Gas Volumes and the Ideal Gas Law GayLussacs


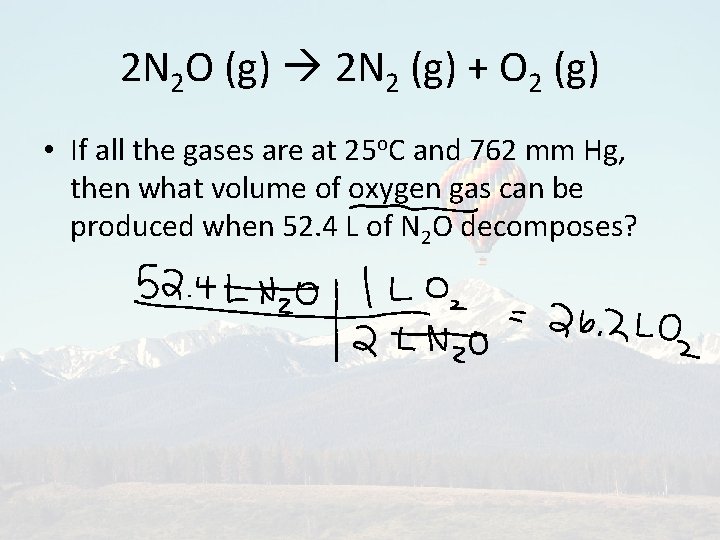













- Slides: 19

Gas Volumes and the Ideal Gas Law

Gay-Lussac’s Law of Combining Volumes • At constant temperature and pressure, the volumes of gaseous reactants and products can be expressed as ratios of small whole numbers. • In other words, in stoichiometric calculations, the coefficients may be used as Volume Ratios instead of molar ratios, IF both compounds involved are gases AND they are at the same temperature as each other.
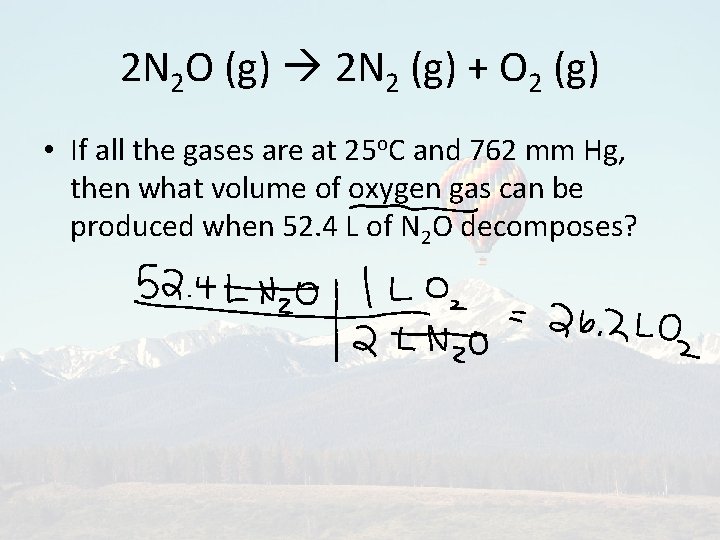
2 N 2 O (g) 2 N 2 (g) + O 2 (g) • If all the gases are at 25 o. C and 762 mm Hg, then what volume of oxygen gas can be produced when 52. 4 L of N 2 O decomposes?

Avogadro’s Law • Equal volumes of gases at the same temperature and pressure contain equal numbers of particles. • A liter of O 2, a liter of He, and a liter of CO 2 all contain exactly the same number of particles if their pressures and temperatures are the same.

• 1 mole of O 2 contains 6. 022 x 1023 molecules of O 2 • 1 mole of He contains 6. 022 x 1023 atoms of He • According to Avogadro’s Law, one mole of any gas will occupy the same volume as one mole of any other gas at the same temperature and pressure.

Standard Molar Volume • IF the gas is at STP, then one mole of a gas will occupy 22. 4 L. • This is true for any gas at STP. • If a gas is at STP, you can use 22. 4 L/mol as a conversion factor to convert from moles to liters and vice versa.

• At STP, what is the volume of 4. 57 mol of nitrogen gas? • A sample of neon has a volume of 13. 4 L at STP. How many moles of the gas are present?

Gas Stoichiometry • You can use both Gay-Lussac’s Law of Combining Volumes and Avogadro’s Law to calculate the stoichiometry of reactions involving gases. • Remember that for Gay-Lussac’s Law, the gases must be at the same temperature and pressure, while the value for Standard Molar Volume can only be used at STP

C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) • What volume of oxygen gas, in liters, is required for the complete combustion of 0. 56 L of propane? Assume that all volume measurements are at the same temperature and pressure.

• What volume of oxygen gas is needed to react completely with 626 m. L of carbon monoxide gas to form gaseous carbon dioxide? What volume of carbon dioxide can be produced?

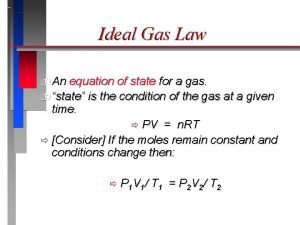
Ideal Gas Law • The Ideal Gas Law is the mathematical relationship among pressure, volume, temperature, and the number of moles of a gas. • For each of the gas laws we used in section 2, we assumed that the number of moles was held constant. • The ideal gas law will reduce to the other gas laws if the appropriate variable are held constant.

PV = n. RT • • • P is pressure V is volume in liters n is the number of moles of the gas T is the temperature in Kelvins And R is the ideal gas constant. – The value of the ideal gas constant depends on what pressure unit is used in the equation

PV=n. RT • If you rearrange the equation to solve for R, you can find the value for R by substituting the known values for ideal conditions


• What is the pressure in atm exerted by a 0. 375 mol sample of nitrogen gas in a 7. 0 L container at 30. o. C?

• A tank of hydrogen gas has a volume of 25. 0 L and holds 16. 2 g while exerting a pressure of 9. 85 atm. What is the temperature of the gas, in Celsius?

• A container holds 0. 500 mol of neon gas at a pressure of 99. 4 k. Pa and a temperature of 28 o. C. What is the volume of the container?

• A 1. 2 L container holds a gas sample at a pressure of 765 mm. Hg and a temperature of 21 o. C. How many moles are in the sample? • If the gas is argon, what is the mass of the sample?
• A gas sample that has a mass of 0. 993 g occupies 0. 570 L. Given that the temperature is 281 K and the pressure is 1. 4 atm, what is the molar mass of the gas?
 Gaylussacs
Gaylussacs Daltons gas law
Daltons gas law Differences between ideal gas and real gas
Differences between ideal gas and real gas Difference between ideal gas and real gas
Difference between ideal gas and real gas Relationship between pressure and volume
Relationship between pressure and volume Ideal gas vs perfect gas
Ideal gas vs perfect gas Imaginary gas
Imaginary gas Computational fluid dynamics
Computational fluid dynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Ideal gas law with mass
Ideal gas law with mass What's an ideal gas
What's an ideal gas Ideal gas constant
Ideal gas constant Which equation agrees with the ideal gas law?
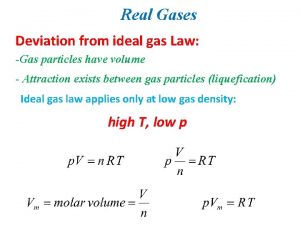
Which equation agrees with the ideal gas law? Deviation from ideal gas
Deviation from ideal gas Pv nrt
Pv nrt Deviations from ideal gas law
Deviations from ideal gas law P v n r t
P v n r t Ideal gas law
Ideal gas law Initial pressure
Initial pressure