Hematology Mastery in Multiple Myeloma Part I Smoldering







- Slides: 24

Hematology Mastery in Multiple Myeloma

Part I: Smoldering Multiple Myeloma and Induction Therapy for Patients with Newly Diagnosed Multiple Myeloma Moderator: Sagar Lonial, MD Panelists: Jonathan Kaufman, MD and Ajay Nooka, MD

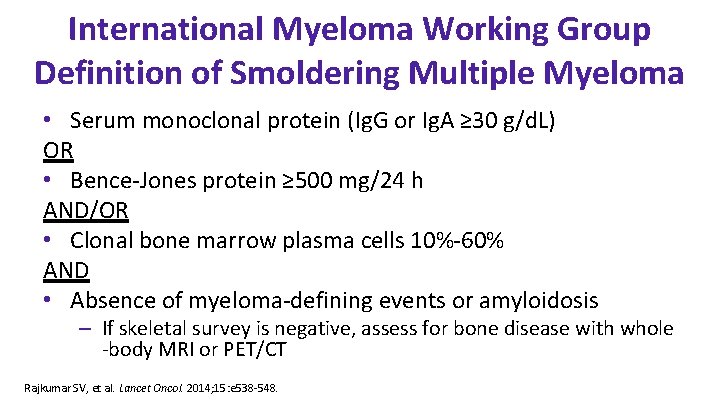
International Myeloma Working Group Definition of Smoldering Multiple Myeloma • Serum monoclonal protein (Ig. G or Ig. A ≥ 30 g/d. L) OR • Bence-Jones protein ≥ 500 mg/24 h AND/OR • Clonal bone marrow plasma cells 10%-60% AND • Absence of myeloma-defining events or amyloidosis – If skeletal survey is negative, assess for bone disease with whole -body MRI or PET/CT Rajkumar SV, et al. Lancet Oncol. 2014; 15: e 538 -548.

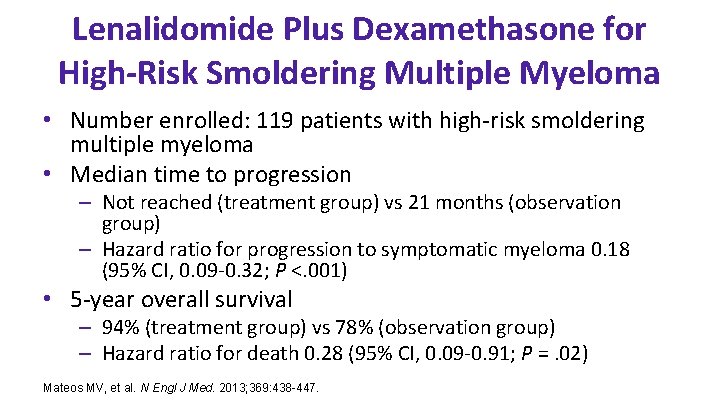
Lenalidomide Plus Dexamethasone for High-Risk Smoldering Multiple Myeloma • Number enrolled: 119 patients with high-risk smoldering multiple myeloma • Median time to progression – Not reached (treatment group) vs 21 months (observation group) – Hazard ratio for progression to symptomatic myeloma 0. 18 (95% CI, 0. 09 -0. 32; P <. 001) • 5 -year overall survival – 94% (treatment group) vs 78% (observation group) – Hazard ratio for death 0. 28 (95% CI, 0. 09 -0. 91; P =. 02) Mateos MV, et al. N Engl J Med. 2013; 369: 438 -447.

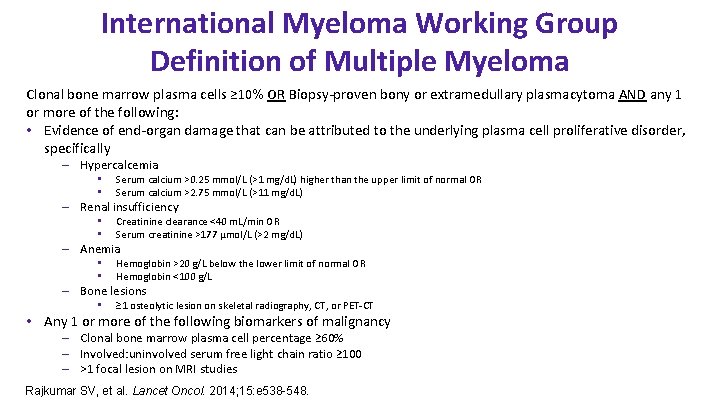
International Myeloma Working Group Definition of Multiple Myeloma Clonal bone marrow plasma cells ≥ 10% OR Biopsy-proven bony or extramedullary plasmacytoma AND any 1 or more of the following: • Evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically – Hypercalcemia • • Serum calcium >0. 25 mmol/L (>1 mg/d. L) higher than the upper limit of normal OR Serum calcium >2. 75 mmol/L (>11 mg/d. L) – Renal insufficiency • • Creatinine clearance <40 m. L/min OR Serum creatinine >177 μmol/L (>2 mg/d. L) – Anemia • • Hemoglobin >20 g/L below the lower limit of normal OR Hemoglobin <100 g/L – Bone lesions • ≥ 1 osteolytic lesion on skeletal radiography, CT, or PET-CT • Any 1 or more of the following biomarkers of malignancy – Clonal bone marrow plasma cell percentage ≥ 60% – Involved: uninvolved serum free light chain ratio ≥ 100 – >1 focal lesion on MRI studies Rajkumar SV, et al. Lancet Oncol. 2014; 15: e 538 -548.

CRAB Criteria • • C: hyper. Calcemia R: Renal failure A: Anemia B: Bone lesions Rajkumar SV, et al. Lancet Oncol. 2014; 15: e 538 -548.

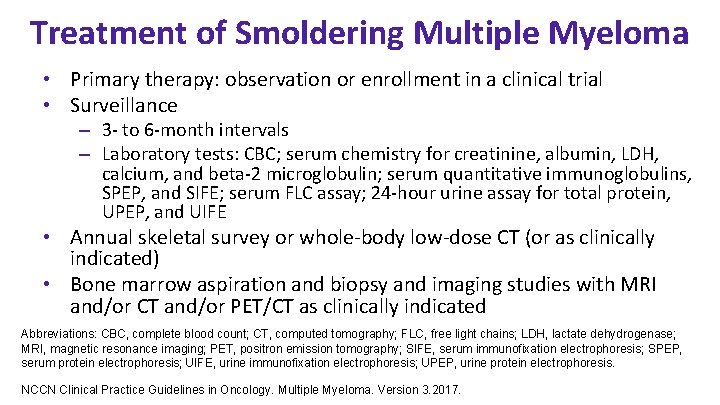
Treatment of Smoldering Multiple Myeloma • Primary therapy: observation or enrollment in a clinical trial • Surveillance – 3 - to 6 -month intervals – Laboratory tests: CBC; serum chemistry for creatinine, albumin, LDH, calcium, and beta-2 microglobulin; serum quantitative immunoglobulins, SPEP, and SIFE; serum FLC assay; 24 -hour urine assay for total protein, UPEP, and UIFE • Annual skeletal survey or whole-body low-dose CT (or as clinically indicated) • Bone marrow aspiration and biopsy and imaging studies with MRI and/or CT and/or PET/CT as clinically indicated Abbreviations: CBC, complete blood count; CT, computed tomography; FLC, free light chains; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PET, positron emission tomography; SIFE, serum immunofixation electrophoresis; SPEP, serum protein electrophoresis; UIFE, urine immunofixation electrophoresis; UPEP, urine protein electrophoresis. NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3. 2017.

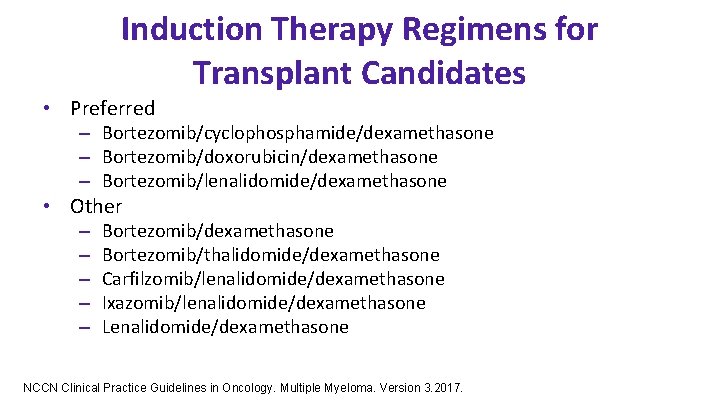
Induction Therapy Regimens for Transplant Candidates • Preferred – Bortezomib/cyclophosphamide/dexamethasone – Bortezomib/doxorubicin/dexamethasone – Bortezomib/lenalidomide/dexamethasone • Other – – – Bortezomib/dexamethasone Bortezomib/thalidomide/dexamethasone Carfilzomib/lenalidomide/dexamethasone Ixazomib/lenalidomide/dexamethasone Lenalidomide/dexamethasone NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3. 2017.

SWOG S 0777—Results • • Number enrolled: 473 patients with newly diagnosed multiple myeloma Median progression-free survival – 43 mo with bortezomib, lenalidomide, and dexamethasone (VRD) vs 30 mo with lenalidomide and dexamethasone alone (RD) – HR 0. 712, 96% CI 0. 56 -0. 906, one-sided P =. 0018 • Median overall survival – 75 mo with VRD vs 64 mo with RD – HR 0. 709, 95% CI 0. 524 -0. 959, two-sided P =. 025 • Overall response rates (partial response or better) – 82% with VRD vs 72% with RD • Complete response or better – 16% with VRD vs 8% with RD Durie BG, et al. Lancet. 2017; 389: 519 -527.

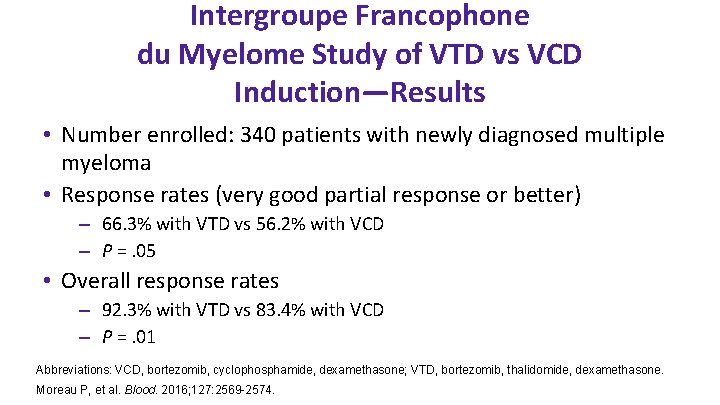
Intergroupe Francophone du Myelome Study of VTD vs VCD Induction—Results • Number enrolled: 340 patients with newly diagnosed multiple myeloma • Response rates (very good partial response or better) – 66. 3% with VTD vs 56. 2% with VCD – P =. 05 • Overall response rates – 92. 3% with VTD vs 83. 4% with VCD – P =. 01 Abbreviations: VCD, bortezomib, cyclophosphamide, dexamethasone; VTD, bortezomib, thalidomide, dexamethasone. Moreau P, et al. Blood. 2016; 127: 2569 -2574.

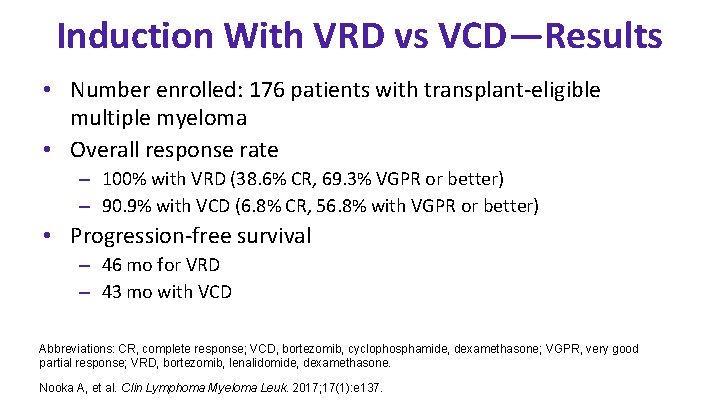
Induction With VRD vs VCD—Results • Number enrolled: 176 patients with transplant-eligible multiple myeloma • Overall response rate – 100% with VRD (38. 6% CR, 69. 3% VGPR or better) – 90. 9% with VCD (6. 8% CR, 56. 8% with VGPR or better) • Progression-free survival – 46 mo for VRD – 43 mo with VCD Abbreviations: CR, complete response; VCD, bortezomib, cyclophosphamide, dexamethasone; VGPR, very good partial response; VRD, bortezomib, lenalidomide, dexamethasone. Nooka A, et al. Clin Lymphoma Myeloma Leuk. 2017; 17(1): e 137.

Induction Therapy Regimens for Transplant Candidates • Immunomodulatory + protease inhibitor triplets – – Bortezomib/lenalidomide/dexamethasone Bortezomib/thalidomide/dexamethasone Carfilzomib/lenalidomide/dexamethasone Ixazomib/lenalidomide/dexamethasone • Chemotherapy + protease inhibitor triplets – Bortezomib/cyclophosphamide/dexamethasone – Bortezomib/doxorubicin/dexamethasone • Doublets – Bortezomib/dexamethasone – Lenalidomide/dexamethasone NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3. 2017.

Part II: Transplantation, Consolidation, and Maintenance Therapy for Multiple Myeloma Moderator: Sagar Lonial, MD Panelists: Jonathan Kaufman, MD and Ajay Nooka, MD

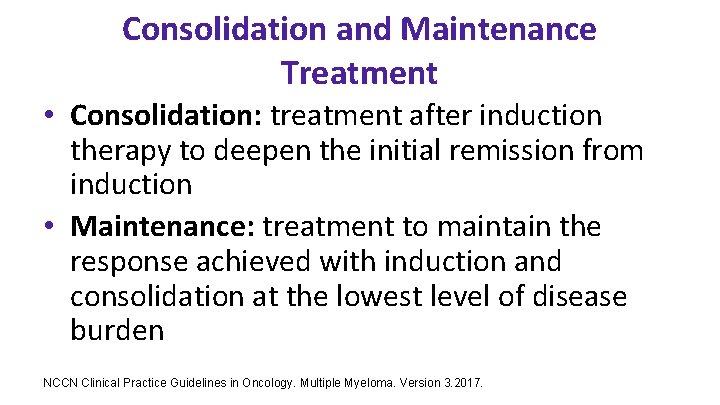
Consolidation and Maintenance Treatment • Consolidation: treatment after induction therapy to deepen the initial remission from induction • Maintenance: treatment to maintain the response achieved with induction and consolidation at the lowest level of disease burden NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3. 2017.

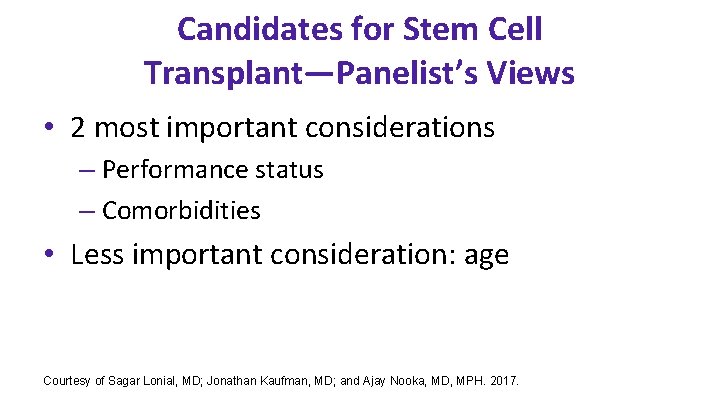
Candidates for Stem Cell Transplant—Panelist’s Views • 2 most important considerations – Performance status – Comorbidities • Less important consideration: age Courtesy of Sagar Lonial, MD; Jonathan Kaufman, MD; and Ajay Nooka, MD, MPH. 2017.

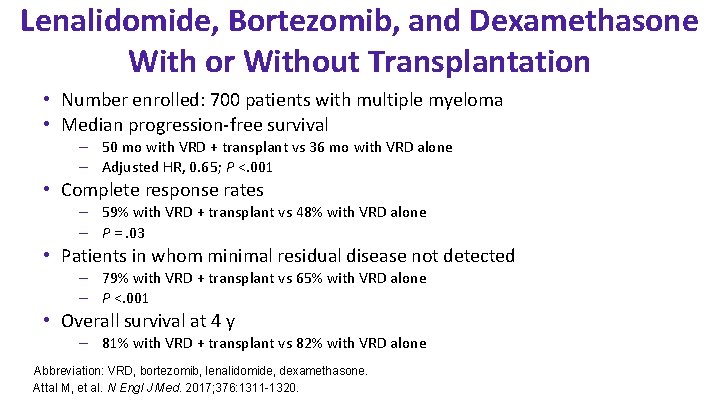
Lenalidomide, Bortezomib, and Dexamethasone With or Without Transplantation • Number enrolled: 700 patients with multiple myeloma • Median progression-free survival – 50 mo with VRD + transplant vs 36 mo with VRD alone – Adjusted HR, 0. 65; P <. 001 • Complete response rates – 59% with VRD + transplant vs 48% with VRD alone – P =. 03 • Patients in whom minimal residual disease not detected – 79% with VRD + transplant vs 65% with VRD alone – P <. 001 • Overall survival at 4 y – 81% with VRD + transplant vs 82% with VRD alone Abbreviation: VRD, bortezomib, lenalidomide, dexamethasone. Attal M, et al. N Engl J Med. 2017; 376: 1311 -1320.

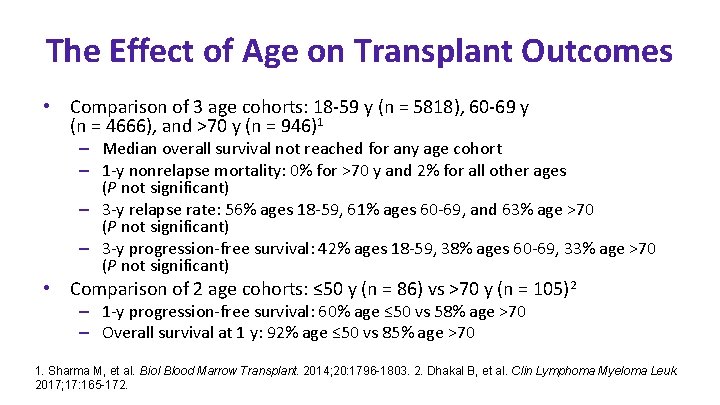
The Effect of Age on Transplant Outcomes • Comparison of 3 age cohorts: 18 -59 y (n = 5818), 60 -69 y (n = 4666), and >70 y (n = 946)1 – Median overall survival not reached for any age cohort – 1 -y nonrelapse mortality: 0% for >70 y and 2% for all other ages (P not significant) – 3 -y relapse rate: 56% ages 18 -59, 61% ages 60 -69, and 63% age >70 (P not significant) – 3 -y progression-free survival: 42% ages 18 -59, 38% ages 60 -69, 33% age >70 (P not significant) • Comparison of 2 age cohorts: ≤ 50 y (n = 86) vs >70 y (n = 105)2 – 1 -y progression-free survival: 60% age ≤ 50 vs 58% age >70 – Overall survival at 1 y: 92% age ≤ 50 vs 85% age >70 1. Sharma M, et al. Biol Blood Marrow Transplant. 2014; 20: 1796 -1803. 2. Dhakal B, et al. Clin Lymphoma Myeloma Leuk. 2017; 17: 165 -172.

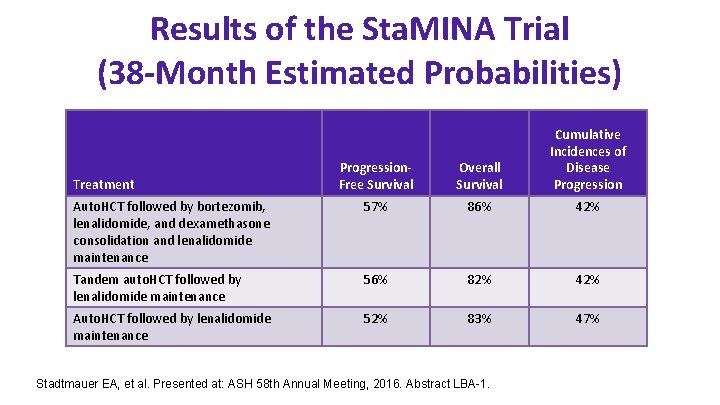
Results of the Sta. MINA Trial (38 -Month Estimated Probabilities) Progression. Free Survival Overall Survival Cumulative Incidences of Disease Progression Auto. HCT followed by bortezomib, lenalidomide, and dexamethasone consolidation and lenalidomide maintenance 57% 86% 42% Tandem auto. HCT followed by lenalidomide maintenance 56% 82% 42% Auto. HCT followed by lenalidomide maintenance 52% 83% 47% Treatment Stadtmauer EA, et al. Presented at: ASH 58 th Annual Meeting, 2016. Abstract LBA-1.

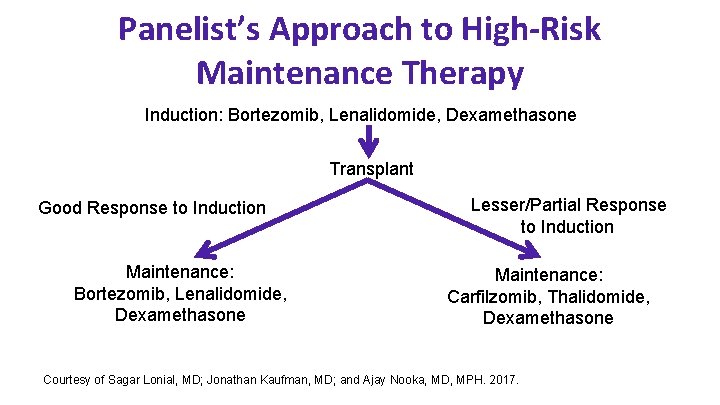
Panelist’s Approach to High-Risk Maintenance Therapy Induction: Bortezomib, Lenalidomide, Dexamethasone Transplant Good Response to Induction Maintenance: Bortezomib, Lenalidomide, Dexamethasone Lesser/Partial Response to Induction Maintenance: Carfilzomib, Thalidomide, Dexamethasone Courtesy of Sagar Lonial, MD; Jonathan Kaufman, MD; and Ajay Nooka, MD, MPH. 2017.

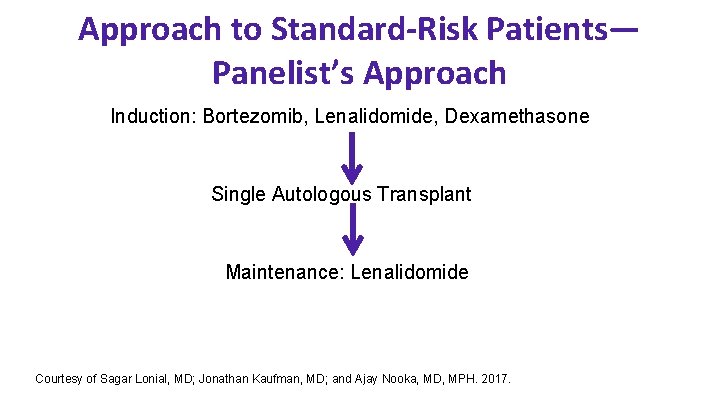
Approach to Standard-Risk Patients— Panelist’s Approach Induction: Bortezomib, Lenalidomide, Dexamethasone Single Autologous Transplant Maintenance: Lenalidomide Courtesy of Sagar Lonial, MD; Jonathan Kaufman, MD; and Ajay Nooka, MD, MPH. 2017.

Part III: Management of Relapsed Multiple Myeloma Moderator: Sagar Lonial, MD Panelists: Jonathan Kaufman, MD and Ajay Nooka, MD

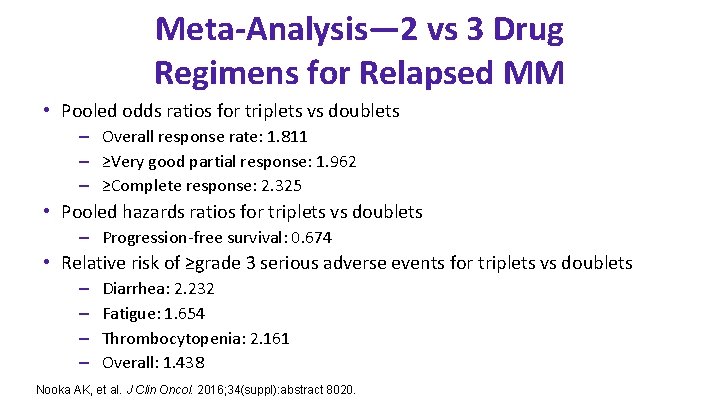
Meta-Analysis— 2 vs 3 Drug Regimens for Relapsed MM • Pooled odds ratios for triplets vs doublets – Overall response rate: 1. 811 – ≥Very good partial response: 1. 962 – ≥Complete response: 2. 325 • Pooled hazards ratios for triplets vs doublets – Progression-free survival: 0. 674 • Relative risk of ≥grade 3 serious adverse events for triplets vs doublets – – Diarrhea: 2. 232 Fatigue: 1. 654 Thrombocytopenia: 2. 161 Overall: 1. 438 Nooka AK, et al. J Clin Oncol. 2016; 34(suppl): abstract 8020.

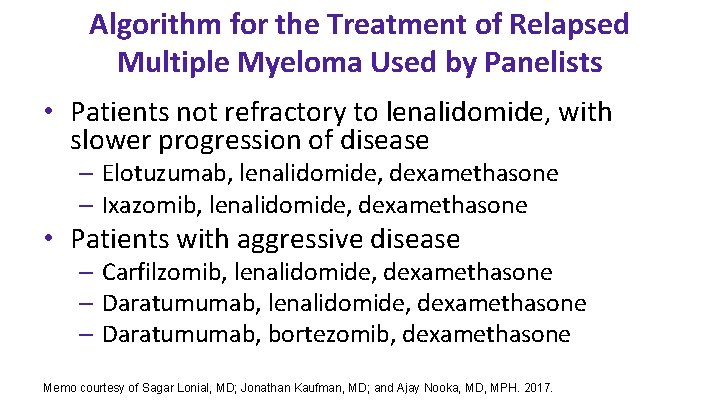
Algorithm for the Treatment of Relapsed Multiple Myeloma Used by Panelists • Patients not refractory to lenalidomide, with slower progression of disease – Elotuzumab, lenalidomide, dexamethasone – Ixazomib, lenalidomide, dexamethasone • Patients with aggressive disease – Carfilzomib, lenalidomide, dexamethasone – Daratumumab, bortezomib, dexamethasone Memo courtesy of Sagar Lonial, MD; Jonathan Kaufman, MD; and Ajay Nooka, MD, MPH. 2017.

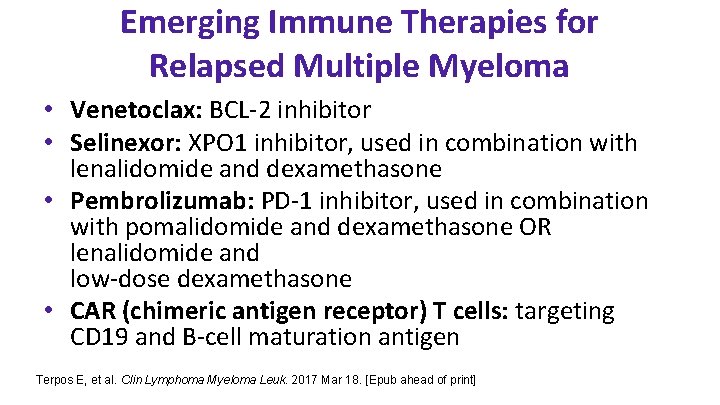
Emerging Immune Therapies for Relapsed Multiple Myeloma • Venetoclax: BCL-2 inhibitor • Selinexor: XPO 1 inhibitor, used in combination with lenalidomide and dexamethasone • Pembrolizumab: PD-1 inhibitor, used in combination with pomalidomide and dexamethasone OR lenalidomide and low-dose dexamethasone • CAR (chimeric antigen receptor) T cells: targeting CD 19 and B-cell maturation antigen Terpos E, et al. Clin Lymphoma Myeloma Leuk. 2017 Mar 18. [Epub ahead of print]
 Smouldering myeloma
Smouldering myeloma Smoldering multiple myeloma
Smoldering multiple myeloma Plasma cell dyscrasia
Plasma cell dyscrasia Mayo clinic multiple myeloma
Mayo clinic multiple myeloma Rulo formasyonu yapan hastalıklar
Rulo formasyonu yapan hastalıklar Waldenstrom macroglobulinemia vs multiple myeloma
Waldenstrom macroglobulinemia vs multiple myeloma Crab criteria multiple myeloma
Crab criteria multiple myeloma Kpd multiple myeloma
Kpd multiple myeloma Mielma
Mielma Mieloma smoldering wikipedia
Mieloma smoldering wikipedia Mieloma smoldering
Mieloma smoldering Discrasie plasmacellulari
Discrasie plasmacellulari Myeloma uk forum
Myeloma uk forum European myeloma network
European myeloma network Myeloma
Myeloma Daratumumab macmillan
Daratumumab macmillan Myeloma euronet
Myeloma euronet Delayed multiple baseline design
Delayed multiple baseline design Advantages and disadvantages of mimd
Advantages and disadvantages of mimd Dr. biren saraiya hematology
Dr. biren saraiya hematology Hematology day
Hematology day Medical hematology student lectures
Medical hematology student lectures American society of hematology
American society of hematology Sucking tube hematology
Sucking tube hematology Quality control in hematology wikipedia
Quality control in hematology wikipedia