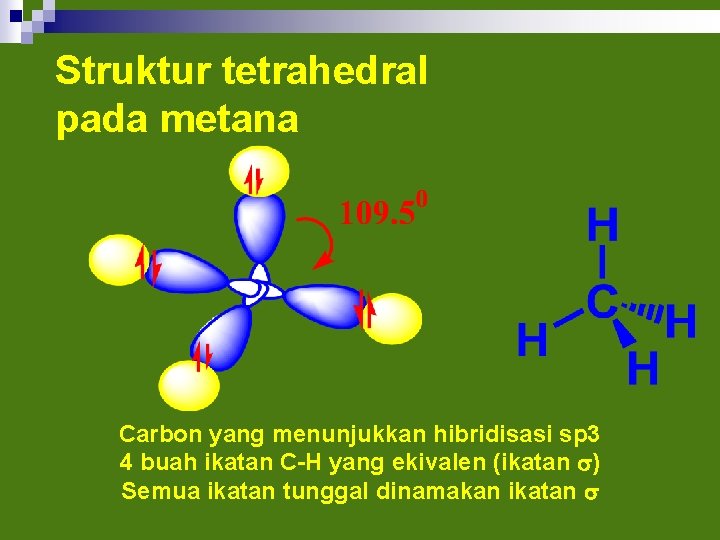
Struktur tetrahedral pada metana Carbon yang menunjukkan hibridisasi sp 3 4 buah ikatan C-H yang ekivalen (ikatan s) Semua ikatan tunggal dinamakan ikatan s

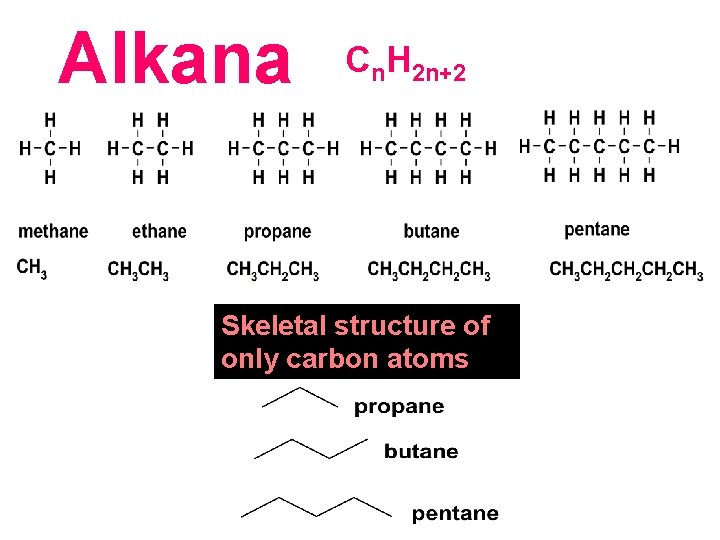
Alkana Cn. H 2 n+2 consist of only carbon and hydrogen bonded by single covalent bonds single Skeletal structure of only carbon atoms

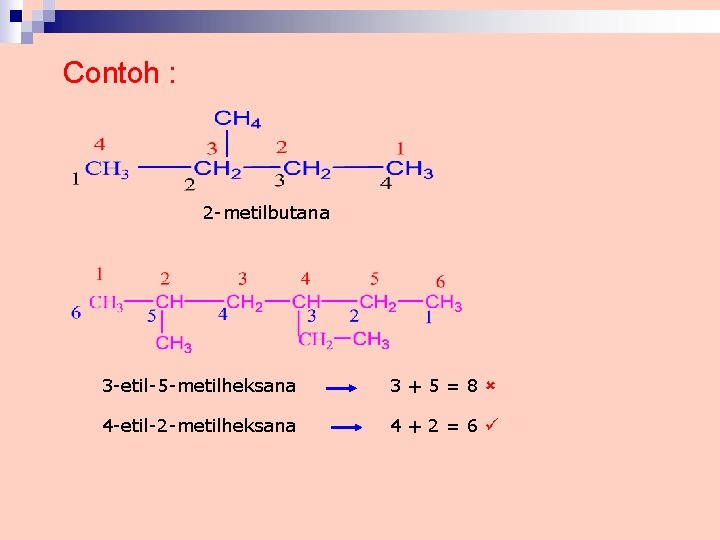
Contoh : 2 -metilbutana 3 -etil-5 -metilheksana 3+5=8 4 -etil-2 -metilheksana 4+2=6

SIFAT FISIK n n n n Senyawa non polar, densitas <1, dengan air membentuk dua lapisan Larut dalam pelarut organik non polar Alkana C 4, berbentuk gas, Alkana Mr >, berbentuk cair, Alkana Mr >>>, berbentuk padat Semakin besar jumlah atom C, Mr molekul semakin >, gaya dispersi tiap molekul >, titik didih semakin tinggi Alkana bercabang td < alkana rantai lurus padanannya Ada pengaruh gaya van der Waals antar molekul Ikatan tunggal mampu berotasi bebas

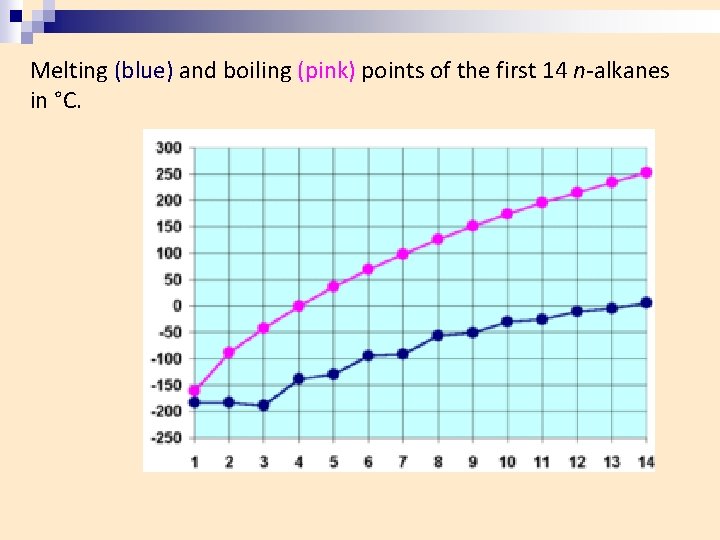
Melting (blue) and boiling (pink) points of the first 14 n-alkanes in °C.

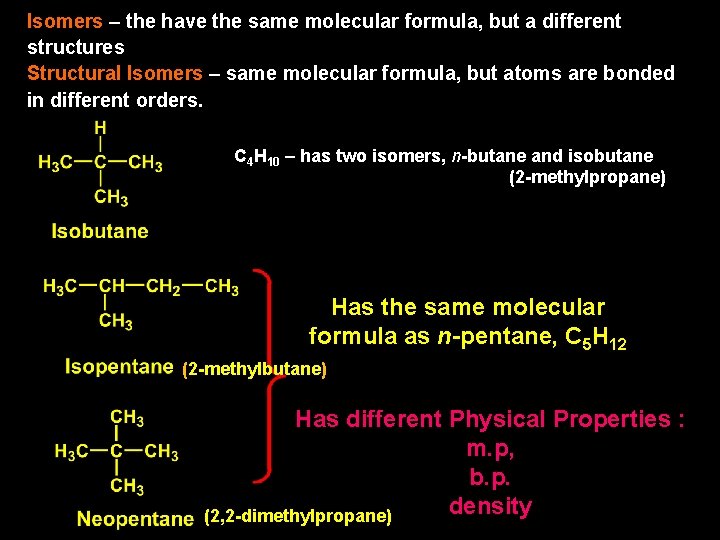
Isomers – the have the same molecular formula, but a different structures Structural Isomers – same molecular formula, but atoms are bonded in different orders. C 4 H 10 – has two isomers, n-butane and isobutane (2 -methylpropane) Has the same molecular formula as n-pentane, C 5 H 12 (2 -methylbutane) Has different Physical Properties : m. p, b. p. density (2, 2 -dimethylpropane)

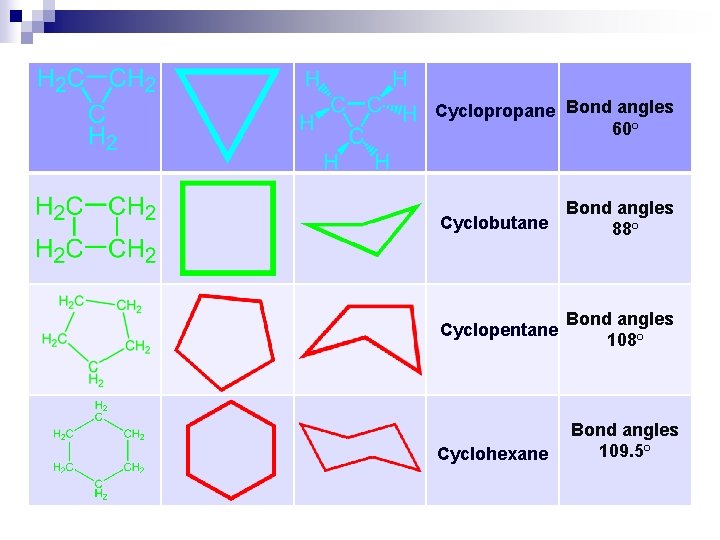
CYCLOALKANES Cn. H 2 n

Cyclopropane Bond angles 60° Cyclobutane Bond angles 88° Cyclopentane Bond angles 108° Cyclohexane Bond angles 109. 5°

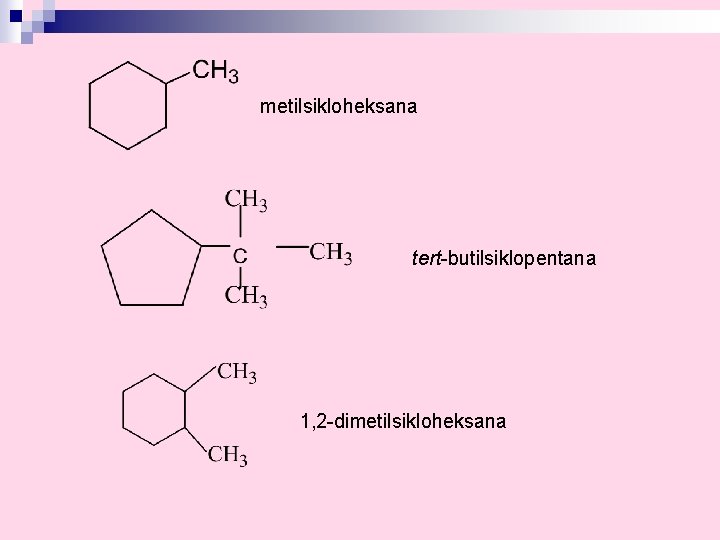
metilsikloheksana tert-butilsiklopentana 1, 2 -dimetilsikloheksana

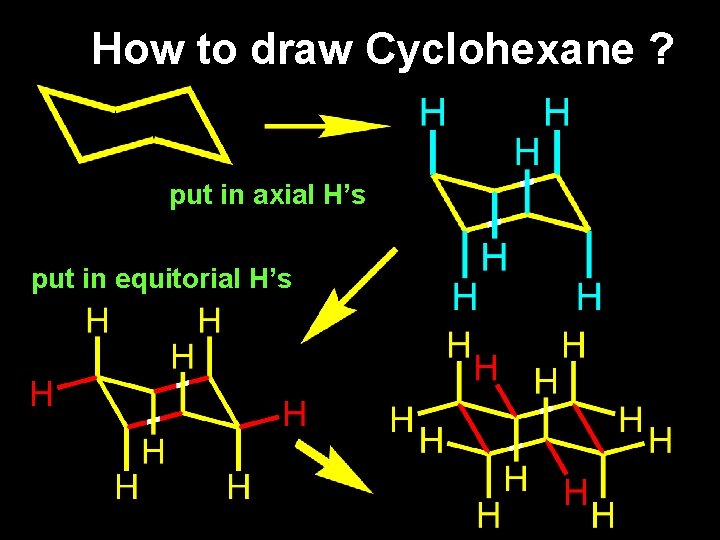
How to draw Cyclohexane ? put in axial H’s put in equitorial H’s

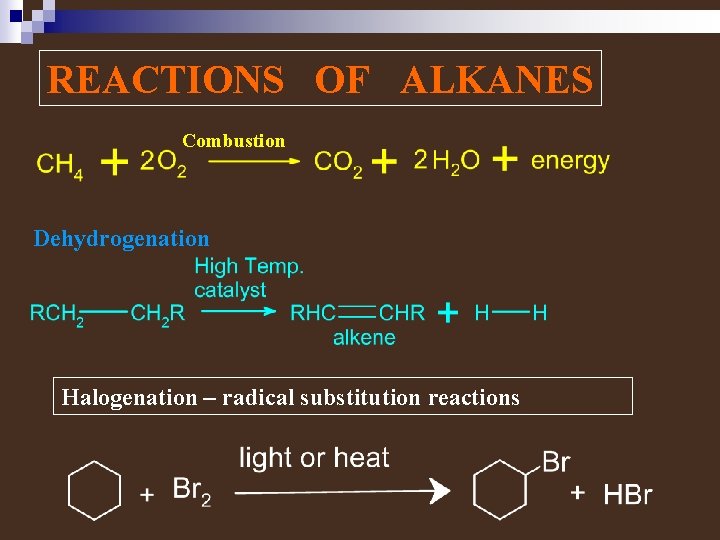
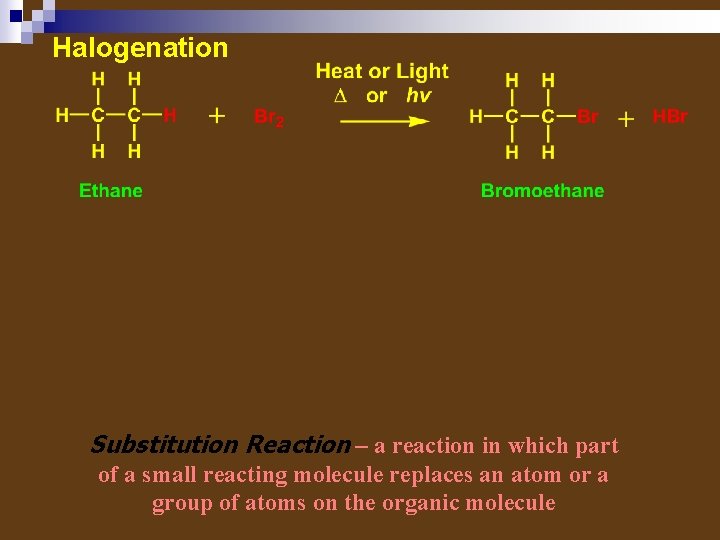
REACTIONS OF ALKANES Combustion Dehydrogenation Halogenation – radical substitution reactions

Halogenation Substitution Reaction – a reaction in which part of a small reacting molecule replaces an atom or a group of atoms on the organic molecule

Alkyl Halides or Haloalkanes 6/5/2021 22

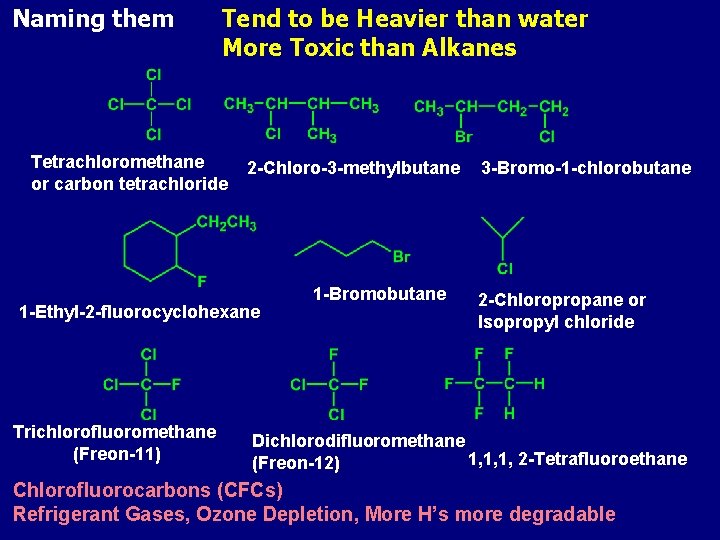
Naming them Tend to be Heavier than water More Toxic than Alkanes Tetrachloromethane or carbon tetrachloride 2 -Chloro-3 -methylbutane 1 -Ethyl-2 -fluorocyclohexane Trichlorofluoromethane (Freon-11) 1 -Bromobutane 3 -Bromo-1 -chlorobutane 2 -Chloropropane or Isopropyl chloride Dichlorodifluoromethane 1, 1, 1, 2 -Tetrafluoroethane (Freon-12) Chlorofluorocarbons (CFCs) Refrigerant Gases, Ozone Depletion, More H’s more degradable

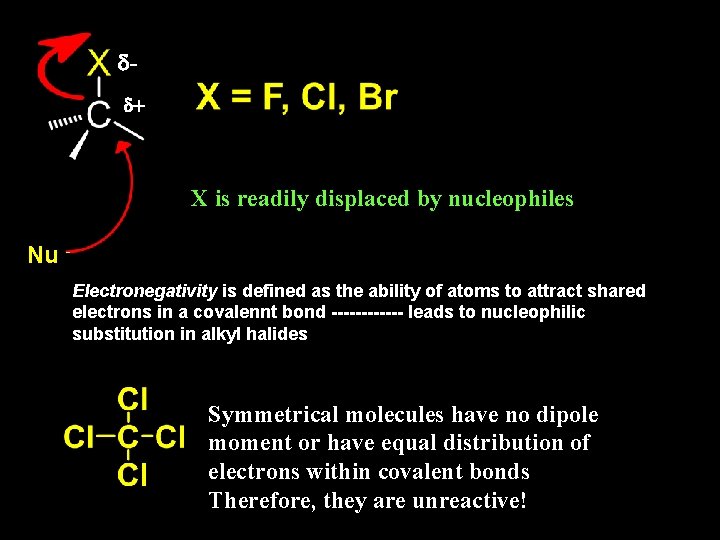
dd+ X is readily displaced by nucleophiles Nu Electronegativity is defined as the ability of atoms to attract shared electrons in a covalennt bond ------ leads to nucleophilic substitution in alkyl halides Symmetrical molecules have no dipole moment or have equal distribution of electrons within covalent bonds Therefore, they are unreactive!

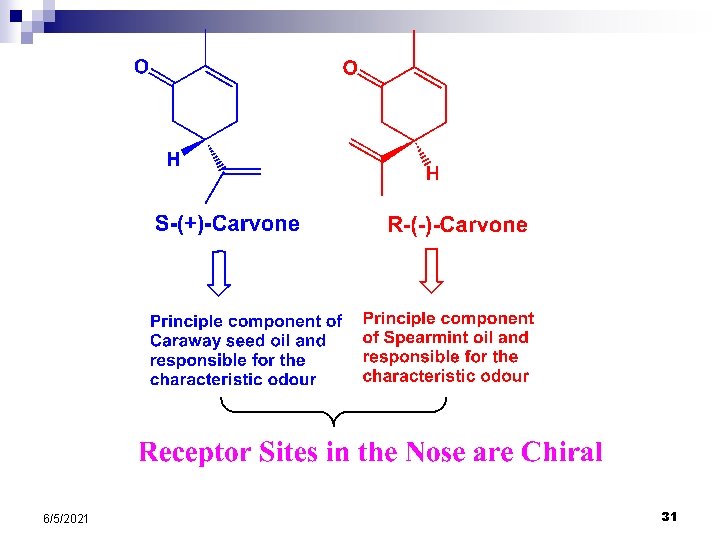
STEREOCHEMISTRY 6/5/2021 25

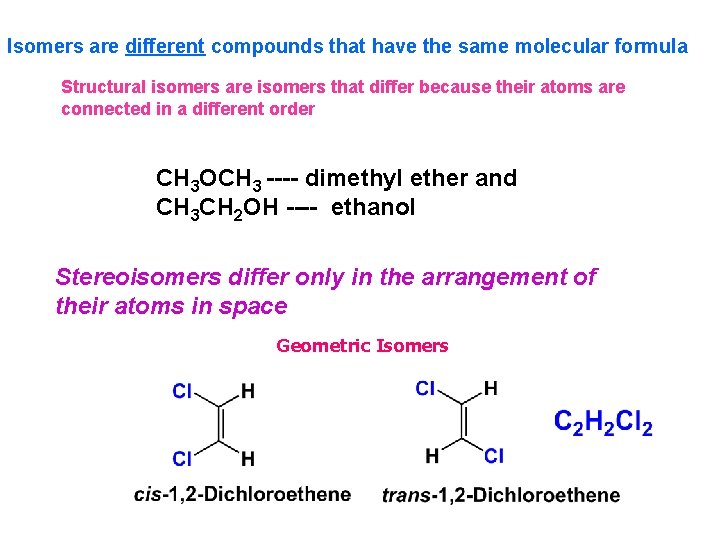
Isomers are different compounds that have the same molecular formula Structural isomers are isomers that differ because their atoms are connected in a different order CH 3 OCH 3 ---- dimethyl ether and CH 3 CH 2 OH ---- ethanol Stereoisomers differ only in the arrangement of their atoms in space Geometric Isomers

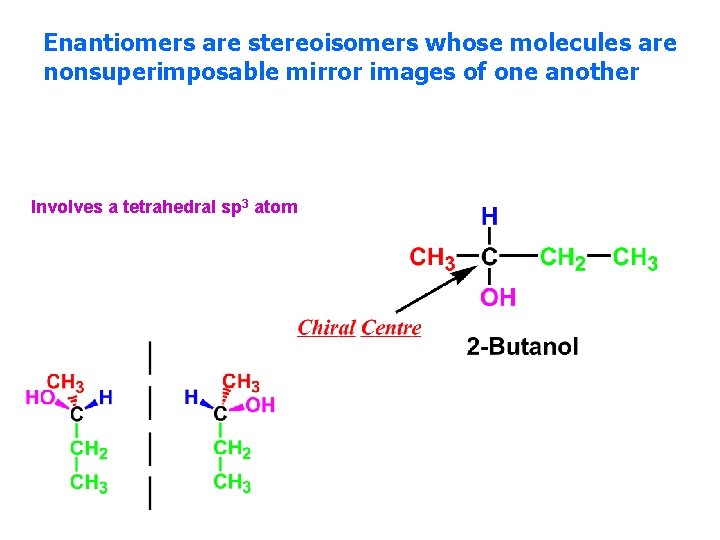
Enantiomers are stereoisomers whose molecules are nonsuperimposable mirror images of one another Involves a tetrahedral sp 3 atom

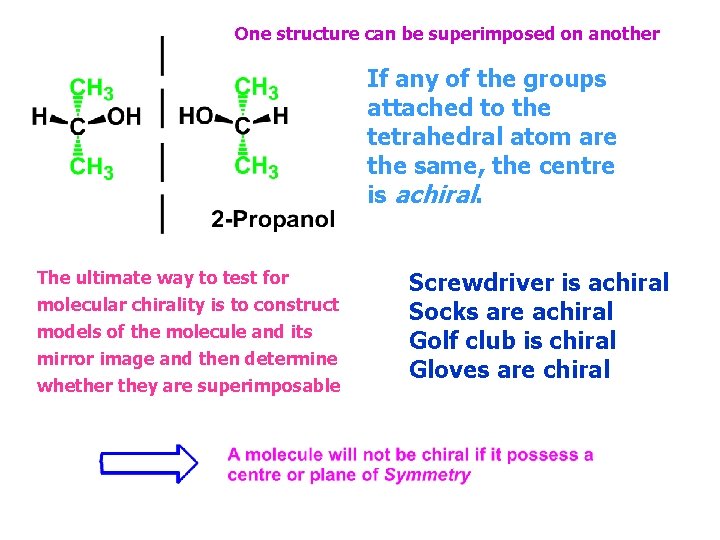
One structure can be superimposed on another If any of the groups attached to the tetrahedral atom are the same, the centre is achiral. The ultimate way to test for molecular chirality is to construct models of the molecule and its mirror image and then determine whether they are superimposable Screwdriver is achiral Socks are achiral Golf club is chiral Gloves are chiral

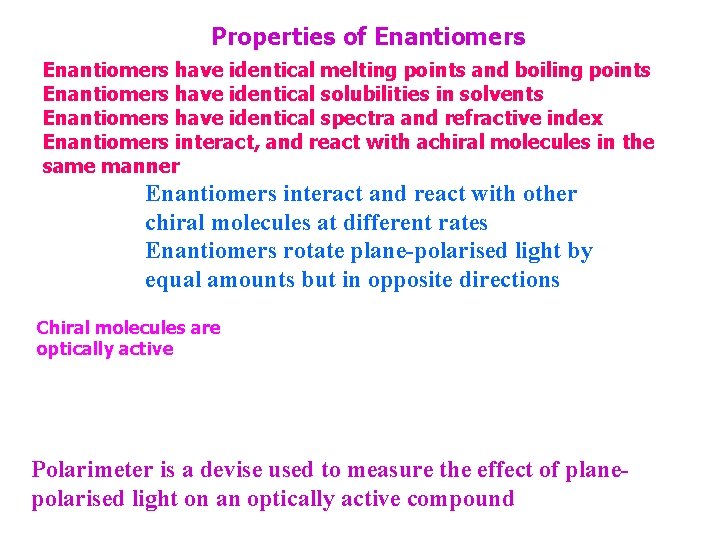
Properties of Enantiomers have identical melting points and boiling points Enantiomers have identical solubilities in solvents Enantiomers have identical spectra and refractive index Enantiomers interact, and react with achiral molecules in the same manner Enantiomers interact and react with other chiral molecules at different rates Enantiomers rotate plane-polarised light by equal amounts but in opposite directions Chiral molecules are optically active Polarimeter is a devise used to measure the effect of planepolarised light on an optically active compound

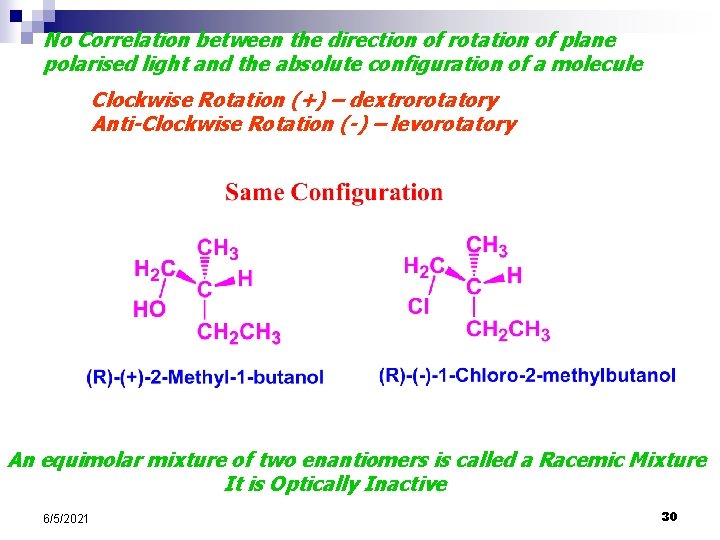
No Correlation between the direction of rotation of plane polarised light and the absolute configuration of a molecule Clockwise Rotation (+) – dextrorotatory Anti-Clockwise Rotation (-) – levorotatory An equimolar mixture of two enantiomers is called a Racemic Mixture It is Optically Inactive 6/5/2021 30

6/5/2021 31
 Materi kuliah ilmu alamiah dasar semester 1
Materi kuliah ilmu alamiah dasar semester 1 Resonansi kimia organik
Resonansi kimia organik Rumus qc dan kc
Rumus qc dan kc Kimia
Kimia Ilmu kimia yang mempelajari kalor adalah
Ilmu kimia yang mempelajari kalor adalah Termokimia
Termokimia Reaksi endoterm
Reaksi endoterm Termokimia adalah cabang ilmu kimia yang mempelajari
Termokimia adalah cabang ilmu kimia yang mempelajari Sediaan galenika
Sediaan galenika Apakah binatang mempunyai roh menurut kristen
Apakah binatang mempunyai roh menurut kristen Inti pati utama dasar cheeseman 1946
Inti pati utama dasar cheeseman 1946 Apakah hal yang harus dimuat dalam anggaran dasar pt
Apakah hal yang harus dimuat dalam anggaran dasar pt Ruang lingkup kajian ilmu kimia adalah
Ruang lingkup kajian ilmu kimia adalah Hakikat ilmu kimia dan keselamatan kerja di laboratorium
Hakikat ilmu kimia dan keselamatan kerja di laboratorium Pengenalan ilmu kimia
Pengenalan ilmu kimia Hubungan ilmu politik dengan ilmu geografi
Hubungan ilmu politik dengan ilmu geografi Hubungan ilmu farmasi dengan ilmu fisika
Hubungan ilmu farmasi dengan ilmu fisika Hubungan antropologi dengan ilmu sejarah
Hubungan antropologi dengan ilmu sejarah Struktur pengetahuan ilmiah dalam filsafat ilmu
Struktur pengetahuan ilmiah dalam filsafat ilmu Hubungan ilmu akhlak dengan ilmu tasawuf
Hubungan ilmu akhlak dengan ilmu tasawuf Falsafah alam tabie
Falsafah alam tabie Huruf mad
Huruf mad Peta konsep perhitungan kimia
Peta konsep perhitungan kimia Nilai ketuhanan sebagai dasar pengembangan ilmu
Nilai ketuhanan sebagai dasar pengembangan ilmu Manusia dan tanggung jawab ilmu budaya dasar
Manusia dan tanggung jawab ilmu budaya dasar Mata kuliah iad
Mata kuliah iad Pengertian ilmu kealaman dasar
Pengertian ilmu kealaman dasar Manusia dan keadilan ilmu budaya dasar
Manusia dan keadilan ilmu budaya dasar Konsep ilmu budaya dasar
Konsep ilmu budaya dasar Ilmu sosial dasar gunadarma
Ilmu sosial dasar gunadarma Rps ilmu alamiah dasar
Rps ilmu alamiah dasar Studi gizi ui
Studi gizi ui