Honors Chemistry Chapter 8 The Mole The Mole




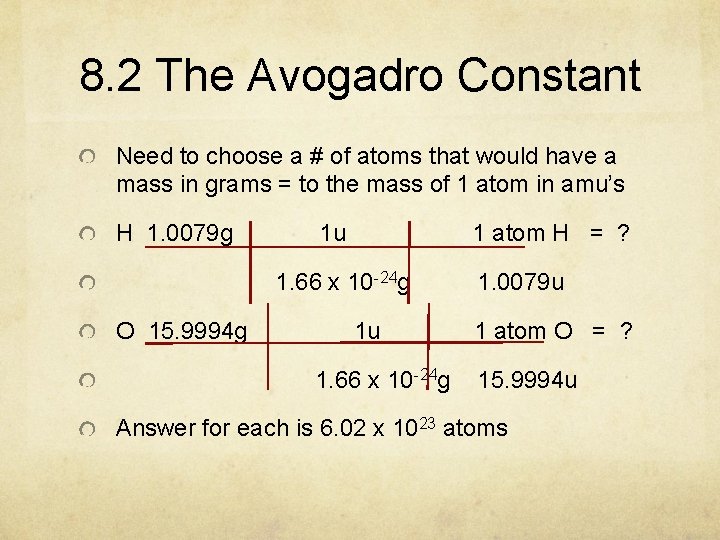













- Slides: 21

Honors Chemistry Chapter 8 The Mole

The Mole Symbol of an elem represents 1 atom of that elem Formula represents 1 molec. or formula unit of the compound These may also represent a group of atoms or formula units Atoms are too small to deal with, so chemists deal w/ large groups of atoms A mole – contains a specific # of atoms or formula units

8. 1 Molecular & Formula Mass H atom’s mass = 1. 67 x 10 -24 g O atom’s mass = 16 x H or 2. 66 x 10 -23 g C atom’s mass = 12 x H or 2. 00 x 10 -23 g Since these #’s are so small, we use a mass scale defined on the atomic level Masses of atoms are compared by using the atomic mass scale Standard is the atomic mass unit (u or amu) Based on 1/12 of a C-12 atom H is 1 u, O is 16 u, H 2 O = 18 u

8. 1 Molecular & Formula Mass Molecular Mass – sum of the atomic masses of all the atoms in a molecule This term is incorrect for ionic compounds Formula Mass – sum of atomic masses of atoms in a formula unit

8. 2 The Avogadro Constant Since chemists can’t count out atoms or molecs, they obtain mass quantities of a subst. It’s important to obtain a relationship betw mass & # of particles Molec & formula masses are in amu’s – too small to meas by ordinary means Need larger unit such as grams
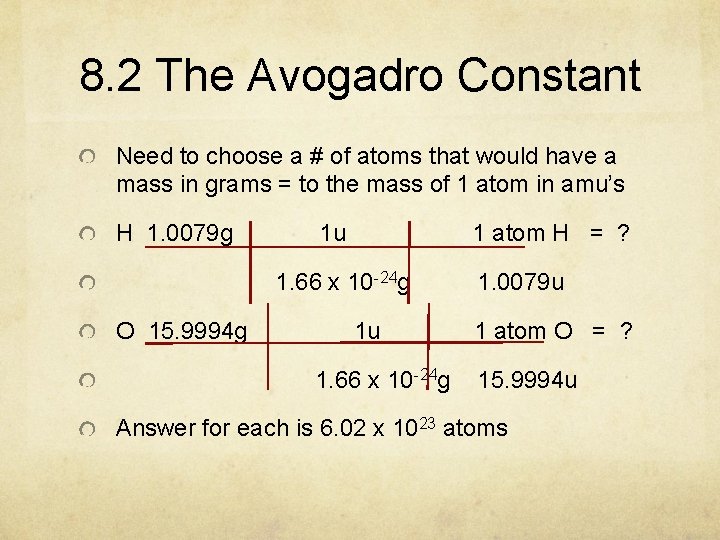
8. 2 The Avogadro Constant Need to choose a # of atoms that would have a mass in grams = to the mass of 1 atom in amu’s H 1. 0079 g 1 u 1 atom H = ? 1. 66 x 10 -24 g O 15. 9994 g 1 u 1. 66 x 10 -24 g 1. 0079 u 1 atom O = ? 15. 9994 u Answer for each is 6. 02 x 1023 atoms

8. 2 The Avogadro Constant 6. 02 x 1023 is called the Avogadro Constant in honor of Amedeo Avogadro, an Italian scientist.

8. 3 The Mole The Avogadro Constant is an SI standard Symbol is NA 6. 02 x 1023 units of anything is call one mole (mol) 1 mole of particles (atoms, ions, molecs, etc) has a mass in grams = to that of one particle in atomic mass units. if 1 mole of a certain type of particle has a mass of 3. 01 g, then a single particle has a mass of 3. 01 u.

8. 3 The Mole 1 mole of molecules = 6. 02 x 1023 molecs 1 mole of atoms = 6. 02 x 1023 atoms 1 mole of formula units = 6. 02 x 1023 formula units 1 mole of ions = 6. 02 x 1023 ions NA can have any of the following units: molecs/mole, atoms/mole, ions/mole, or formula units/mole

8. 3 The Molar Mass – mass of 1 mole of particles (Save room for examples)

8. 4 Moles in Solution Many methods of expressing the relationship betw dissolved subst & the soln. Most common – Molarity (M) – ratio betw the moles of dissoved subst & the vol of soln in dm 3 M = moles of solute dm 3 of soln a one-molar (1 M) soln of salt water contains 1 mole of Na. Cl in 1 dm 3 of soln A. 35 M soln of KNO 3 contains. 35 moles of KNO 3 in 1 dm 3 of soln

8. 4 Moles in Solution ***Note – molarity is expressed in moles per dm 3 of solution, not solvent. to make a 1 M soln of Na. Cl, you do NOT add 1 dm 3 H 2 O to 58. 5 g Na. Cl. The final volume must be 1 dm 3. Dissolve the salt in water, then add enough water to make 1 dm 3 of solution. (Leave room for examples)

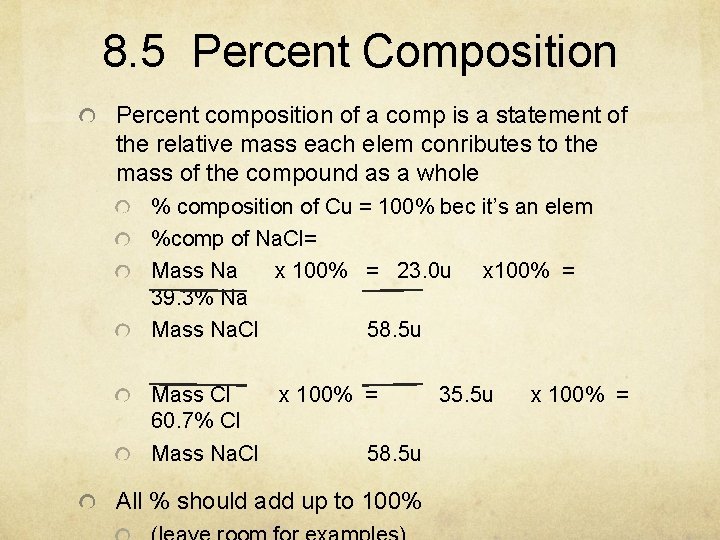
8. 5 Percent Composition Percent composition of a comp is a statement of the relative mass each elem conributes to the mass of the compound as a whole % composition of Cu = 100% bec it’s an elem %comp of Na. Cl= Mass Na x 100% = 23. 0 u x 100% = 39. 3% Na Mass Na. Cl 58. 5 u Mass Cl x 100% = 35. 5 u 60. 7% Cl Mass Na. Cl 58. 5 u All % should add up to 100% x 100% =

8. 6 Empirical Formula - Simplest ratio of the elements in that compound Empirical formulas can be found using experimental data Need only the mass of ea elem in the sample Elems in a compound combine in simple, wholenumber ratios if atoms of elem are present in simple ratios, then the moles of atoms for ea elem in the comp will also be in small, whole-number ratios.

8. 6 Empirical Formula Ex) We have a 2. 50 sample of a comp which contains 0. 900 g Ca and 1. 60 g Cl. Find the empirical formula Step 1 – find the # of moles (leave room in notes) Step 2 – Find the ratio of moles of Ca to moles of Cl Divide each by the smallest # of moles Leave room in notes Step 3 – Find the empirical formula

8. 6 Empirical Formula Empirical formulas can also be found from % composition Assume a 100 g sample & change all % to g Ex) A sample has a % comp of 40. 0% C, 6. 71% H, and 53. 3% O. Find the empirical formula Leave room in notes

8. 6 Empirical Formula Sometimes dividing by the smallest # of moles doesn’t yield a ratio close to a whole # Must multiply all ratios by a whole # to make them whole #’s Ex) a sample of a compound is 66% Ca and 34% P. Find the empirical formula (leave room in notes)

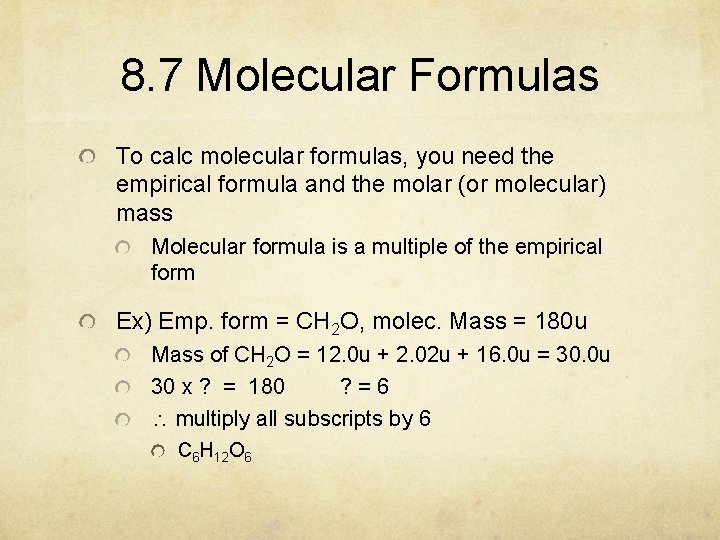
8. 7 Molecular Formulas To calc molecular formulas, you need the empirical formula and the molar (or molecular) mass Molecular formula is a multiple of the empirical form Ex) Emp. form = CH 2 O, molec. Mass = 180 u Mass of CH 2 O = 12. 0 u + 2. 02 u + 16. 0 u = 30. 0 u 30 x ? = 180 ? =6 multiply all subscripts by 6 C 6 H 12 O 6

8. 8 Hydrates Some comps crystallize from a water soln w/ water molecs adhering to ions or molecs & become part of the crystal Hydrates – crystals that contain water molecs Can be dried by heating & driving off the water Then meas how much mass is lost Ni. SO 3 6 H 2 O The means that 6 molecs of water adhere to 1 formula unit.

8. 8 Hydrates To calculate the formula mass of a hydrate, add the mass of the salt (Ni. SO 3) to the mass of the water Ni. SO 3 = 138. 8 u 6 H 2 O = 108. 0 u 246. 8 u = Ni. SO 3 6 H 2 O

8. 8 Hydrates From sample problem on p. 215 or your packet: 10. 407 g hydrate − 9. 520 g dry (anhydrous) Ba. I 2 0. 887 g water Convert mass of dry Ba. I 2 and mass of water to moles (leave room in notes) Find the simplest ratio (divide by smallest #) Ba. I 2 2 H 2 O