GAS LAWS oh the pressurethe volume the temperature















- Slides: 17

GAS LAWS …oh, the pressure…the volume. . . the temperature. . . the number of moles!!!

Pressure ®Results from collisions with walls of container ® 1 atm = 760 mm. Hg = 760 torr = 29. 9 in. Hg = 14. 7 psi

Dalton’s Law. . . ®In a mixture of gases, the sum of the N 2 O 2 partial pressure of CO 2 each gas equals the Ar total pressure of the mixture ®Ptotal = P 1 + P 2 + P 3…

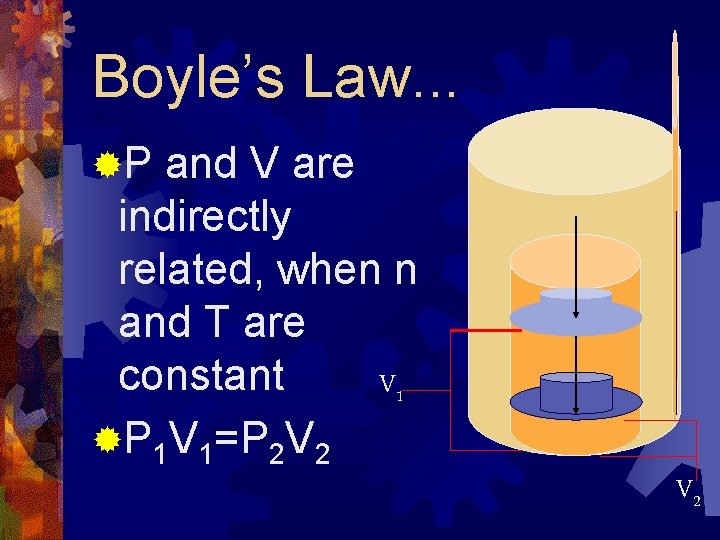
Boyle’s Law. . . ®P and V are indirectly related, when n and T are constant V 1 ®P 1 V 1=P 2 V 2

Charles’s Law. . . ®T and V are directly related, when n and P are constant ®V 1=V 2 T 1 T 2

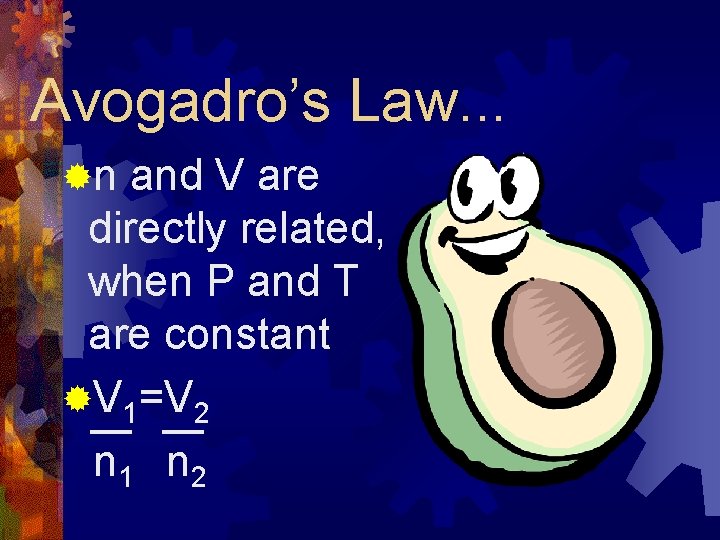
Avogadro’s Law. . . ®n and V are directly related, when P and T are constant ®V 1=V 2 n 1 n 2

Gay-Lussac’s Law. . . ®P and T are directly related, when n and V are constant ®P 1=P 2 T 1 T 2

Practice…#1 ®A given mass of oxygen occupies 500 m. L at 760 mm. Hg at 20°C in a sealed vessel. At what pressure (in mm. Hg) will it occupy 450 m. L at the same temperature?

Practice…#2 ®A sealed sample of nitrogen at 25°C occupies a volume of 5. 65 L at a pressure of 14. 3 psi. If the pressure, at the same temperature, is changed to 14. 7 psi, what is the final volume in L?

Practice…#3 ®At sea level and 0°C, the partial pressure of the nitrogen in clean, dry air is 601 torr when the atmospheric pressure is 760 torr. If oxygen were the only other gas, what would its pressure be in torr?

Practice…#4 ®On a day when the atmospheric pressure is 744 mm. Hg, you collect a sample of oxygen over water at 20°C. By making the water level inside the eudiometer the same as outside, the inside

Practice…#4 (cont. ) ®pressure was known to be 744 mm. Hg, too. The volume of the gas was 325 m. L. Calculate the partial pressure of the oxygen in the bottle, and then calculate what volume the oxygen

Practice…#4 (cont. ) ®would have if all of the water vapor were removed and the gas pressure were to become 760 mm. Hg. (The vapor pressure of water at 20˚C is 17. 535 mm. Hg. )

Practice…#5 ®Some of the total anesthetics used in surgery are gases at 37°C. If 1. 50 L of a gas is used at 20°C, to what volume (in L) does the gas change when the temperature becomes 37°C at the same pressure?

Practice…#6 ® 10 g of helium are in a pliable balloon with a volume of 2. 50 L at 1 atm and 25°C. If the balloon has a slow leak and the volume is arrested at 1. 10 L, what mass of helium will be left in the balloon?

Practice…#7 ®So, you’re working at a fast food joint and you’re told to move a CO 2 canister to storage. You inadvertently move the cylinder from its cozy 27°C to the chilly cold storage temp of -10°C. If the pressure inside the cylinder

Practice…#7 (cont. ) ®was originally 2. 25 atm, what will the pressure (in atm) be once it stabilizes in cold storage?
 Mathematical relationship between temperature and pressure
Mathematical relationship between temperature and pressure Facts about montesquieu
Facts about montesquieu Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Gas laws crash course
Gas laws crash course Direct vs indirect chemistry
Direct vs indirect chemistry Empirical gas laws
Empirical gas laws What is the combined gas law
What is the combined gas law Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Pivnert
Pivnert Gas law conceptual questions
Gas law conceptual questions Boyle's law problems
Boyle's law problems Charles' law worksheet with answers
Charles' law worksheet with answers A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c Different gas laws
Different gas laws Combined gas laws
Combined gas laws