INTRODUCTION TO ORGANIC CHEMISTRY ORGANIC MOLECULES Organic means






- Slides: 17

INTRODUCTION TO ORGANIC CHEMISTRY

ORGANIC MOLECULES § Organic means: “contains carbon” § 90% of all known compounds are organic

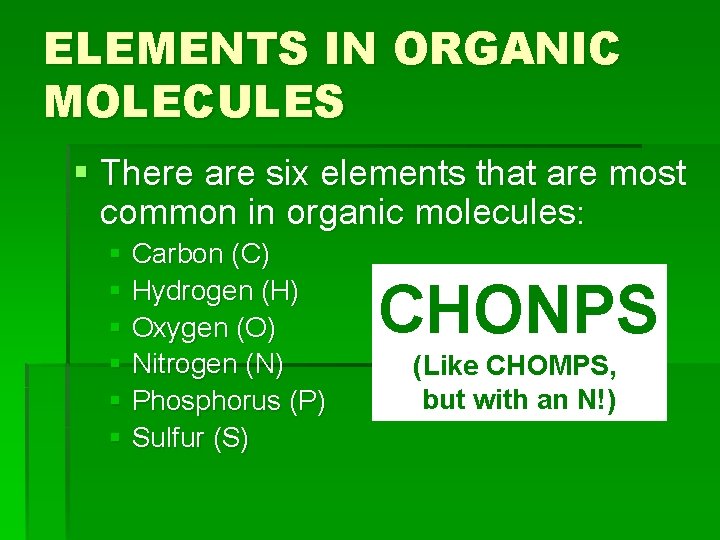
ELEMENTS IN ORGANIC MOLECULES § There are six elements that are most common in organic molecules: § Carbon (C) § Hydrogen (H) § Oxygen (O) § Nitrogen (N) § Phosphorus (P) § Sulfur (S) CHONPS (Like CHOMPS, but with an N!)


CARBON § Carbon can form covalent bonds with 4 other atoms. § Because Carbon can bond with four other atoms, Carbon can help make many different structures. This helps to ensure diversity of life on earth! H H C H H

COVALENT BONDS § In covalent bonds, atoms share electrons § They are the strongest type of chemical bond § Atoms can share one, two or three electrons.

TYPES OF COVALENT BONDS § Share one electron: Single bond C-C § Share two electrons: Double bond C=C § Share three electrons: Triple bond CΞ C

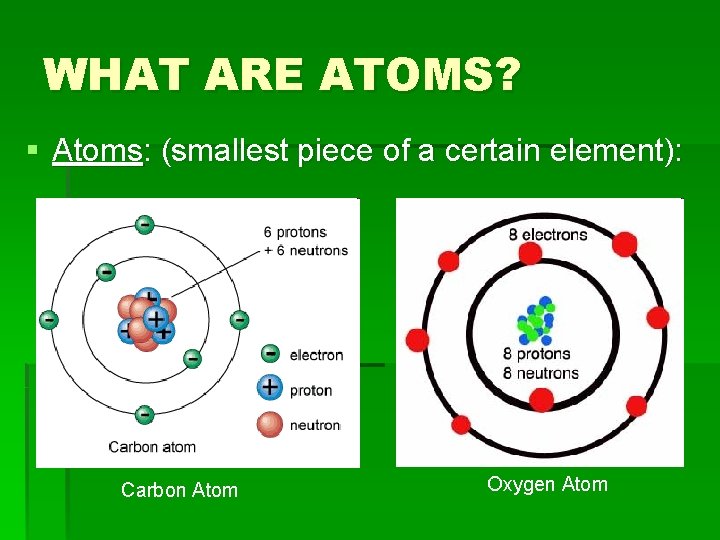
WHAT ARE ATOMS? § Atoms: (smallest piece of a certain element): Carbon Atom Oxygen Atom

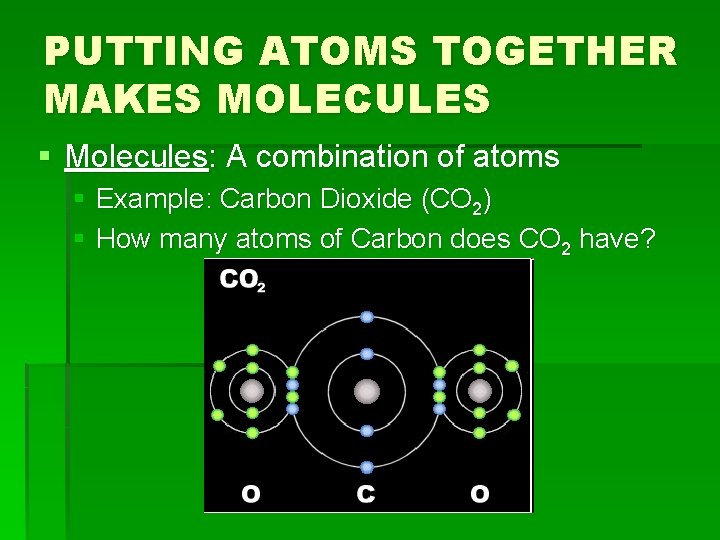
PUTTING ATOMS TOGETHER MAKES MOLECULES § Molecules: A combination of atoms § Example: Carbon Dioxide (CO 2) § How many atoms of Carbon does CO 2 have?

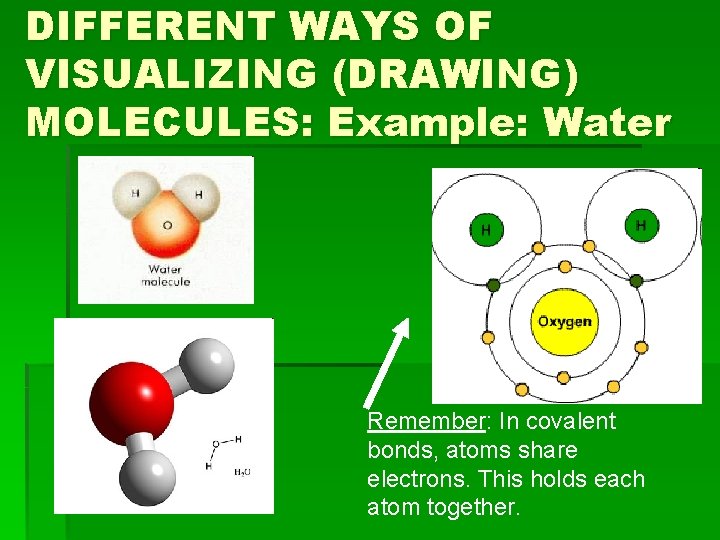
DIFFERENT WAYS OF VISUALIZING (DRAWING) MOLECULES: Example: Water Remember: In covalent bonds, atoms share electrons. This holds each atom together.

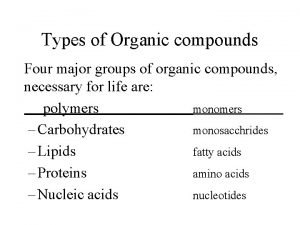
THE FOUR MOST COMMON TYPES OF ORGANIC MOLECULES § Carbohydrates: such as sugar and pasta § Lipids: such as butter and olive oil § Proteins: such as meat, nuts and soy § Nucleic Acids: such as DNA

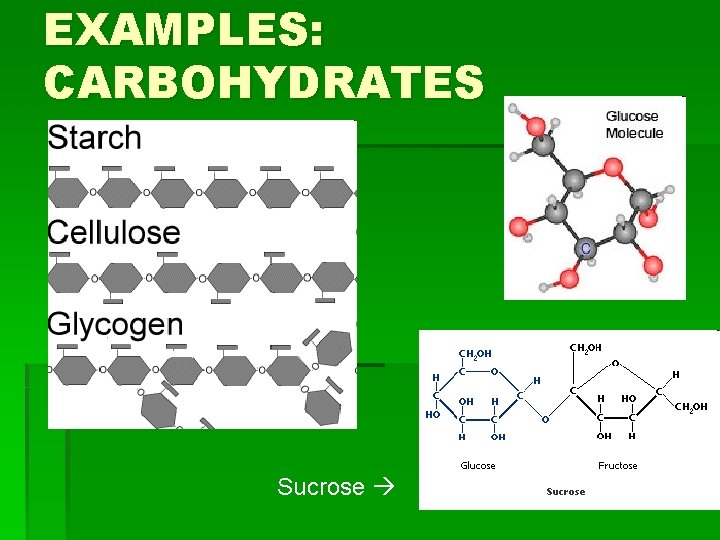
EXAMPLES: CARBOHYDRATES Sucrose

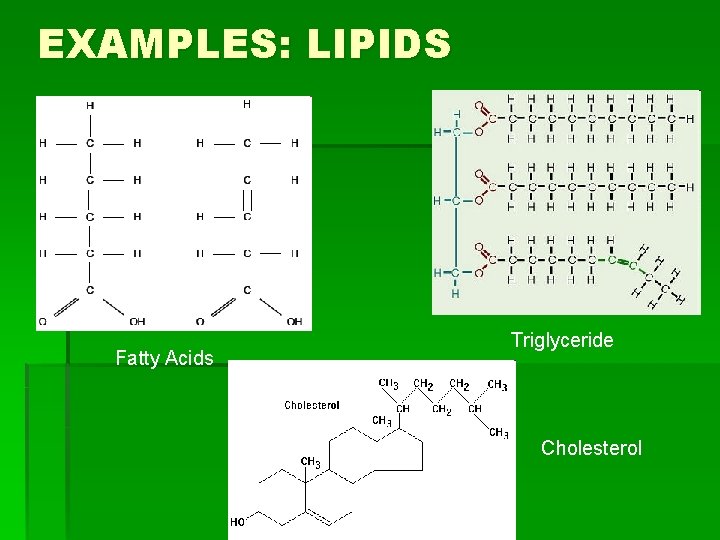
EXAMPLES: LIPIDS Fatty Acids Triglyceride Cholesterol

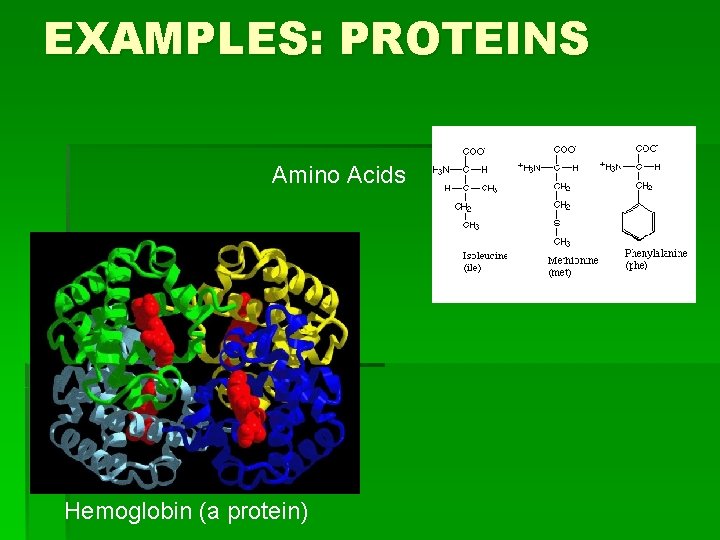
EXAMPLES: PROTEINS Amino Acids Hemoglobin (a protein)

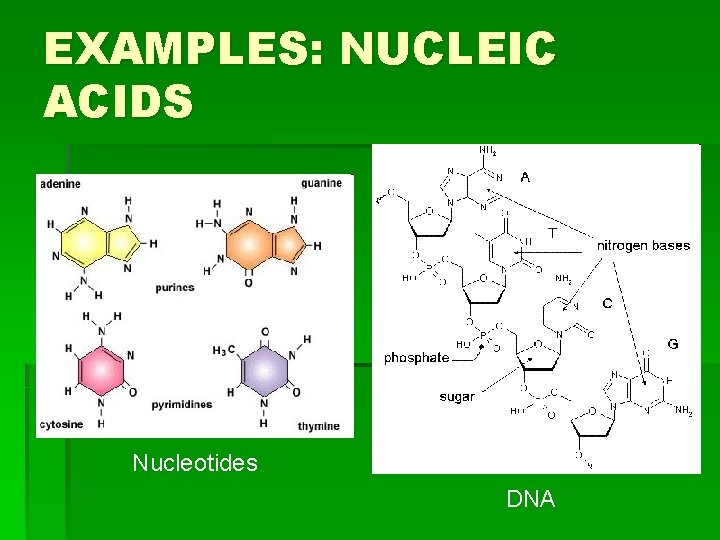
EXAMPLES: NUCLEIC ACIDS Nucleotides DNA

HOW TO REMEMBER STRUCTURES § Carbohydrates: § Have a 5 -Carbon ring form, or often have many rings joined in a line (or branching from each other) by an oxygen molecule § Lipids: § Have long hydrocarbon chains (chains of hydrogen and carbon atoms), and steroids have rings joined at the side.

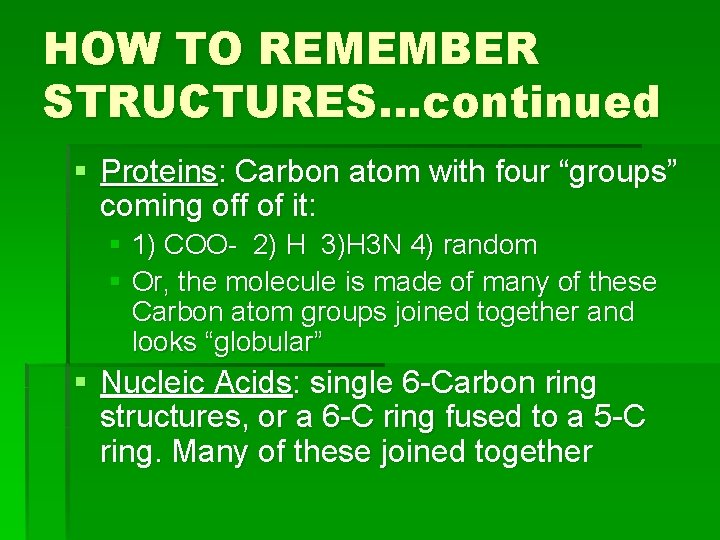
HOW TO REMEMBER STRUCTURES…continued § Proteins: Carbon atom with four “groups” coming off of it: § 1) COO- 2) H 3)H 3 N 4) random § Or, the molecule is made of many of these Carbon atom groups joined together and looks “globular” § Nucleic Acids: single 6 -Carbon ring structures, or a 6 -C ring fused to a 5 -C ring. Many of these joined together
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Importance of organic chemistry
Importance of organic chemistry Four types of organic compounds
Four types of organic compounds Organic vs inorganic
Organic vs inorganic Are unsaturated fats solid at room temperature
Are unsaturated fats solid at room temperature Chemsheets as 1017
Chemsheets as 1017 Chemistry molecules
Chemistry molecules Examples of ab2e type molecules
Examples of ab2e type molecules Shapes of molecules a level chemistry
Shapes of molecules a level chemistry Chemsheets shapes of molecules
Chemsheets shapes of molecules Founder of organic chemistry
Founder of organic chemistry Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Functional group vs homologous series
Functional group vs homologous series Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry