Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3







































- Slides: 83

Grotzinger • Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks © 2011 by W. H. Freeman and Company

Chapter 3: Earth Materials: Minerals and Rocks

About Earth Materials • All Earth materials are composed of atoms bound together. • Minerals are composed of atoms bonded together and are the building blocks of rocks. • Rocks are composed of minerals and they record various geologic processes.

Lecture Outline 1. What are minerals? 2. 2. The structure of matter 3. 3. The formation of minerals 4. 4. Classes of rock-forming minerals 5. 5. Physical properties of minerals 6. 6. What are rocks?

Lecture Outline 7. The rock cycle: interactions between the plate tectonic and climate systems 8. Concentrations of valuable mineral resources

1. What Are Minerals? Minerals are the building blocks of rocks.

1. What Are Minerals? Geologists define mineral as a naturally occurring, solid, crystalline substance, usually inorganic, with a specific chemical composition.

1. What Are Minerals? Naturally occurring = found in nature Solid, crystalline substance = atoms are arranged in orderly patterns Usually inorganic = not a product of living tissue With a specific chemical formula = unique chemical composition

Thought questions for this chapter Coal, a natural organic substance that forms from decaying vegetation, is not considered to be a mineral. However, when coal is heated to high temperatures and buried under high pressures, it is transformed into the mineral graphite. Why is it, then, that coal is not considered a mineral, but graphite is? Explain your reasoning.

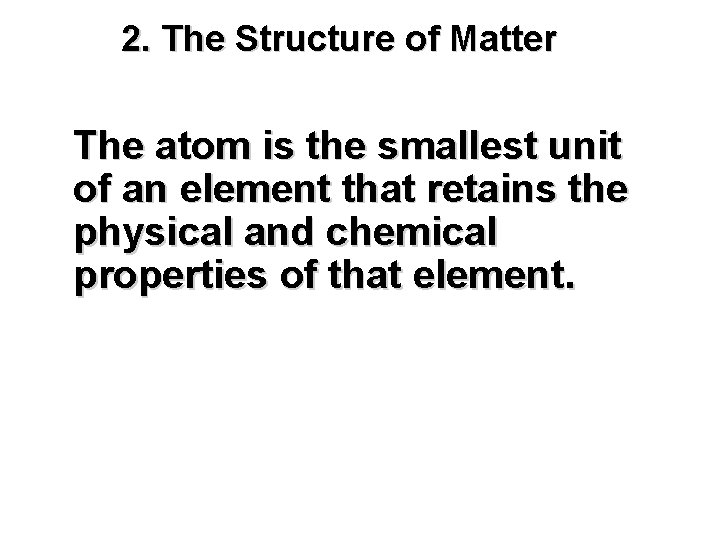
2. The Structure of Matter The atom is the smallest unit of an element that retains the physical and chemical properties of that element.

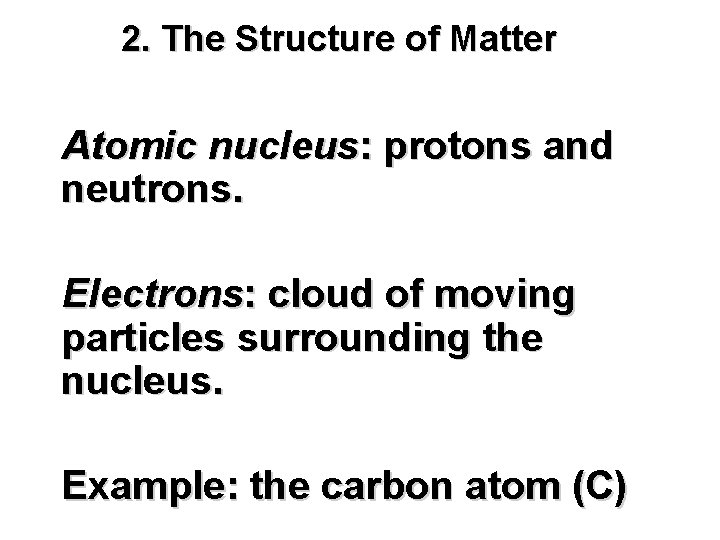
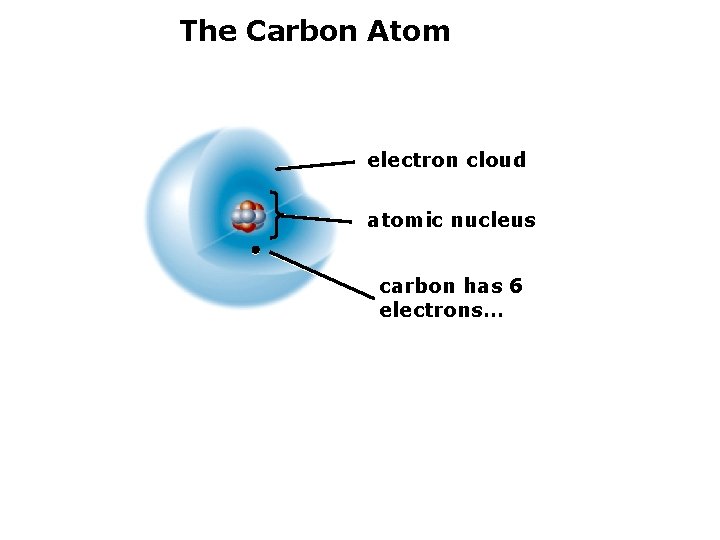
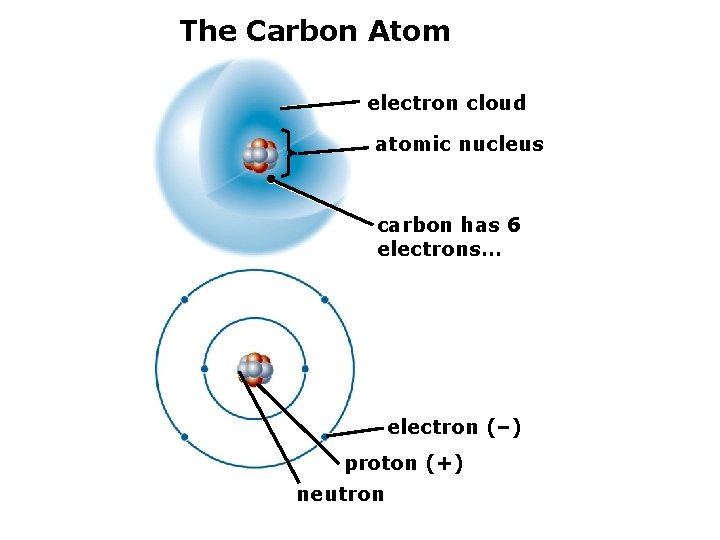
2. The Structure of Matter Atomic nucleus: protons and neutrons. Electrons: cloud of moving particles surrounding the nucleus. Example: the carbon atom (C)

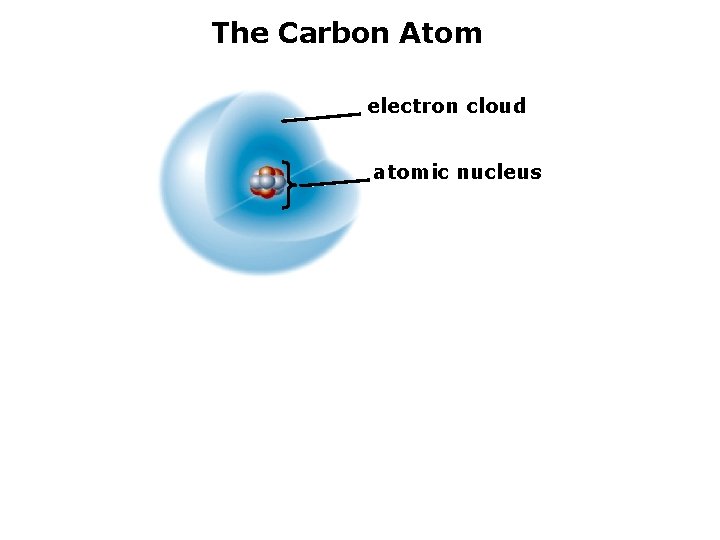
The Carbon Atom electron cloud atomic nucleus

The Carbon Atom electron cloud atomic nucleus carbon has 6 electrons…

The Carbon Atom electron cloud atomic nucleus carbon has 6 electrons… electron (–) proton (+) neutron

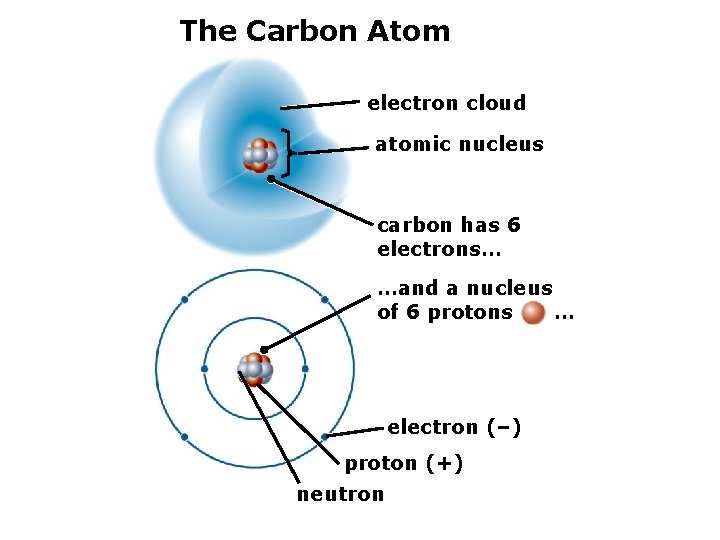
The Carbon Atom electron cloud atomic nucleus carbon has 6 electrons… …and a nucleus of 6 protons … electron (–) proton (+) neutron

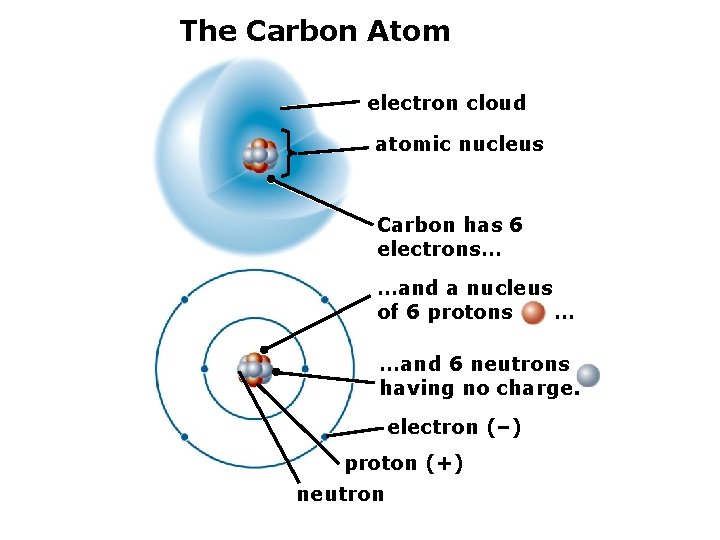
The Carbon Atom electron cloud atomic nucleus Carbon has 6 electrons… …and a nucleus of 6 protons … …and 6 neutrons having no charge. electron (–) proton (+) neutron

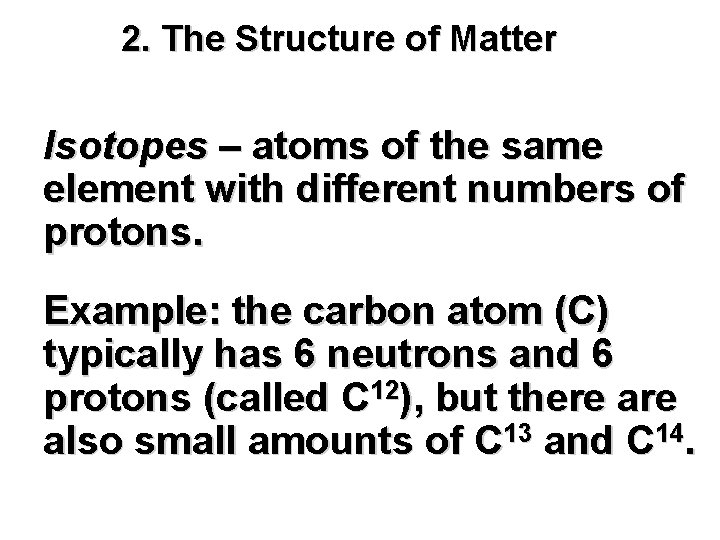
2. The Structure of Matter Isotopes – atoms of the same element with different numbers of protons. Example: the carbon atom (C) typically has 6 neutrons and 6 protons (called C 12), but there also small amounts of C 13 and C 14.

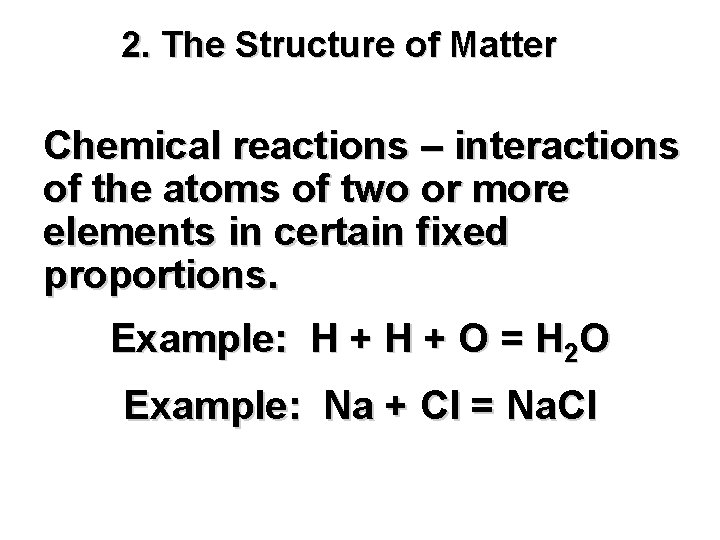
2. The Structure of Matter Chemical reactions – interactions of the atoms of two or more elements in certain fixed proportions. Example: H + O = H 2 O Example: Na + Cl = Na. Cl

2. The Structure of Matter Chemical compounds that are minerals form by: electron sharing or electron transfer

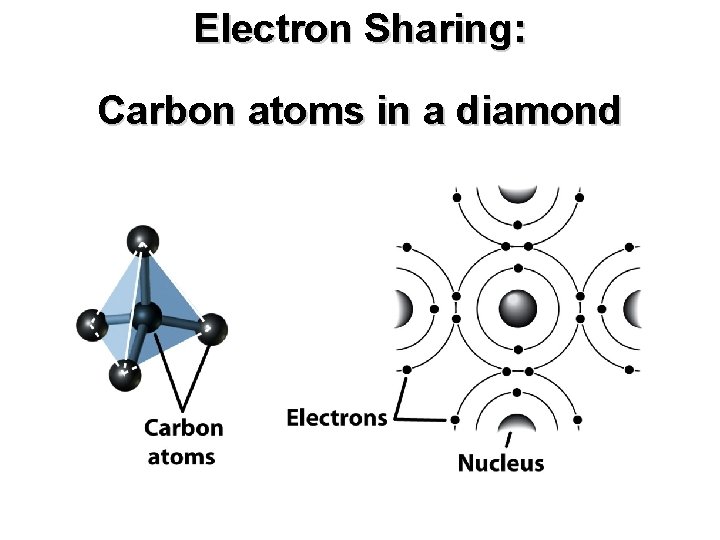
Electron Sharing: Carbon atoms in a diamond

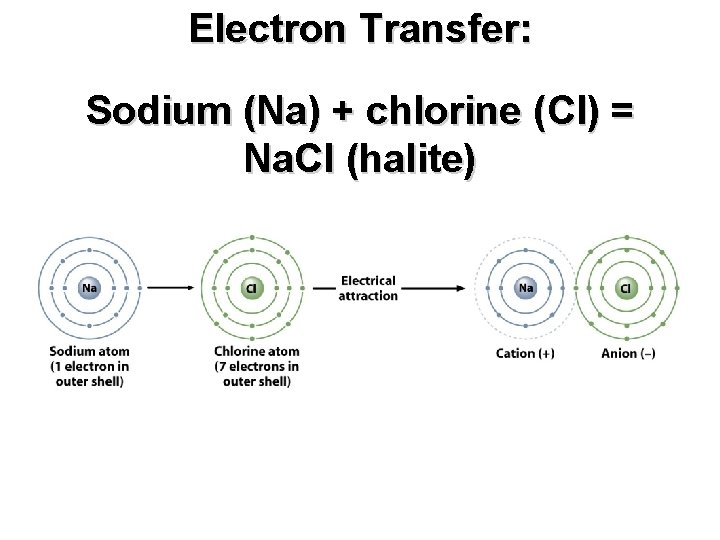
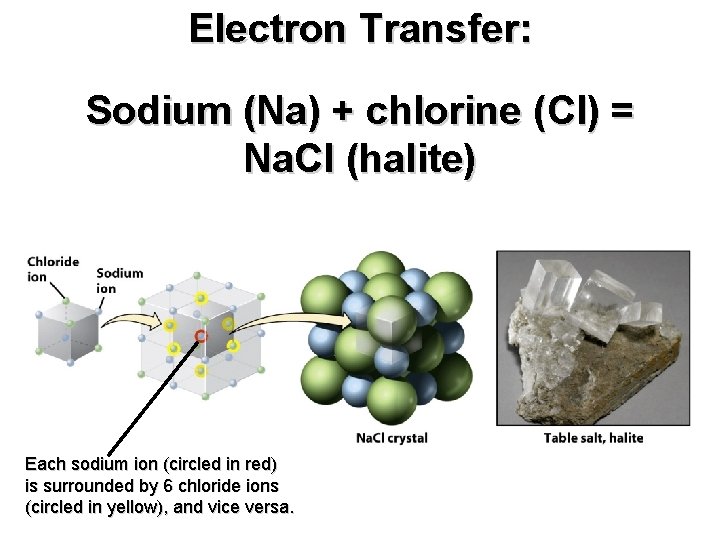
Electron Transfer: Sodium (Na) + chlorine (Cl) = Na. Cl (halite)

Electron Transfer: Sodium (Na) + chlorine (Cl) = Na. Cl (halite) Each sodium ion (circled in red) is surrounded by 6 chloride ions (circled in yellow), and vice versa.

3. The Structure of Minerals How do minerals form? Crystallization – atoms come together in the proper proportion and proper arrangement

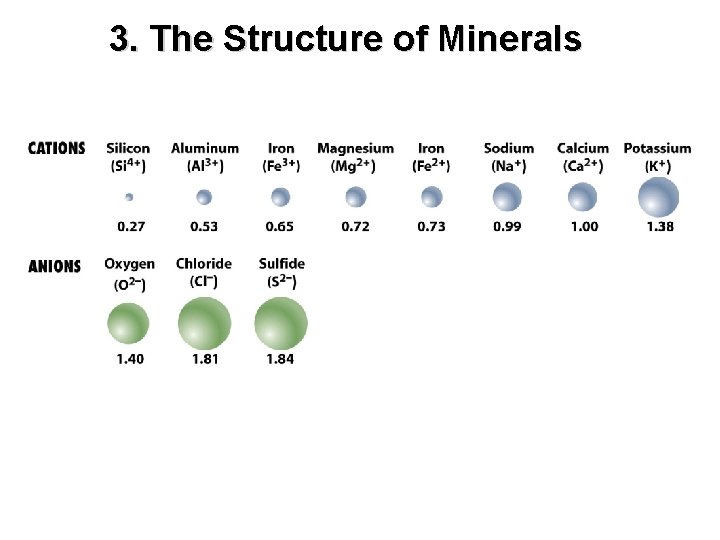
3. The Structure of Minerals Electrical charges of atomic ions Cation – positively charged Anion – negatively charged Atomic ions arrange themselves according to charge and size.

3. The Structure of Minerals The forces of electrical attraction between protons (+) and electrons (-) that hold minerals and other chemical compounds together covalent bonds ionic bonds metallic bonds

3. The Structure of Minerals

3. The Structure of Minerals When do minerals form? • During cooling of molten rock • During evaporation of water • Upon changes in temperature and pressure on existing minerals

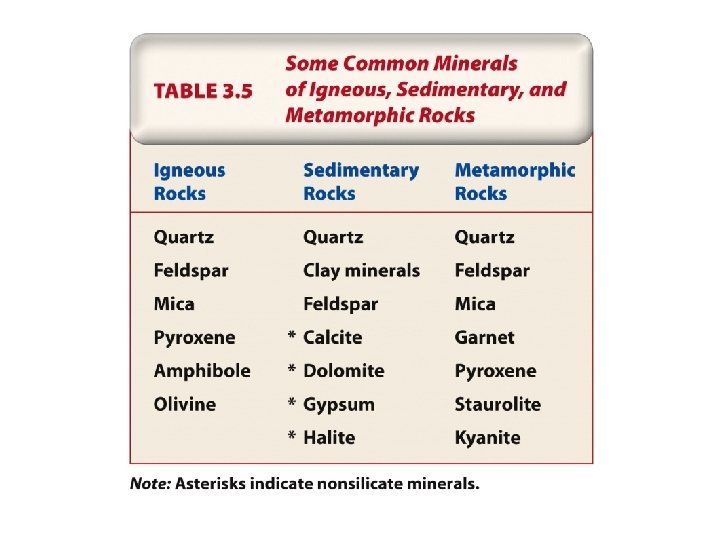
4. Classes of Rock-forming Minerals Chemical classes of minerals: • • • Silicates – contain O and Si Carbonates – contain C and O Oxides – contain O and metallic cations • Sulfides – contain S and metallic cations • Sulfates – contain SO 4 and metallic cations

4. Classes of Rock-forming Minerals Chemical classes (cont. ): • Halides – contain Cl, F, I, or Br • Hydroxides – contain OH • Native elements – masses of all the same element metallically bonded

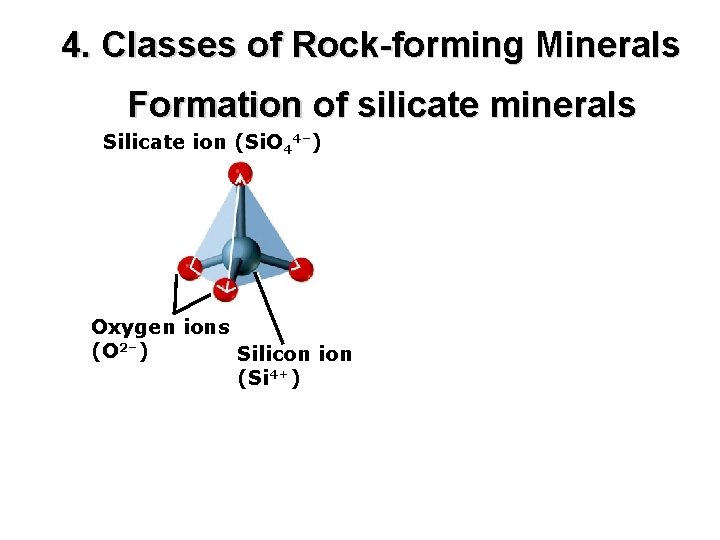
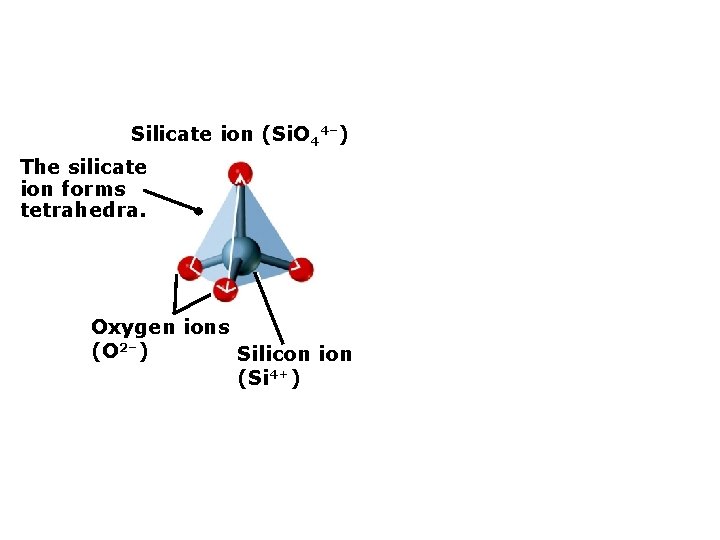
4. Classes of Rock-forming Minerals Formation of silicate minerals Silicate ion (Si. O 44–) Oxygen ions (O 2–) Silicon ion (Si 4+)

Silicate ion (Si. O 44–) The silicate ion forms tetrahedra. Oxygen ions (O 2–) Silicon ion (Si 4+)

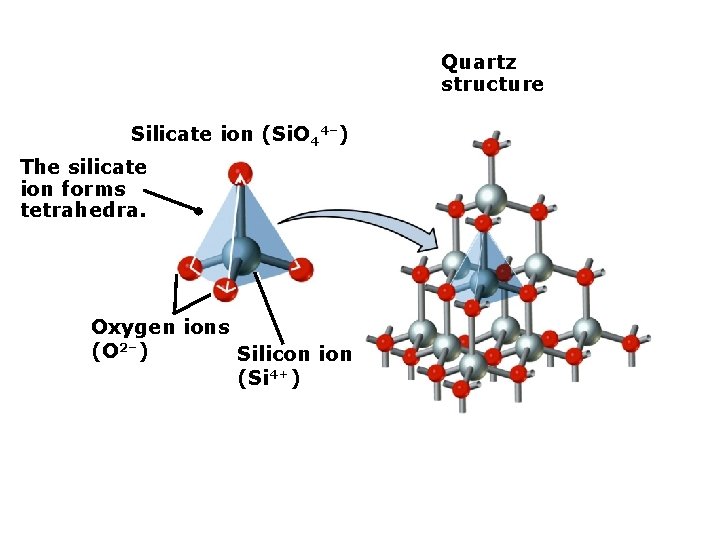
Quartz structure Silicate ion (Si. O 44–) The silicate ion forms tetrahedra. Oxygen ions (O 2–) Silicon ion (Si 4+)

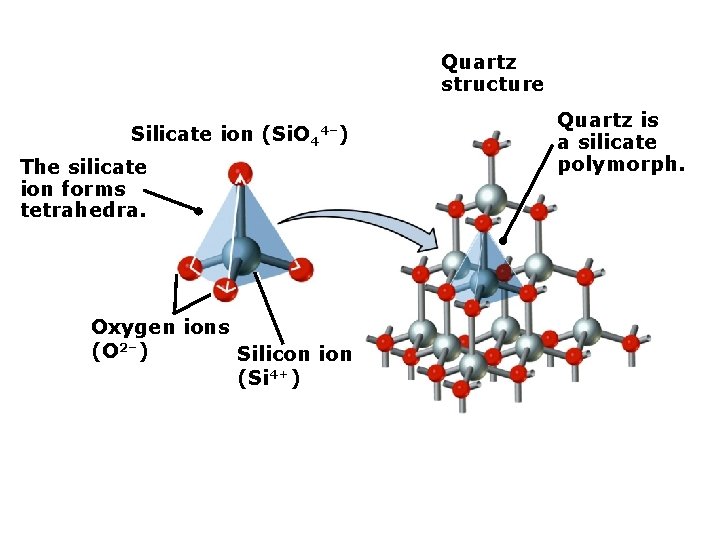
Quartz structure Silicate ion (Si. O 4 4–) The silicate ion forms tetrahedra. Oxygen ions (O 2–) Silicon ion (Si 4+) Quartz is a silicate polymorph.

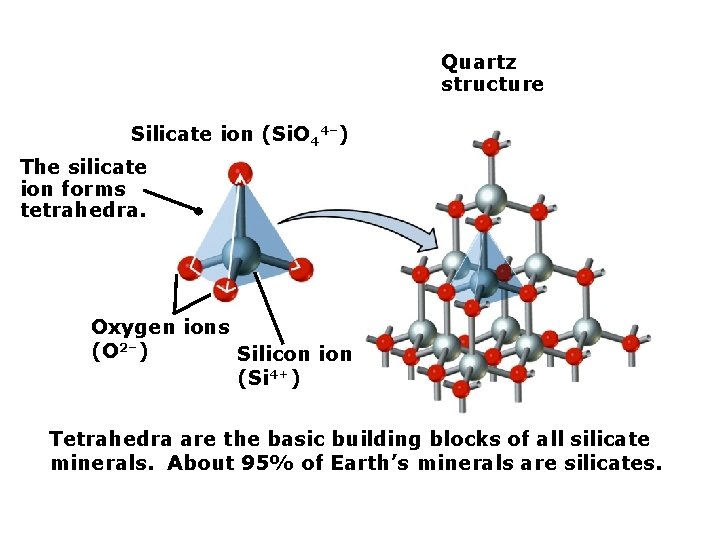
Quartz structure Silicate ion (Si. O 44–) The silicate ion forms tetrahedra. Oxygen ions (O 2–) Silicon ion (Si 4+) Tetrahedra are the basic building blocks of all silicate minerals. About 95% of Earth’s minerals are silicates.

Thought questions for this chapter Draw a simple diagram to show silicon and oxygen in silicate minerals share electrons.

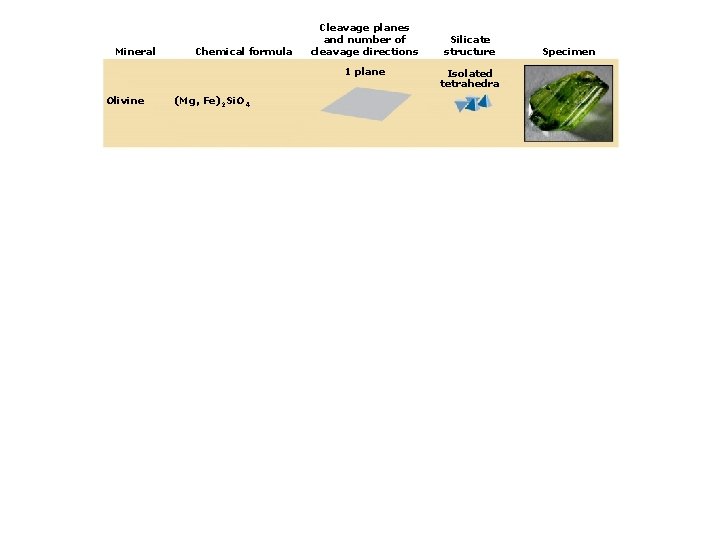
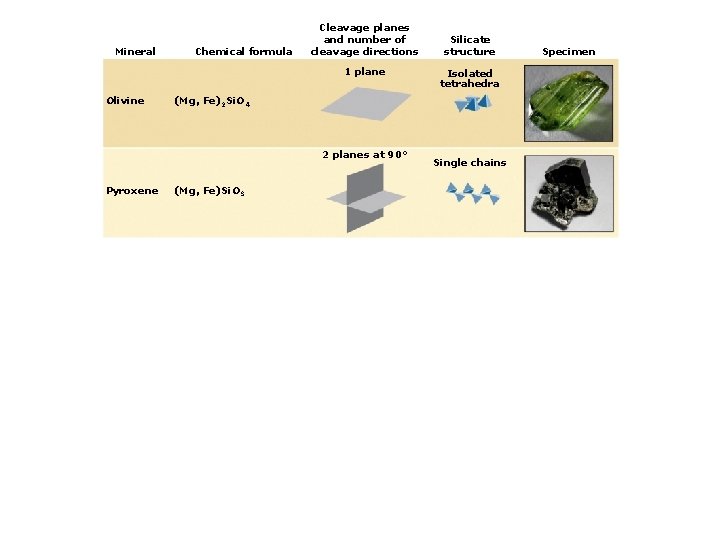
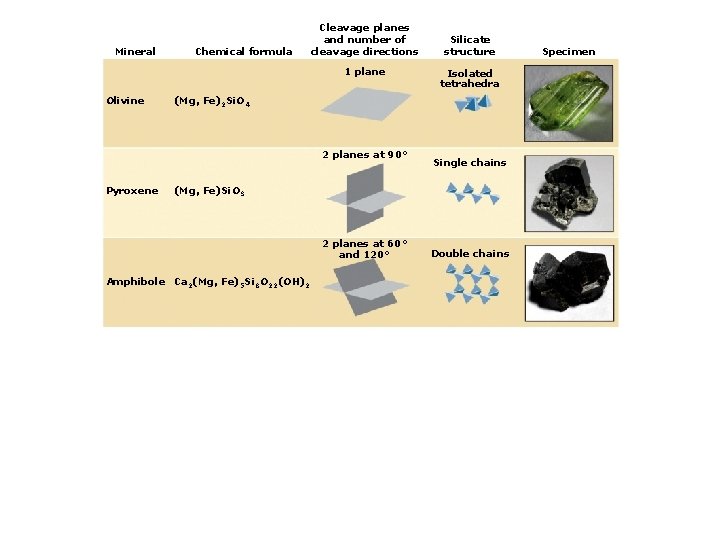
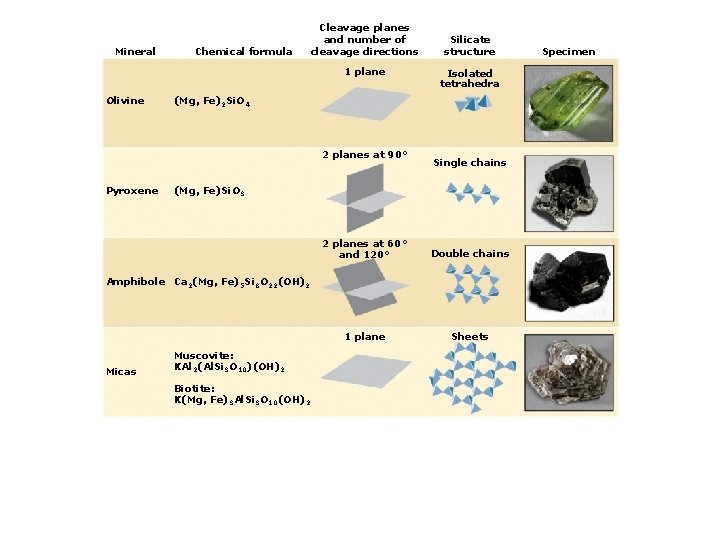
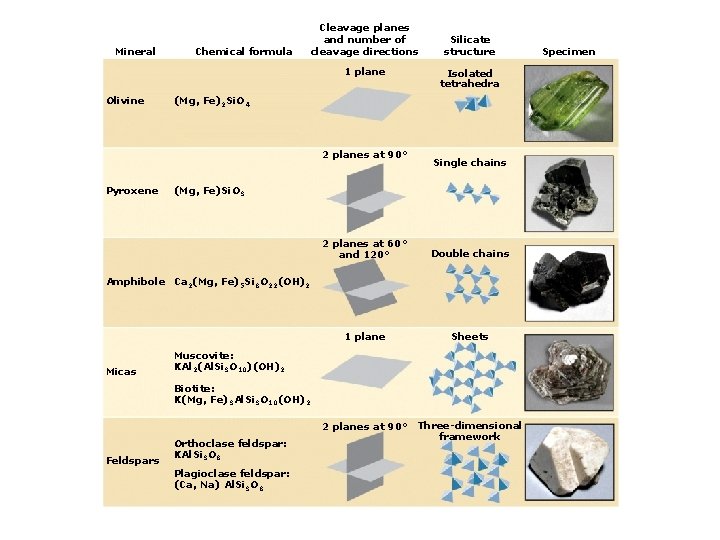
4. Classes of Rock-forming Minerals Types of silicate minerals: Isolated silica tetrahedra Single-chain linkages Double-chain linkages Sheet linkages Frameworks

Mineral Chemical formula Cleavage planes and number of cleavage directions 1 plane Olivine (Mg, Fe)2 Si. O 4 Silicate structure Isolated tetrahedra Specimen

Mineral Chemical formula Cleavage planes and number of cleavage directions 1 plane Olivine Isolated tetrahedra (Mg, Fe)2 Si. O 4 2 planes at 90° Pyroxene Silicate structure (Mg, Fe)Si. O 3 Single chains Specimen

Mineral Chemical formula Cleavage planes and number of cleavage directions 1 plane Olivine Isolated tetrahedra (Mg, Fe)2 Si. O 4 2 planes at 90° Pyroxene Silicate structure Single chains (Mg, Fe)Si. O 3 2 planes at 60° and 120° Amphibole Ca 2(Mg, Fe)5 Si 8 O 22(OH)2 Double chains Specimen

Mineral Chemical formula Cleavage planes and number of cleavage directions 1 plane Olivine Isolated tetrahedra (Mg, Fe)2 Si. O 4 2 planes at 90° Pyroxene Silicate structure Single chains (Mg, Fe)Si. O 3 2 planes at 60° and 120° Double chains 1 plane Sheets Amphibole Ca 2(Mg, Fe)5 Si 8 O 22(OH)2 Micas Muscovite: KAl 2(Al. Si 3 O 10)(OH)2 Biotite: K(Mg, Fe)3 Al. Si 3 O 10(OH)2 Specimen

Mineral Chemical formula Cleavage planes and number of cleavage directions 1 plane Olivine Isolated tetrahedra (Mg, Fe)2 Si. O 4 2 planes at 90° Pyroxene Silicate structure Single chains (Mg, Fe)Si. O 3 2 planes at 60° and 120° Double chains 1 plane Sheets 2 planes at 90° Three-dimensional framework Amphibole Ca 2(Mg, Fe)5 Si 8 O 22(OH)2 Micas Muscovite: KAl 2(Al. Si 3 O 10)(OH)2 Biotite: K(Mg, Fe)3 Al. Si 3 O 10(OH)2 Feldspars Orthoclase feldspar: KAl. Si 3 O 8 Plagioclase feldspar: (Ca, Na) Al. Si 3 O 8 Specimen

Thought questions for this chapter Diopside, a pyroxene, has the formula (Ca, Mg)2 Si 2 O 6. What does that tell you about its crystal structure and cation substitution? What physical properties of sheet silicates are related to their crystal structure?

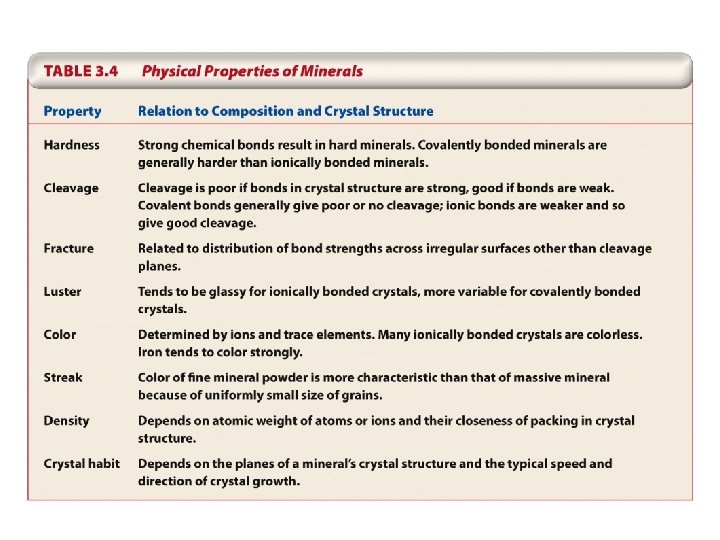
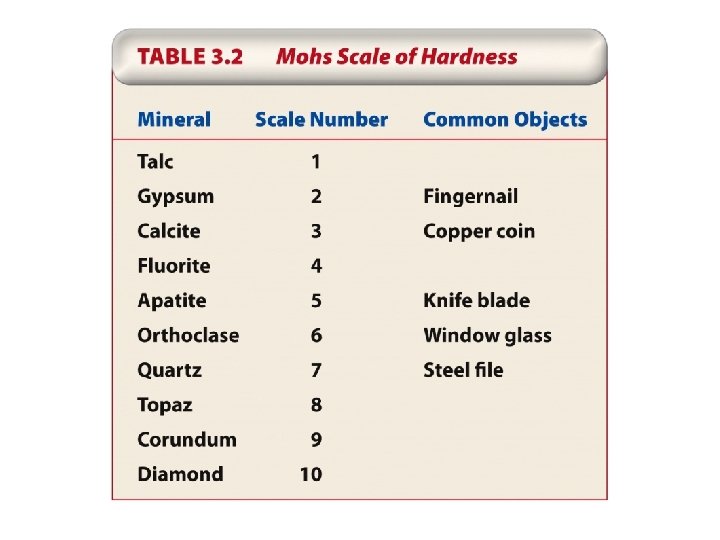
5. Physical Properties of Minerals Hardness Cleavage Fracture Luster Color Streak Density Crystal habit



5. Physical Properties of Minerals Uses of physical properties: Mineral identification Industrial application of minerals

5. Physical Properties of Minerals Mica and its cleavage

5. Physical Properties of Minerals Pyrite and its crystal habit

5. Physical Properties of Minerals Calcite and its cleavage

5. Physical Properties of Minerals

5. Physical Properties of Minerals Hematite and its streak

Thought questions for this chapter Aragonite, with a density of 2. 9 g/cm 3, has exactly the same chemical composition as calcite, which has a density of 2. 7 g/cm 3. Other things being equal, which of these two minerals is more likely to have formed under high pressure? There at least seven physical properties one can use to identify an unknown mineral. Which ones are most useful in discriminating between minerals that look similar? Describe a strategy that would allow you to prove that an unknown clear calcite crystal is not the same mineral as a known clear crystal of quartz.

Thought questions for this chapter Choose two minerals from Appendix 4 that you think might make good abrasive or grinding stones for sharpening steel, and describe the physical properties that cause you to believe they would be suitable for that purpose.

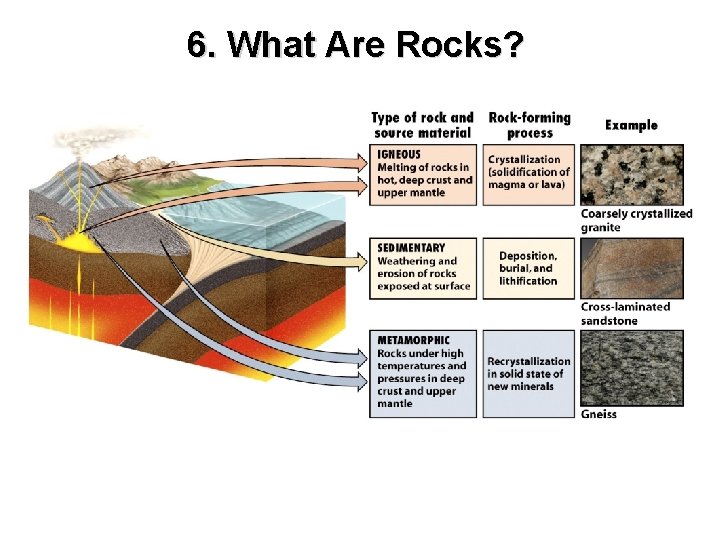
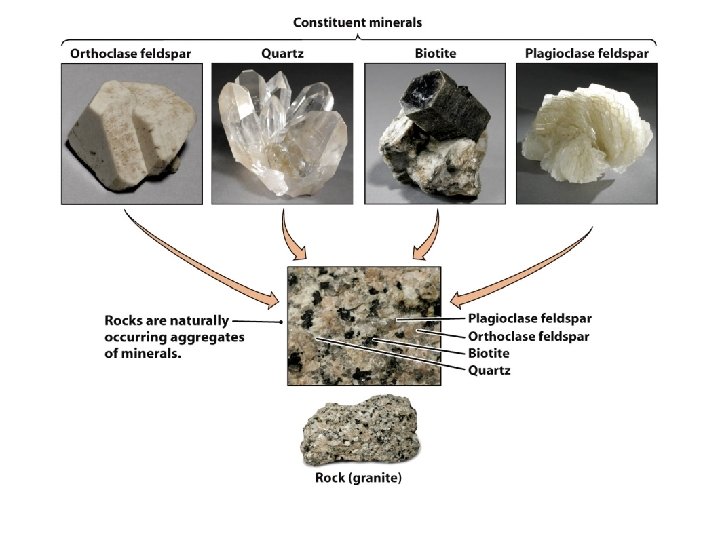
6. What Are Rocks? Rocks are naturally occurring solid aggregates of minerals, or in some cases, non-mineral solid matter. Identity is determined by: texture composition

6. What Are Rocks? Rocks are classified into three groups: Igneous Sedimentary Metamorphic

6. What Are Rocks?


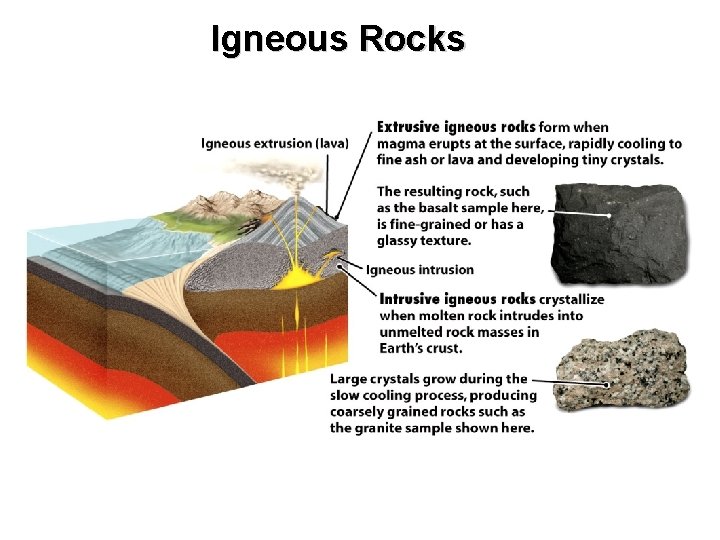
Igneous Rocks


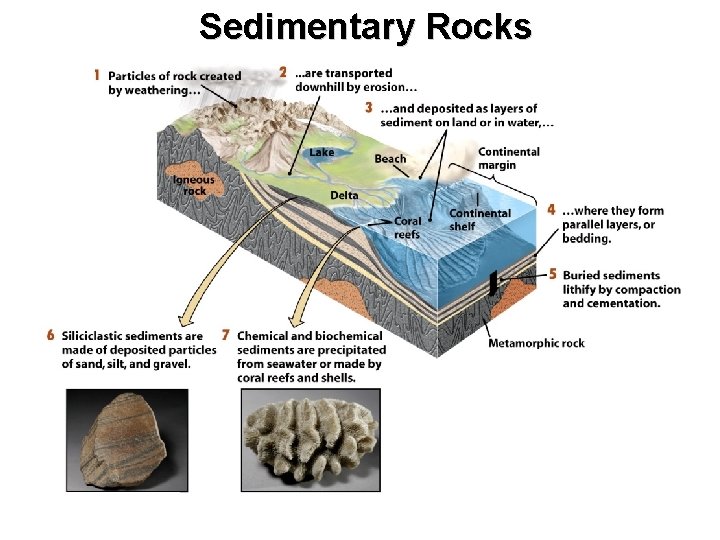
Sedimentary Rocks

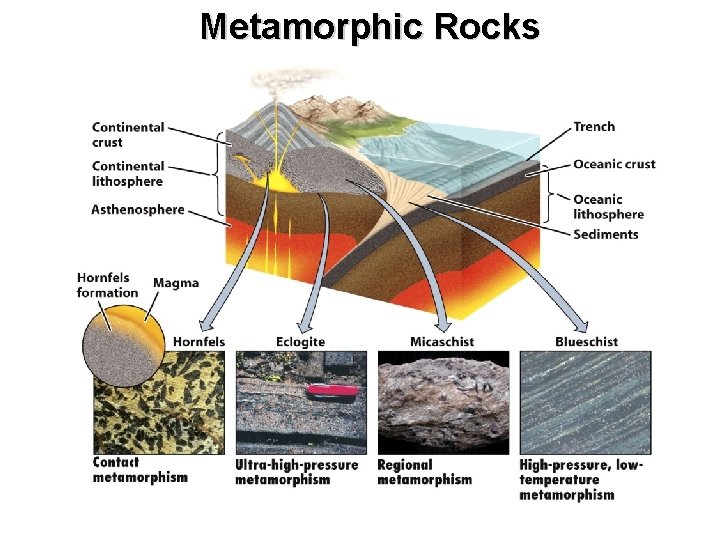
Metamorphic Rocks

Thought questions for this chapter In some bodies of granite, we can find very large crystals, some as much as a meter across, yet these crystals tend to have few crystal faces. What can you deduce about the conditions under which these large crystals grew? Which igneous intrusion would you expect to have a wider contact metamorphic zone: one intruded by a very hot magma or one intruded by a cooler magma? Where are igneous rocks most likely to be found? How could you be certain that the rocks were igneous and not sedimentary or metamorphic?

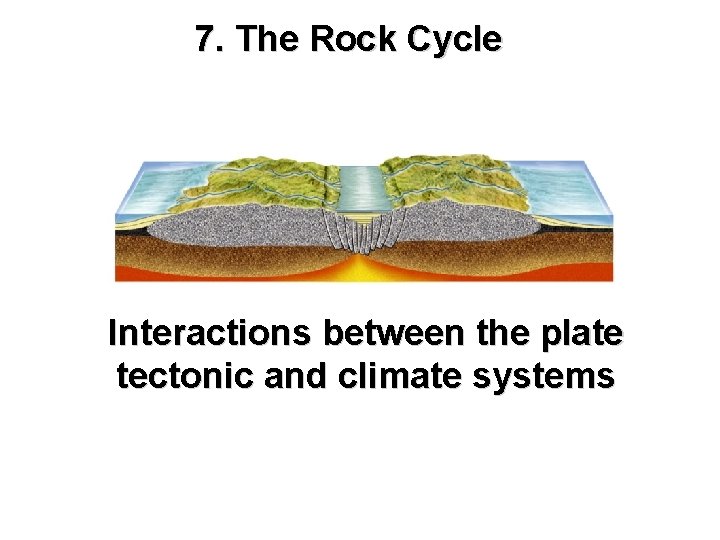
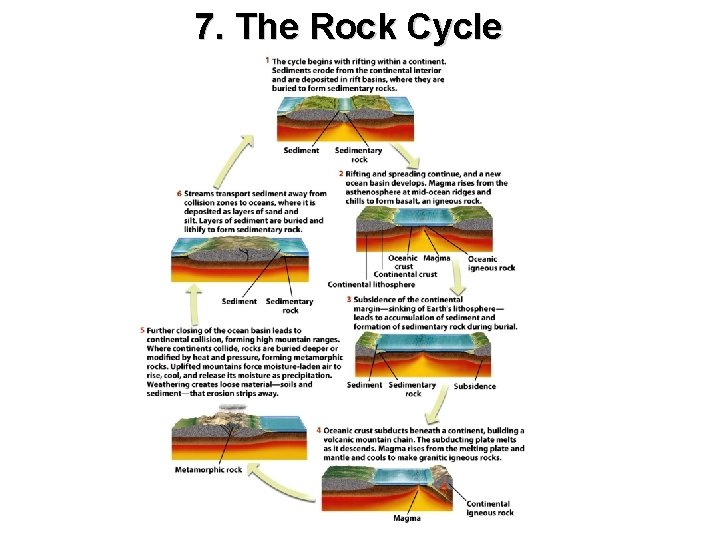
7. The Rock Cycle Interactions between the plate tectonic and climate systems

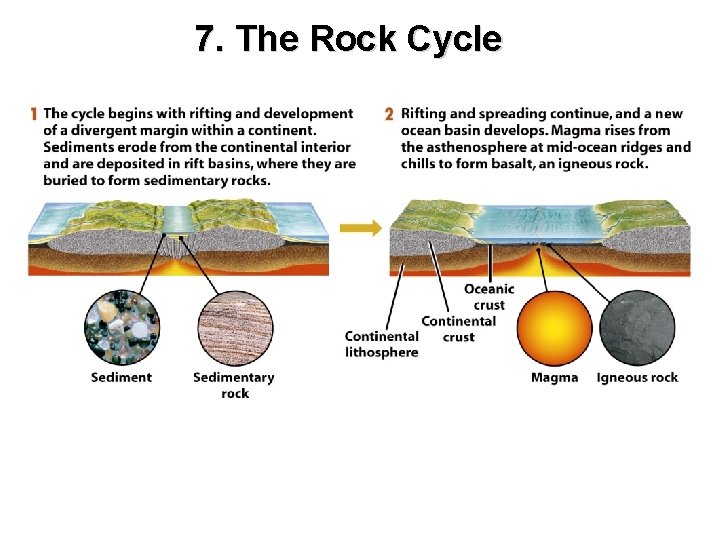
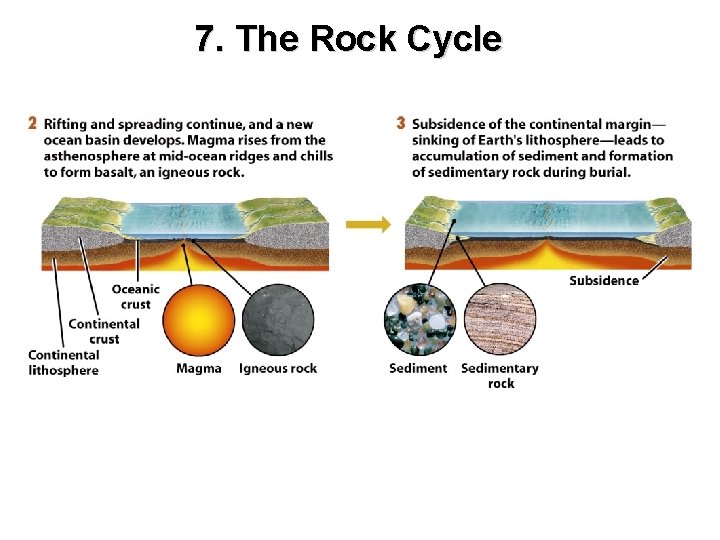
7. The Rock Cycle

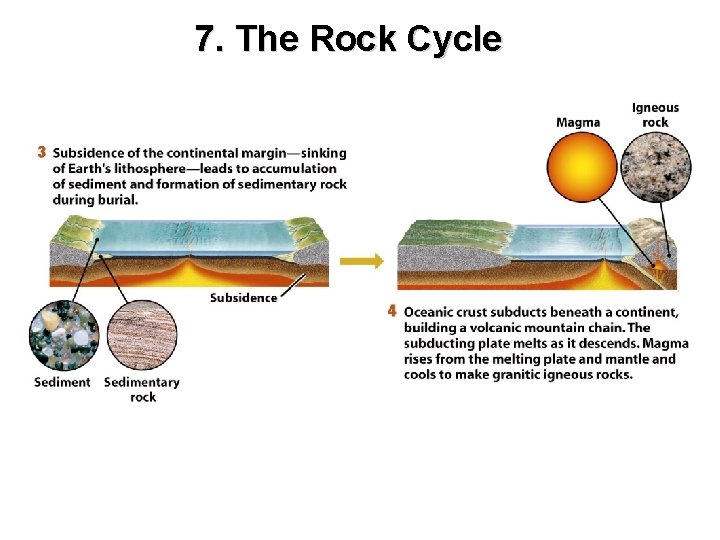
7. The Rock Cycle

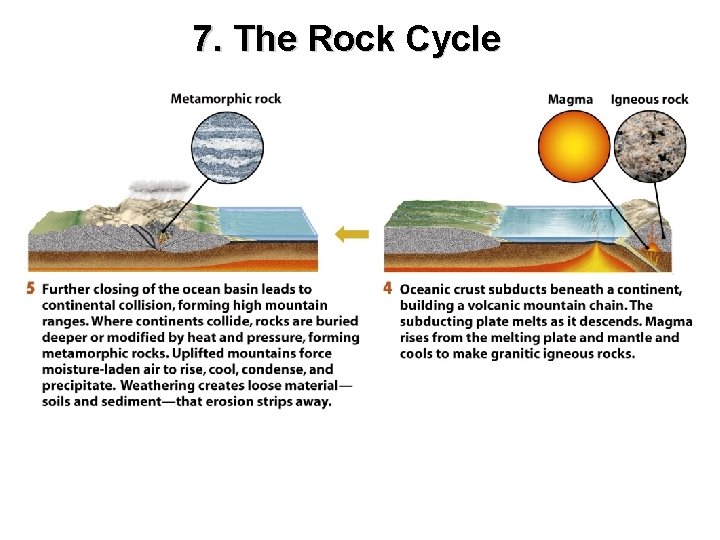
7. The Rock Cycle

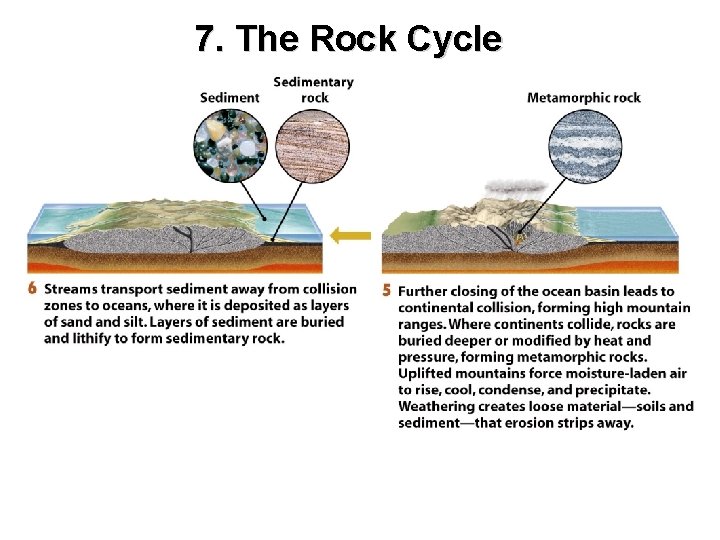
7. The Rock Cycle

7. The Rock Cycle

7. The Rock Cycle

Thought questions for this chapter What geologic processes transform a sedimentary rock into an igneous rock? Describe the geologic processes by which an igneous rock is transformed into a metamorphic rock and then exposed to erosion. Using the rock cycle, trace the path from a magma to a granitic intrusion to a metamorphic gneiss to a sandstone. Be sure to include the roles of the plate tectonics climate systems and the specific processes that create rocks.

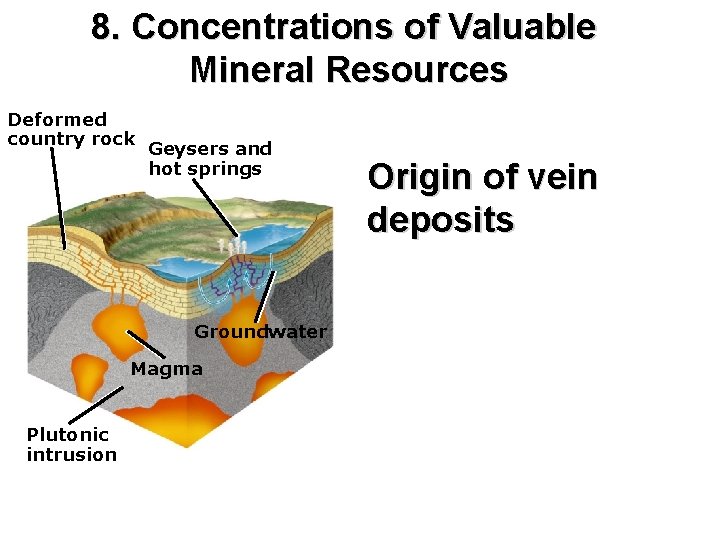
8. Concentrations of Valuable Mineral Resources Types of ore minerals: Vein deposits Disseminated deposits Igneous deposits Sedimentary deposits

8. Concentrations of Valuable Mineral Resources Deformed country rock Geysers and hot springs Groundwater Magma Plutonic intrusion Origin of vein deposits

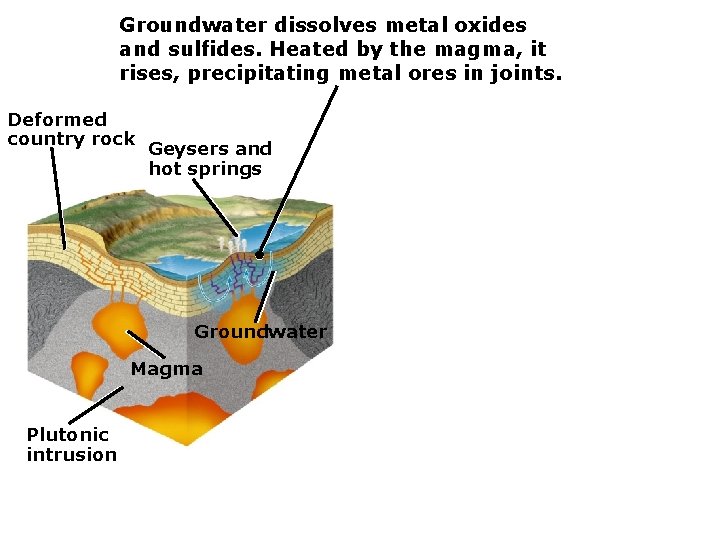
Groundwater dissolves metal oxides and sulfides. Heated by the magma, it rises, precipitating metal ores in joints. Deformed country rock Geysers and hot springs Groundwater Magma Plutonic intrusion

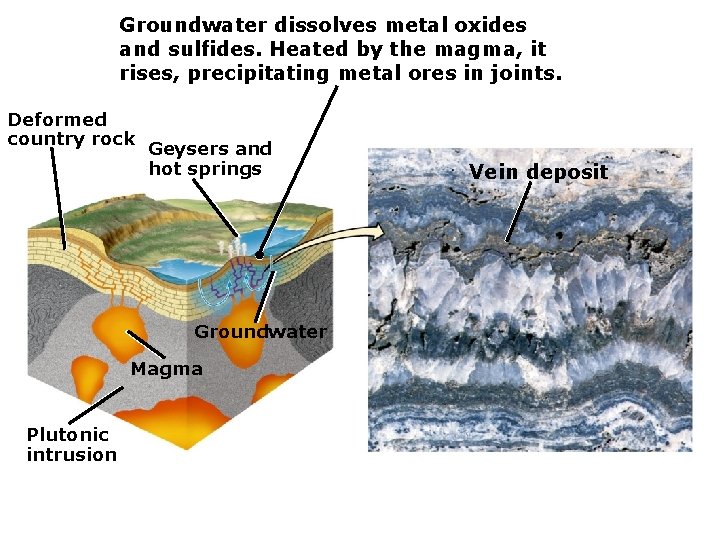
Groundwater dissolves metal oxides and sulfides. Heated by the magma, it rises, precipitating metal ores in joints. Deformed country rock Geysers and hot springs Groundwater Magma Plutonic intrusion Vein deposit

8. Concentrations of Valuable Mineral Resources Typical sulfide minerals from vein deposits

8. Concentrations of Valuable Mineral Resources Open-pit mine for disseminated deposits of copper-bearing minerals.

8. Concentrations of Valuable Mineral Resources Igneous deposits Chromite layers (dark) in layered igneous rock

8. Concentrations of Valuable Mineral Resources Sedimentary deposits: Copper, iron, other metals Gold, diamonds, other heavy minerals (placers)

Thought questions for this chapter Back in the late 1800 s, gold miners used to pan for gold by placing sediment from rivers in a pan and filtering water through the pan while swirling the pan’s contents. The miners wanted to be certain that they had found real gold and not pyrite (“fool’s gold”). Why did this method work? What mineral property does the process of panning for gold use? What is another possible method for distinguishing between gold and pyrite?

Key terms and concepts Anion Atomic mass Atomic number Bedding Biological sediment Carbonate Cation Chemical sediments Cleavage Color Contact metamorphism Covalent bond Crystal habit

Key terms and concepts Density Disseminated deposit Electron sharing Electron transfer Erosion Fracture Grain Hardness Hydrothermal solution Igneous rock Ionic bond Isotope Lithification

Key terms and concepts Luster Magma Metallic bond Metamorphic rock Mineralogy Mohs scale of hardness Ore Oxides Polymorph Precipitate Regional metamorphism Rock cycle

Key terms and concepts Sedimentary rock Silicate Siliclastic sediments Specific gravity Streak Sulfate Sulfide Texture Trace element Vein Weathering