Gas Laws Volume V v The volume of










- Slides: 32

Gas Laws

Volume (V) v The volume of a gas is simply the volume of the container it is contained in. v The metric unit of volume, liter (L), is often used. v There might also be problems that use cubic meters as the unit for volume. • 1 L = 1 m 3

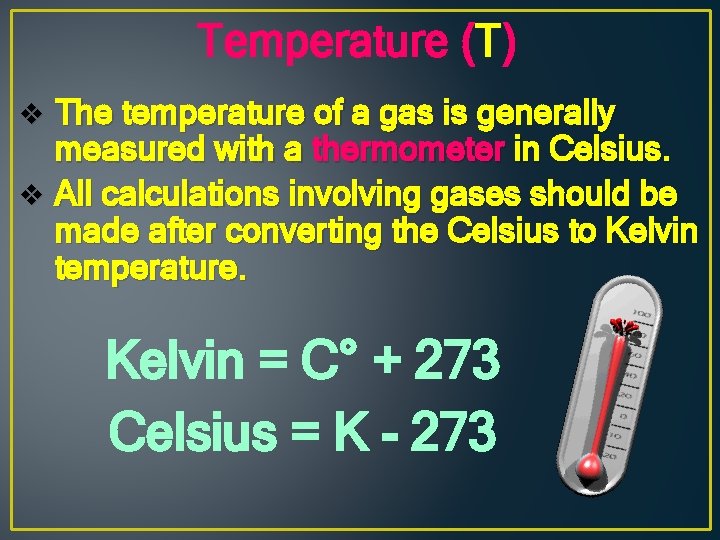
Temperature (T) v The temperature of a gas is generally measured with a thermometer in Celsius. v All calculations involving gases should be made after converting the Celsius to Kelvin temperature. Kelvin = C° + 273 Celsius = K - 273

Pressure (P) v The pressure of a gas is the force exerted on the wall of the container, in which a gas is trapped. v There are several units for pressure depending on the instrument used to measure it including: 1) atmospheres (atm) 2) Millimeters of Mercury (mm. Hg) 3) Kilopascal (k. Pa) 4) Torr

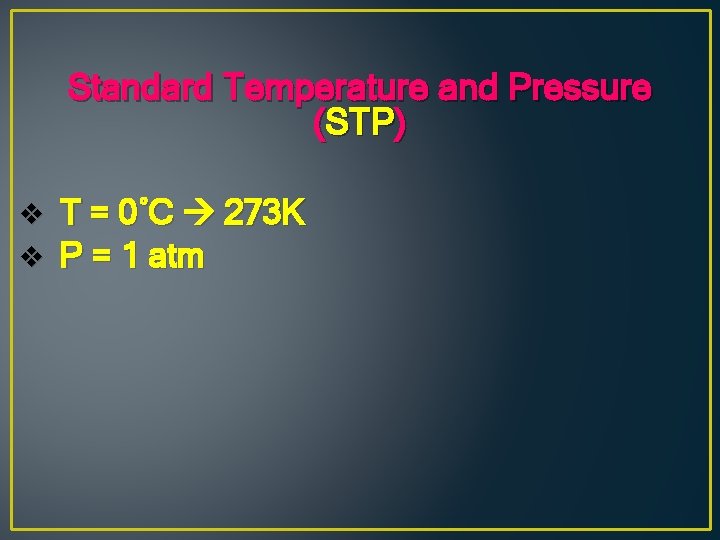
Standard Temperature and Pressure (STP) v v T = 0˚C 273 K P = 1 atm

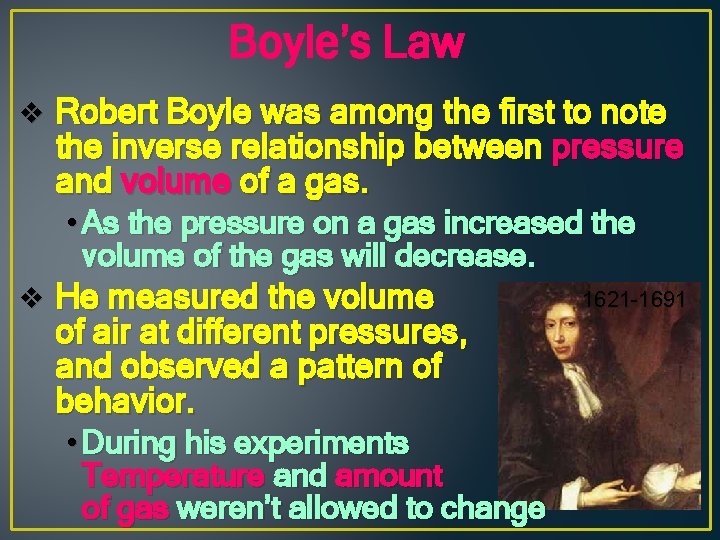
Boyle’s Law v Robert Boyle was among the first to note the inverse relationship between pressure and volume of a gas. • As the pressure on a gas increased the volume of the gas will decrease. v He measured the volume of air at different pressures, and observed a pattern of behavior. • During his experiments Temperature and amount of gas weren’t allowed to change 1621 -1691

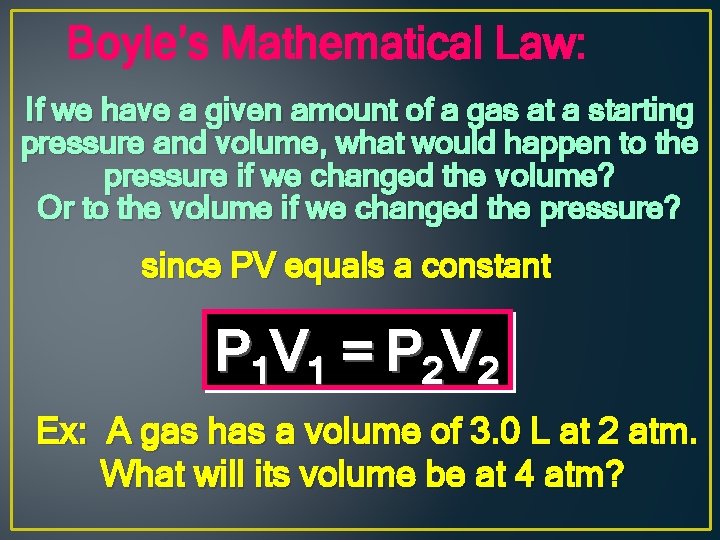
Boyle’s Mathematical Law: If we have a given amount of a gas at a starting pressure and volume, what would happen to the pressure if we changed the volume? Or to the volume if we changed the pressure? since PV equals a constant P 1 V 1 = P 2 V 2 Ex: A gas has a volume of 3. 0 L at 2 atm. What will its volume be at 4 atm?

Boyle’s Mathematical Law: 1) List the variables or clues given: Ø P 1 = 2 atm Ø V 1 = 3. 0 L Ø P 2 = 4 atm Ø V 2 = ? 2) determine which law is being represented: P 1 V 1 = V 2 P 2 3) Plug in the variables & calculate: (2 atm)(3. 0 L) = (4 atm) (V 2)

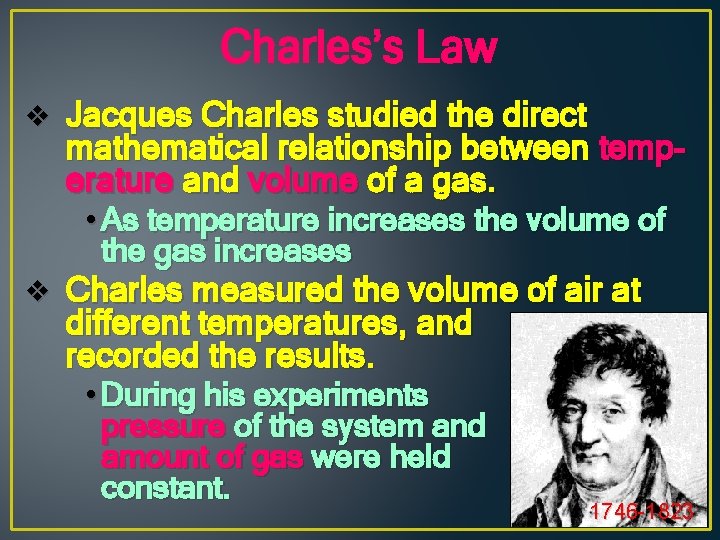
Charles’s Law v Jacques Charles studied the direct mathematical relationship between temperature and volume of a gas. • As temperature increases the volume of the gas increases v Charles measured the volume of air at different temperatures, and recorded the results. • During his experiments pressure of the system and amount of gas were held constant. 1746 -1823

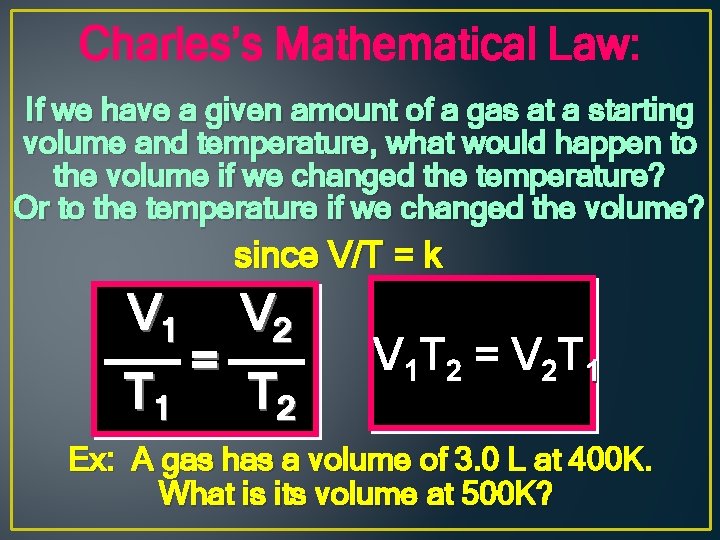
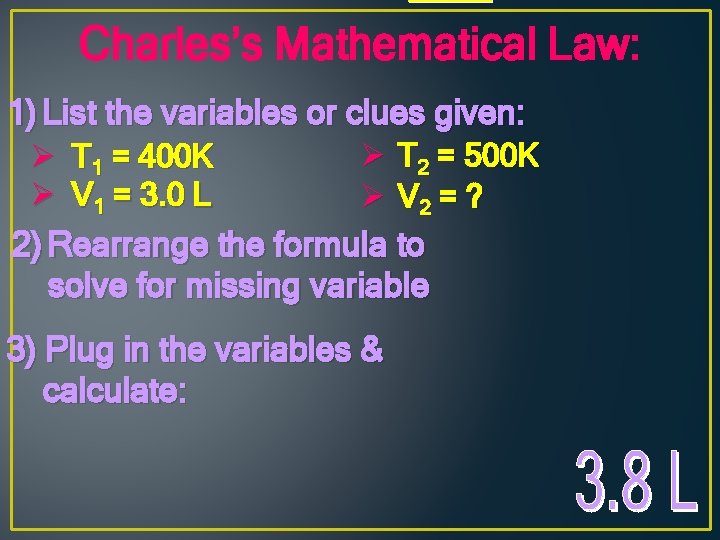
Charles’s Mathematical Law: If we have a given amount of a gas at a starting volume and temperature, what would happen to the volume if we changed the temperature? Or to the temperature if we changed the volume? since V/T = k V 1 T 1 = V 2 T 2 V 1 T 2 = V 2 T 1 Ex: A gas has a volume of 3. 0 L at 400 K. What is its volume at 500 K?

Charles’s Mathematical Law: 1) List the variables or clues given: Ø T 2 = 500 K Ø V 2 = ? 2) Rearrange the formula to solve for missing variable Ø T 1 = 400 K Ø V 1 = 3. 0 L 3) Plug in the variables & calculate:

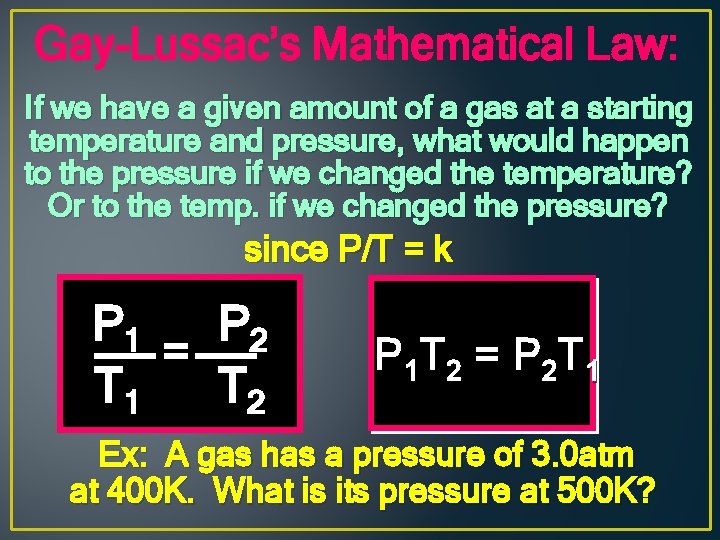
Gay-Lussac’s Mathematical Law: If we have a given amount of a gas at a starting temperature and pressure, what would happen to the pressure if we changed the temperature? Or to the temp. if we changed the pressure? since P/T = k P 1 P 2 = T 1 T 2 P 1 T 2 = P 2 T 1 Ex: A gas has a pressure of 3. 0 atm at 400 K. What is its pressure at 500 K?

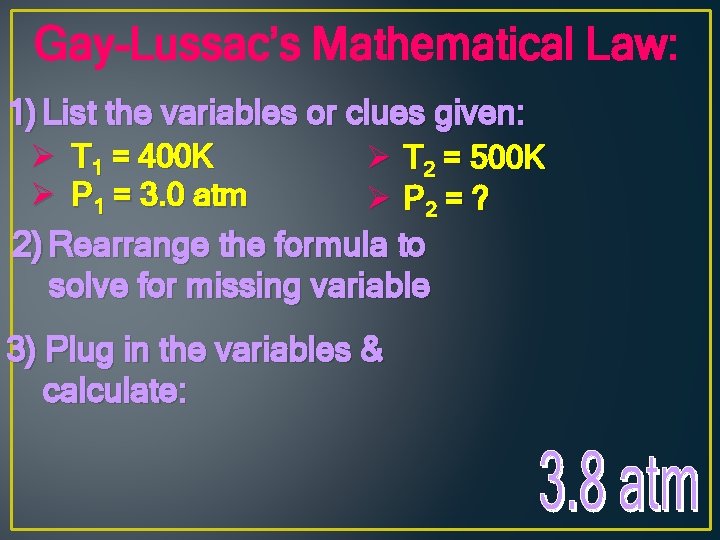
Gay-Lussac’s Mathematical Law: 1) List the variables or clues given: Ø T 1 = 400 K Ø P 1 = 3. 0 atm Ø T 2 = 500 K Ø P 2 = ? 2) Rearrange the formula to solve for missing variable 3) Plug in the variables & calculate:

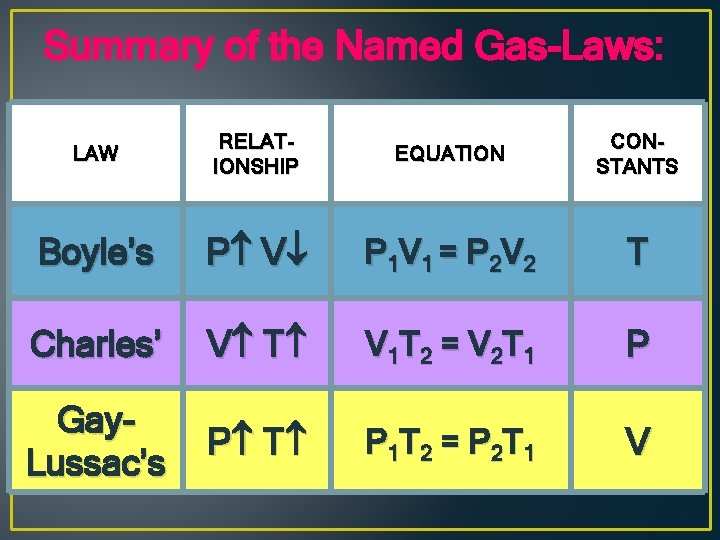
Summary of the Named Gas-Laws: LAW RELATIONSHIP EQUATION CONSTANTS Boyle’s P V P 1 V 1 = P 2 V 2 T Charles’ V T V 1 T 2 = V 2 T 1 P Gay. Lussac’s P T P 1 T 2 = P 2 T 1 V

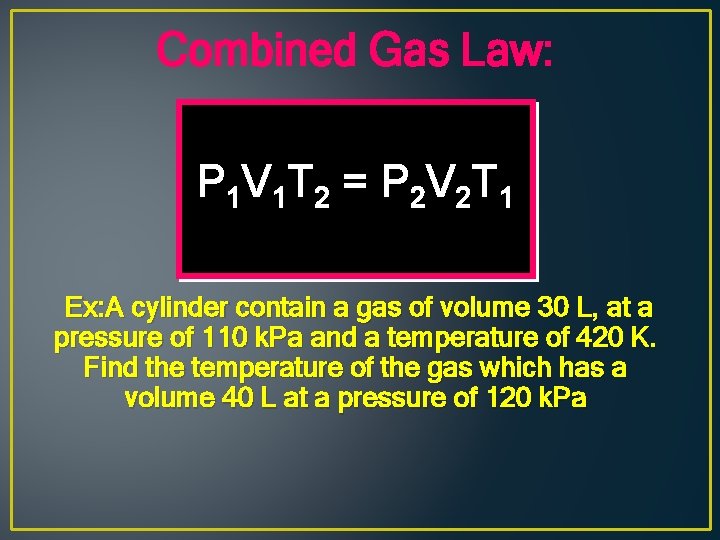
Combined Gas Law: P 1 V 1 T 2 = P 2 V 2 T 1 Ex: A cylinder contain a gas of volume 30 L, at a pressure of 110 k. Pa and a temperature of 420 K. Find the temperature of the gas which has a volume 40 L at a pressure of 120 k. Pa

Combined Gas Law: 1) List the variables or clues given: Ø T 1 = 420 K Ø V 1 = 30 L Ø P 1 = 110 k. Pa Ø T 2 = ? Ø V 2 = 40 L Ø P 2 = 120 k. Pa 2) Rearrange the formula to solve for missing variable 3) Plug in the variables & calculate:

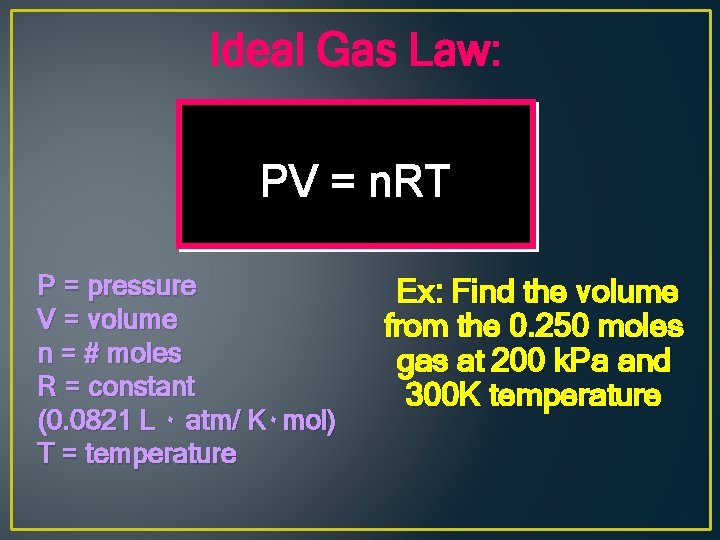
Ideal Gas Law: PV = n. RT P = pressure V = volume n = # moles R = constant (0. 0821 L ۰ atm/ K۰ mol) T = temperature Ex: Find the volume from the 0. 250 moles gas at 200 k. Pa and 300 K temperature

Ideal Gas Law: 1) List the variables or clues given: Ø Ø Ø P = 200 k. Pa V=? n = 0. 250 mol R= 0. 0821 T = 300 K 2) Rearrange the formula to solve for missing variable 3) Plug in the variables & calculate:

Gas Stoichiometry: v At STP, 1 mol of gas = 22. 4 liters v We can add this to our Stoichiometry graphic organizer!

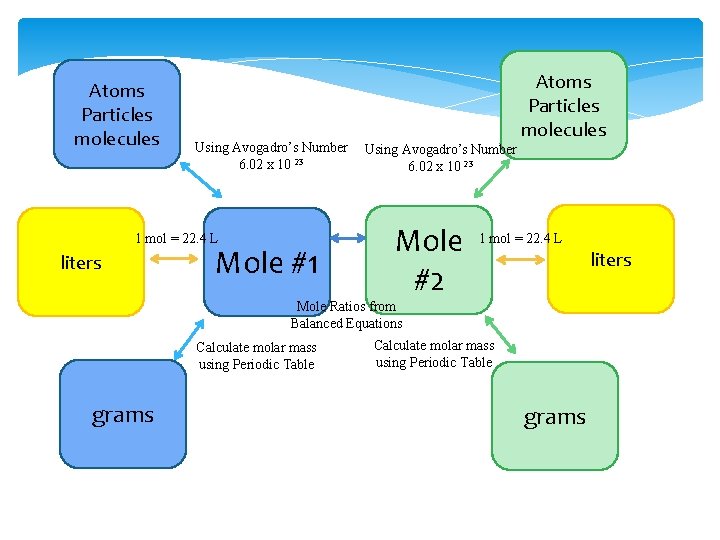
Atoms Particles molecules Using Avogadro’s Number 6. 02 x 10 23 1 mol = 22. 4 L liters Mole #1 Atoms Particles molecules Using Avogadro’s Number 6. 02 x 10 23 Mole #2 1 mol = 22. 4 L liters Mole Ratios from Balanced Equations Calculate molar mass using Periodic Table grams

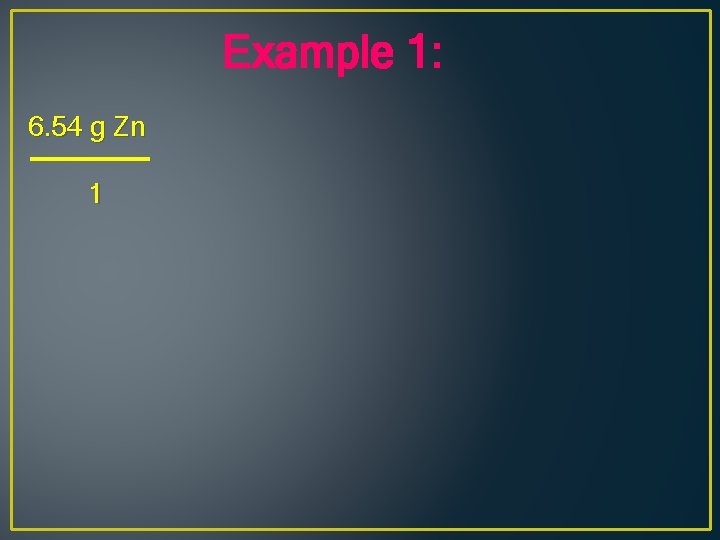
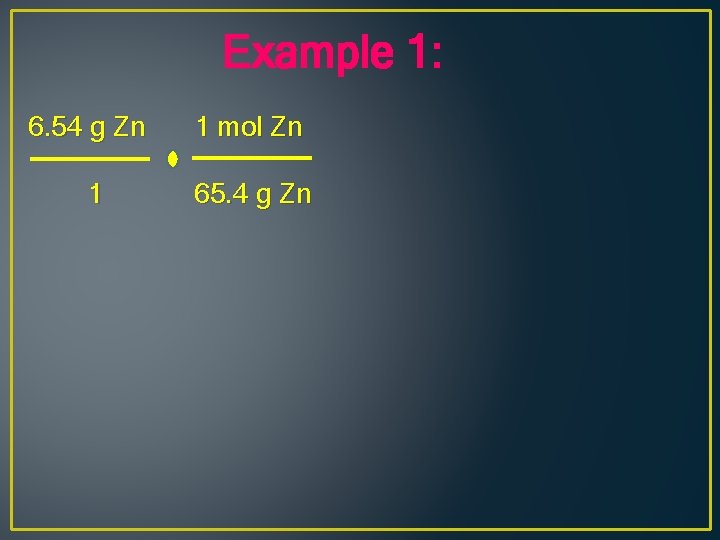
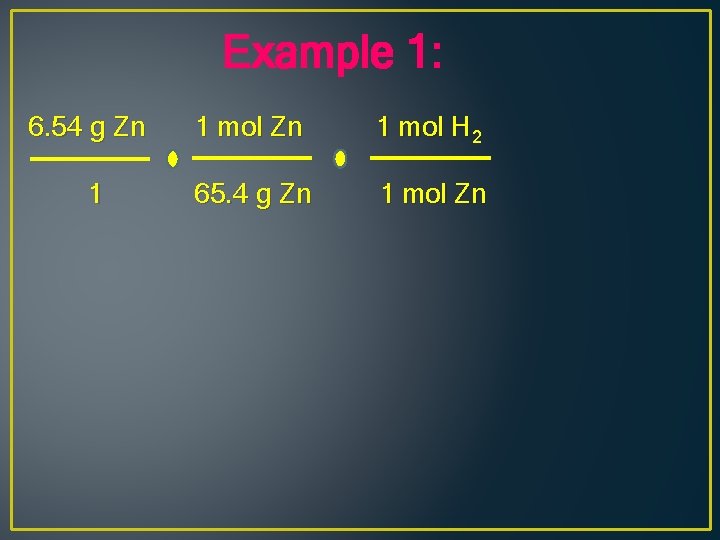
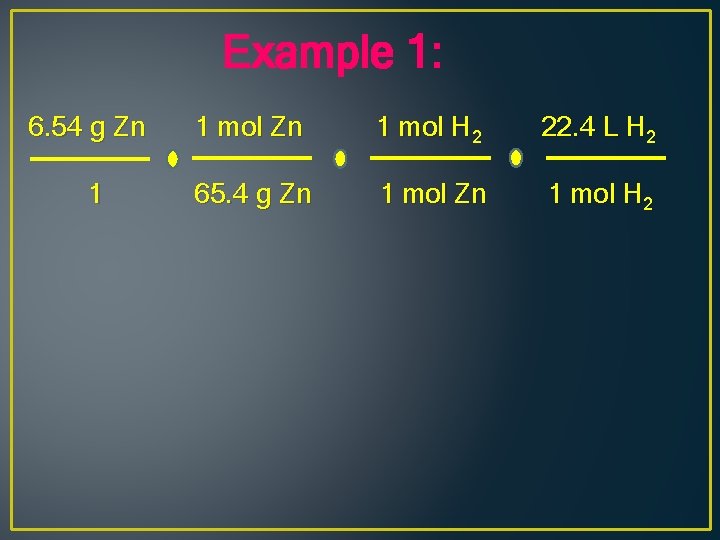
Example 1: What volume of hydrogen at STP can be produced when 6. 54 g of Zn reacts with hydrochloric acid, HCl? Zn + 2 HCl H 2 + Zn. Cl 2

Example 1: 6. 54 g Zn 1

Example 1: 6. 54 g Zn 1 mol Zn 1 65. 4 g Zn

Example 1: 6. 54 g Zn 1 mol H 2 1 65. 4 g Zn 1 mol Zn

Example 1: 6. 54 g Zn 1 mol H 2 22. 4 L H 2 1 65. 4 g Zn 1 mol H 2

Example 1: 6. 54 g Zn 1 mol H 2 22. 4 L H 2 1 65. 4 g Zn 1 mol H 2

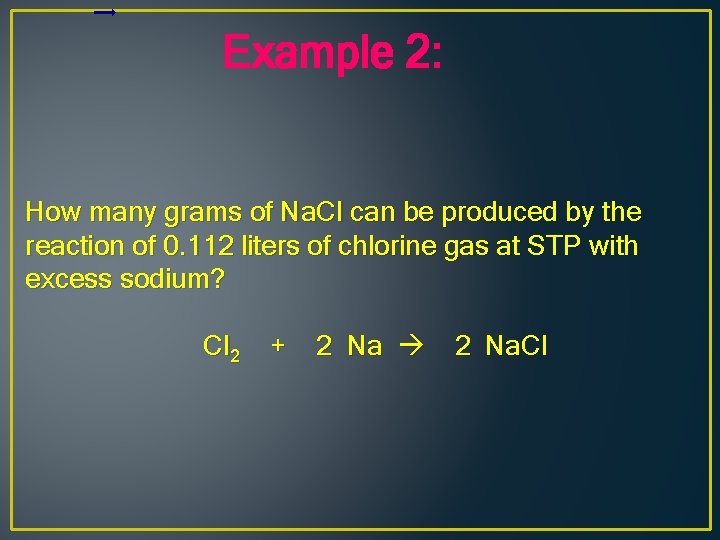
Example 2: How many grams of Na. Cl can be produced by the reaction of 0. 112 liters of chlorine gas at STP with excess sodium? Cl 2 + 2 Na. Cl

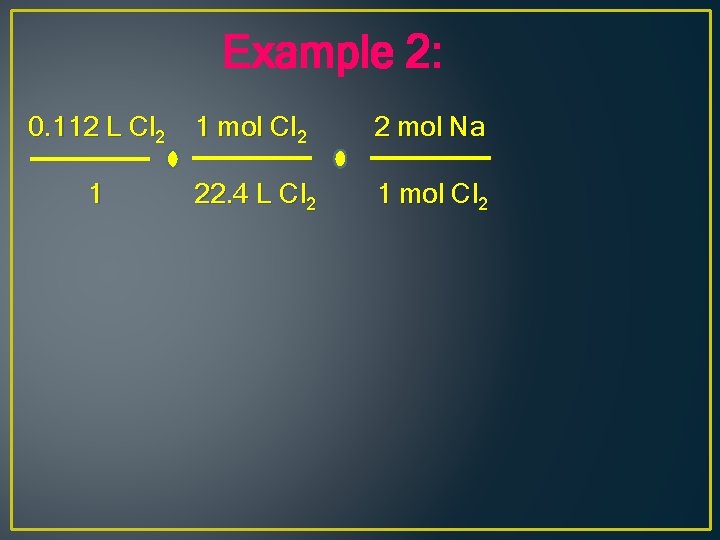
Example 2: 0. 112 L Cl 2 1

Example 2: 0. 112 L Cl 2 1 mol Cl 2 1 22. 4 L Cl 2

Example 2: 0. 112 L Cl 2 1 mol Cl 2 2 mol Na 1 22. 4 L Cl 2 1 mol Cl 2

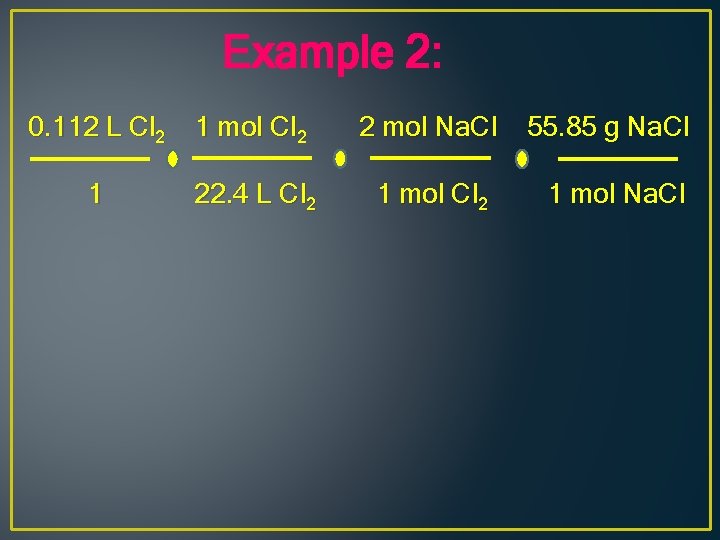
Example 2: 0. 112 L Cl 2 1 mol Cl 2 2 mol Na. Cl 55. 85 g Na. Cl 1 22. 4 L Cl 2 1 mol Na. Cl

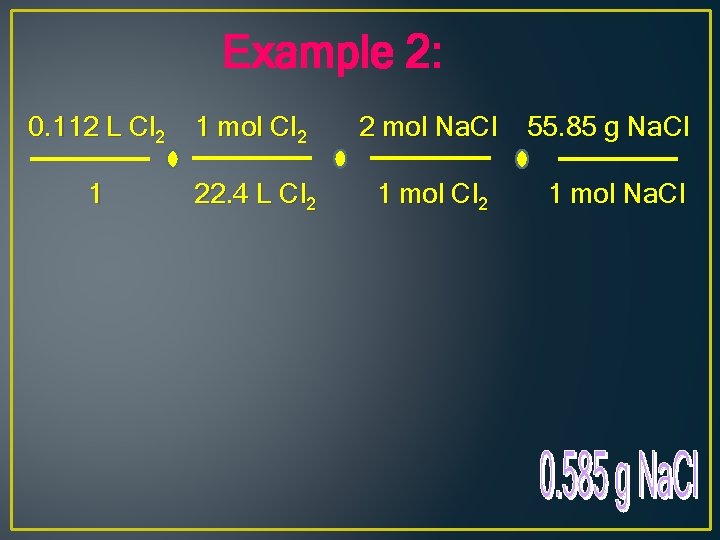
Example 2: 0. 112 L Cl 2 1 mol Cl 2 2 mol Na. Cl 55. 85 g Na. Cl 1 22. 4 L Cl 2 1 mol Na. Cl
 Charles de secondat
Charles de secondat Gas laws crash course
Gas laws crash course Direct and indirect relationship
Direct and indirect relationship Empirical gas laws
Empirical gas laws Ideal gas law
Ideal gas law All the gas laws
All the gas laws Different gas laws
Different gas laws Pivnert
Pivnert Conceptual problems examples
Conceptual problems examples Boyle's law example
Boyle's law example Ideal gas law equation example
Ideal gas law equation example 3 gas laws
3 gas laws Different gas laws
Different gas laws Combined gas laws
Combined gas laws Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle State charle's law.
State charle's law. Charles law formula
Charles law formula Ap chemistry gas laws
Ap chemistry gas laws Gas laws graphic organizer
Gas laws graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Gay lussac's law in real life
Gay lussac's law in real life Kmt gas laws
Kmt gas laws Gas law equations
Gas law equations Empirical gas law
Empirical gas law Combined gas law definition
Combined gas law definition Ideal gas law
Ideal gas law Is the volume of a liquid definite or indefinite
Is the volume of a liquid definite or indefinite Pseudo reduced specific volume
Pseudo reduced specific volume Imaginary gas
Imaginary gas Differences between ideal gas and real gas
Differences between ideal gas and real gas Ideal gas vs perfect gas
Ideal gas vs perfect gas Conclusion of bhopal gas tragedy
Conclusion of bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy