Gas Laws Chapter 11 Section 2 Boyles Law

Gas Laws Chapter 11 Section 2
Boyle’s Law • Boyle’s law shows the relationship between pressure and volume • The relationship is inverse • Volume pressure • Mathematically: PV = k • k is a constant
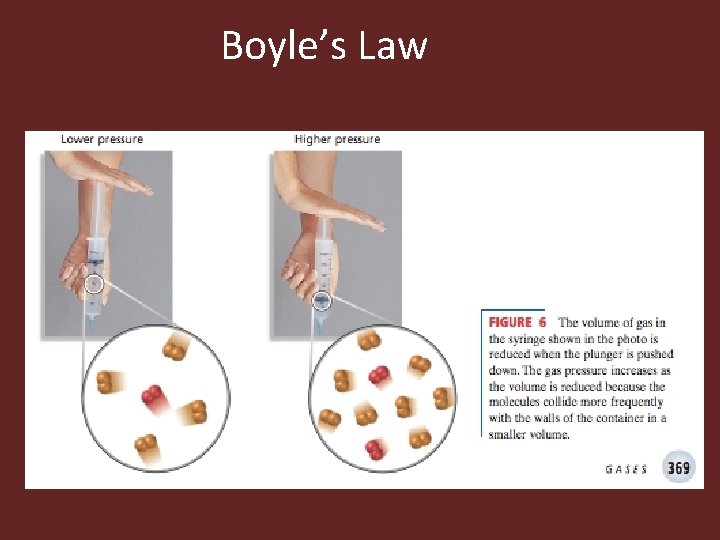
Boyle’s Law
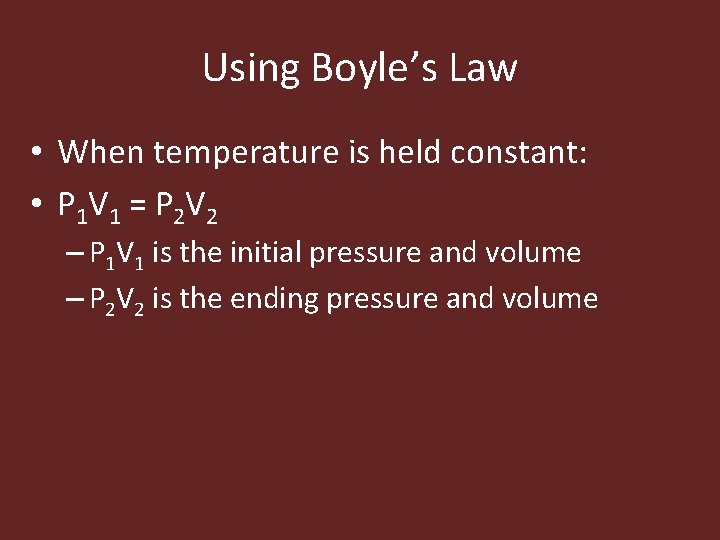
Using Boyle’s Law • When temperature is held constant: • P 1 V 1 = P 2 V 2 – P 1 V 1 is the initial pressure and volume – P 2 V 2 is the ending pressure and volume

Problem 1 • A sample of oxygen gas has a volume of 150. 0 m. L when its pressure is 0. 947 atm. What will the volume of the gas be at a pressure of 0. 987 atm if the temperature remains constant? • Identify every quantity in the problem with the units

Problem Solution • • • P 1 = 0. 947 atm V 1 = 150. 0 m. L P 2 = 0. 987 atm V 2 = ? Use Boyle’s law to solve • Answer: 144 m. L O 2
Charles’s Law • Charles’s law show the relationship between volume and temperature • The relationship is direct • Temperature volume • V = k. T or V/T = k

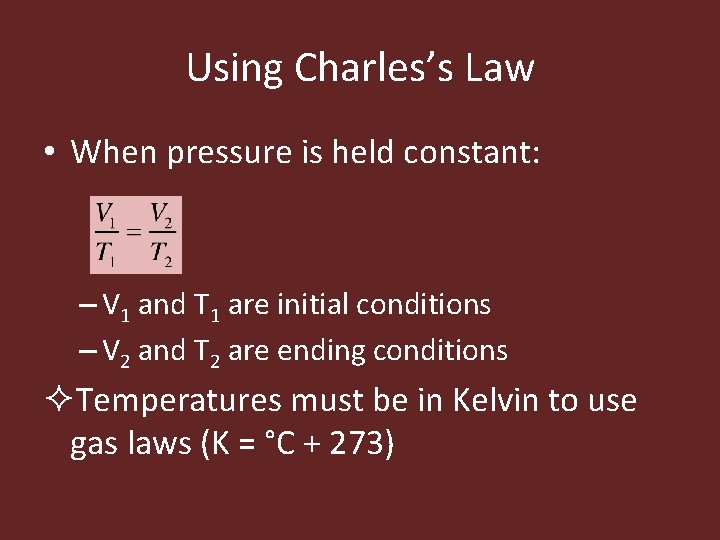
Using Charles’s Law • When pressure is held constant: – V 1 and T 1 are initial conditions – V 2 and T 2 are ending conditions ²Temperatures must be in Kelvin to use gas laws (K = °C + 273)

Problem 2 • A sample of neon gas occupies a volume of 752 m. L at 25°C. What volume will the gas occupy at 50°C if the pressure remains constant? • Identify every quantity in the problem with the units

Solution • • V 1 = 752 m. L T 1 = 25°C + 273 = 298 K T 2 = 50°C + 273 = 323 K Use Charles’s law to solve • Answer: 815 m. L Ne
Gay-Lussac’s Law • Gay-Lussac’s law shows the relationship between pressure and temperature • The relationship is direct • Temperature pressure • P = k. T or P/T = k
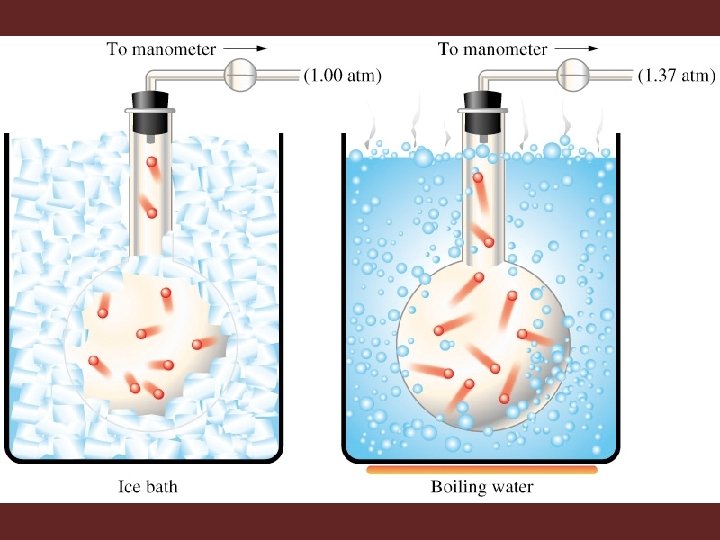

Using Gay-Lussac’s Law • When volume is held constant: – P 1 and T 1 are initial conditions – P 2 and T 2 are ending conditions ²Temperatures must be in Kelvin to use gas laws (K = °C + 273)

Problem 3 • The gas in a container is at a pressure of 3. 00 atm at 25°C. Directions on the container warn the user not to keep it in a place where the temperature exceeds 52°C. What would the gas pressure in the container be at 52°C? • Identify every quantity in the problem with the units

Solution • • • P 1 = 3. 00 atm T 1 = 25°C + 273 = 298 K T 2 = 52°C + 273 = 325 K P 2 = ? Use Gay-Lussac’s law to solve • Answer: 3. 27 atm
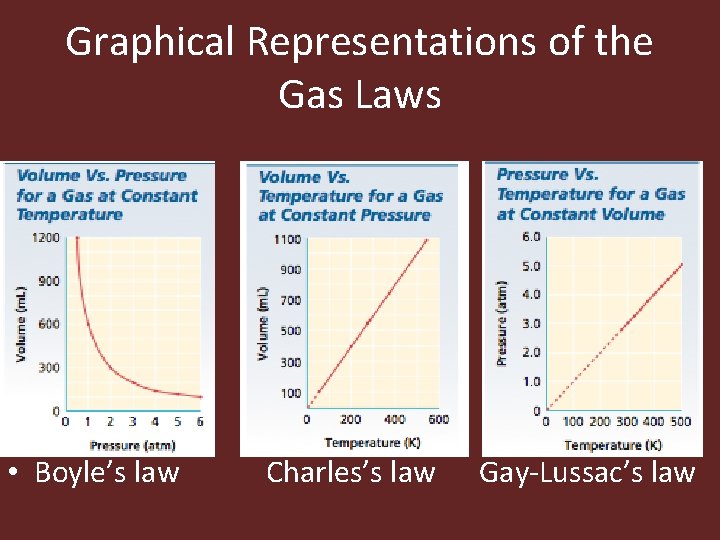
Graphical Representations of the Gas Laws • Boyle’s law Charles’s law Gay-Lussac’s law

Combined Gas Law • A gas sample frequently undergoes changes in temperature, pressure, and volume all at the same time. • The amount of gas remains constant • Combined gas law: expresses the relationship between pressure, volume, and temperature of a fixed amount of gas
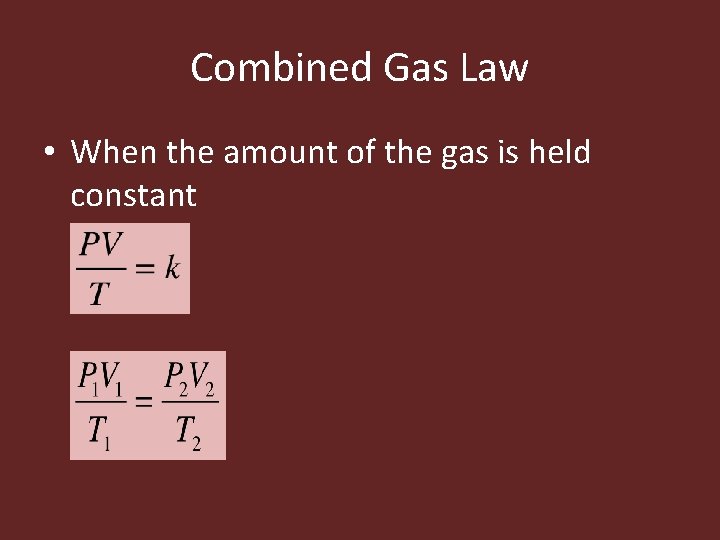
Combined Gas Law • When the amount of the gas is held constant

Problem 4 • A helium-filled balloon has a volume of 50. 0 L at 25°C and 1. 08 atm. What volume will it have at 0. 855 atm and 10. 0°C? • Identify every quantity in the problem with the units

Solution • • P 1 = 1. 08 atm T 1 = 25°C + 273 = 298 K V 1 = 50. 0 L P 2 = 0. 855 atm T 2 = 10. 0°C + 273 = 283 K V 2 = ? Use the combined gas law to solve

Solution • 60. 0 L He
- Slides: 22