FLUIDS AND ELECTROLYTES MAINTENANCE FLUIDS Maintenance intravenous IV










































- Slides: 60

FLUIDS AND ELECTROLYTES

MAINTENANCE FLUIDS • Maintenance intravenous (IV) fluids are used in children who cannot be fed enterally. • Along with maintenance fluids, children may require concurrent replacement fluids if they have excessive ongoing losses, such as may occur with drainage from a nasogastric tube. • In addition, if dehydration is present, the patient also needs to receive deficit replacement.

• Maintenance fluids are composed of a solution of water, glucose, sodium, potassium, and chloride. • This solution replaces electrolyte losses from the urine and stool, as well as water losses from the urine, stool, skin, and lungs.


• Maintenance fluids do not provide adequate calories, protein, fat, minerals, or vitamins. • Because of inadequate calories, a child on maintenance IV fluids loses 0. 5 o/o to 1 o/o of real weight each day.

• Daily water losses are measurable (urine and stool) and not measurable (insensible losses from the skin and lungs). • Failure to replace these losses leads to a thirsty, uncomfortable child and, ultimately, a dehydrated child.



• Sodium and potassium are given in maintenance fluids to replace losses from urine and stool.

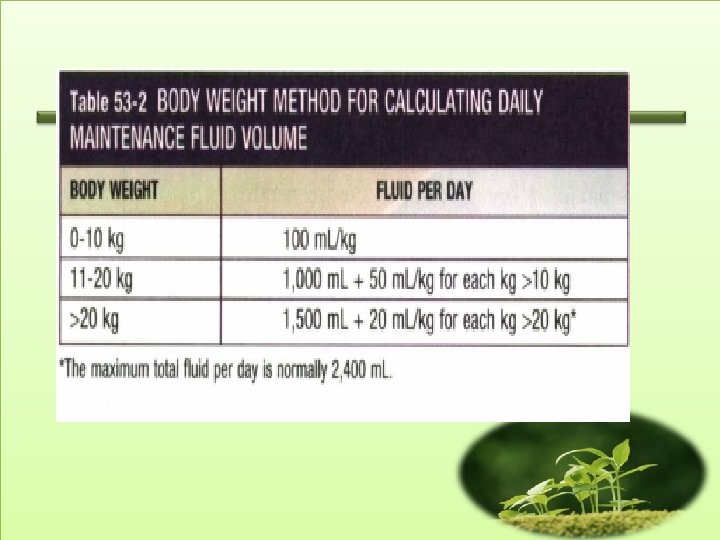
• After calculation of water needs and electrolvte needs, children typical. Iy received either 5% dextrose( D 5)'in 1/4 normal saline (NS) plus 20 m. Eql. L of potassium chloride(KCl) or D 5 in 1/2 NS plus 20 m. Eql. L of KCl. • Children weighing less than 10 kg do best with the solution containing 1/4 NS (38. 5 m. Eq/L) because of their high water needs per kilogram. • In contrast, larger children and adults may receive the solution with 1/2 NS (77 m. Eq/L).

Replacment Therapy

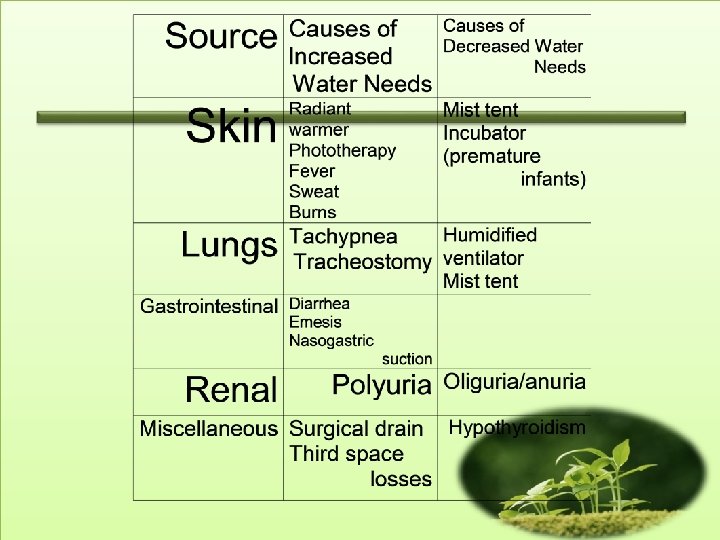
• • Components of maintenance therapy Urin: 60% Insensible losses (skin and lungs): 35% Stool: 5%

• Insensible losses, composed of evaporative losses from the skin and lungs, represent approximately one third of total maintenance water. • Sweating is not insensible and, in contrast to evaporative losses, sweat contains water and electrolytes.

• A variety of clinical situations modify normal maintenance water balance. • Evaporative skin water losses can be significant in neonates, especially premature infants who are under radiant warmers or undergoing phototherapy. • Burns can result in massive losses of water and electrolytes. • Fever leads to a predictable increase in insensible losses, causing a I 0%to 15% increase in maintenance water needs for each 1'C increase in temperature greater than 38'C. • Tachypnea or a tracheostomy increases evaporative losses from the lungs.


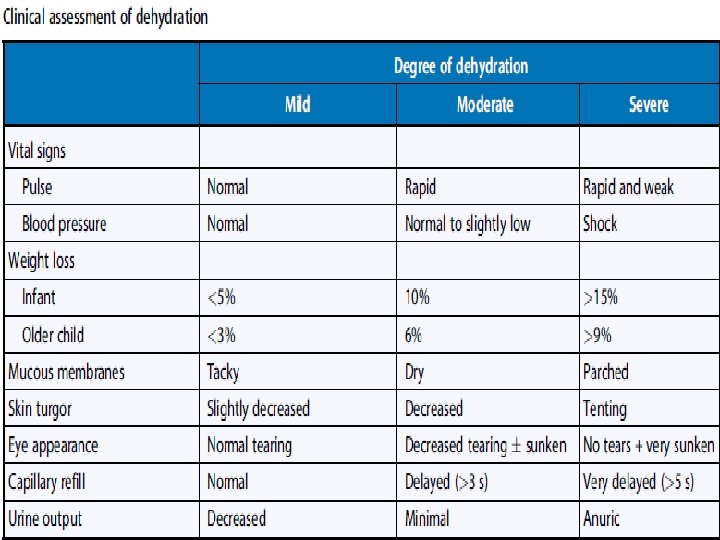
DEHYDRATION • Dehydration, most often due to gastroenteriris, is common in children. • The first step in caring for a child with dehydration is to assess the degree of dehydration. • The degree of dehydration dictates the urgency of the situation and the volume of fluid needed for rehydration.

• An infant with mild dehydration (3%to 5% of body weight dehydrated) has few clinical signs or symptoms. • The infant may be thirsty; the alert parent may notice a decline in urine output. • The history describes decreased intake and increased fluid losses.

• An infant with moderate dehydration has demonstrable physical signs and symptoms. • Intravascular space depletion is evident by an increased heart rate and reduced urine output. • The patient is 10% dehydrated and needs fairly prompt inrervention.

• An infant with severe dehydration is gravely ill. • The decrease in blood pressure indicates that vital organs may be receiving inadequate perfusion (shock). • The infant is at least 15% dehydrated and should receive immediate and aggressive intravenous (IV) therapy.

• Mild, moderate, and severe dehydration represent 3%, 6 %, a nd 9% of body weight lost in older children and adults. • This difference is because water is a higher percentage of body weight in infants.


• Clinical assessment of dehydration is only an estimate; the patient must be continually reevaluated during therapy. • The degree of dehydration is underestimated in hypernatremic dehydration because the osmotically driven shift of water from the intracellular space to the extracellular space helps to preserve the intravascular volume.

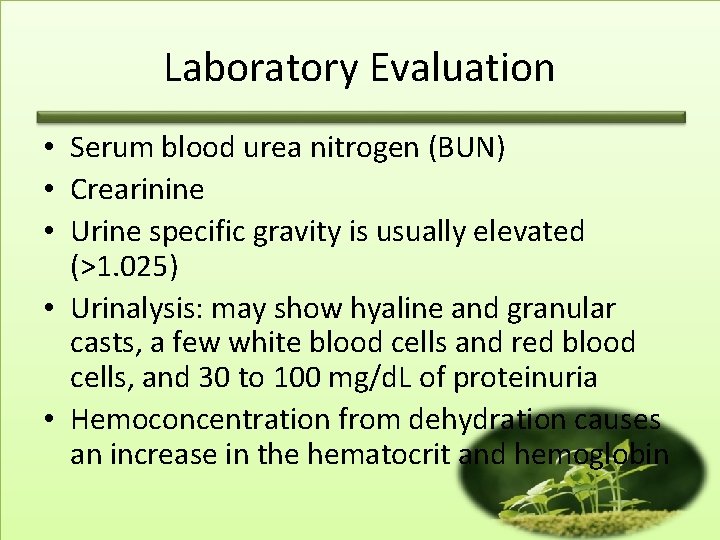
Laboratory Evaluation • Serum blood urea nitrogen (BUN) • Crearinine • Urine specific gravity is usually elevated (>1. 025) • Urinalysis: may show hyaline and granular casts, a few white blood cells and red blood cells, and 30 to 100 mg/d. L of proteinuria • Hemoconcentration from dehydration causes an increase in the hematocrit and hemoglobin

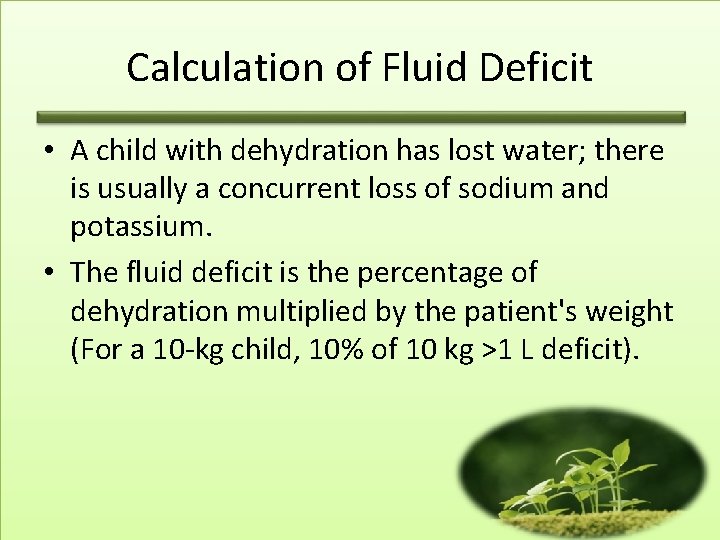
Calculation of Fluid Deficit • A child with dehydration has lost water; there is usually a concurrent loss of sodium and potassium. • The fluid deficit is the percentage of dehydration multiplied by the patient's weight (For a 10 -kg child, 10% of 10 kg >1 L deficit).

Approach to Dehydration • The child with dehydration requires acute intervenrion to ensure that there is adequate tissue perfusion. • This resuscitation phase requires rapid restoration of the circulating intravascular volume which should be done with an isotonic solution, such as normal saline (NS) or ringer's lactate. • Blood is an appropriate fluid choice for a child with acute blood loss. • The child is given a fluid bolus, usually 20 m. Llkg of the isotonic solution, over about 20 minutes. • A child with severe dehydration may require multiple fluid boluses and may need to receive fluid at a faster rate. • The initial resuscitation and rehydration is complete when che child has an adequate intravascular volume.

• Typically the child has some general clinical improvement, including a lower heart rate, normalization of the blood pressure, improved perfusion, and a more alert affect.

• With adequate intravascular volume, it is now appropriate to plan the fluid therapy for the next 24 hours. • In order to assure that the intravascular volume is restored, the patient receives an additional 20 m. L/kg bolus of isotonic fluid over 2 hours. • The child's total fluid needs are added together (maintenance + deficit). • The volume of isotonic fluids the patient has received as acute resuscitation is subtracted from this total. • The remaining fluid volume is then administered over 24 hours.

Fluid Management of Dehydration • Restore intravascular voiume Normal saline: 20 ml/kg over 20 minutes Repeat as needed • Rapid volume repletion: 2 O m. Llkg normal saline (maximum : 1 L) over 2 hours • Calculate 24 -hosr fluid needs: maintenance + deficit volume • Subtract isotonic fluid already administered from 24 -hour fluid needs • Administer remaining volume over 24 hours using D 5 1/2 normal saline + 2 O m. Eq/LKCl • Replace ongoing losses as they occur

Monitoring and Management of Dehidration • All calculations in fluid therapy are only approximations. • Thus, the patient needs to be monitored during treatment with therapy modifications based on the clinical situation.

Monitoring Therapy • Vital signs Pulse Blood pressure • Intake and outpur Fluid balance Urine output and specific graviuy • Physical examination Weight Clinical signs of depletion or overload • Electrolytes

Treatment of Hypernatremic Dehydration • • • Restore intravascular volume Normal saline: 20 ml/kg over 20 minutes (repeat until intravascular volume restored) Determine time for correction based on initial sodium concentration [Na] 145 -157 m. Eq/L: 24 hr [Na] 158 -170 m. Eq/L: 48 hr Na] 171 -183 m. Eq/L: 72 hr [Na] 18 a-196 m. Eq/L: 84 hr Administer fluid at a constant rate over the time for correction Typical fluid: D 5 1/2 normal saline (wirh 20 m. Eq/L pocassium chloride unless contraindicated) Typical rate: 1. 25 -1. 5 times maintenance Follow serum sodium concentration Adjust fluid based on clinical status and serum sodium concentration Signs of volume depletion: administer normal saline (20 m. Llkg) Sodium decreases too rapidly Increase sodium concentration of IV fluid or Decrease rare of IV fluid Sodium decreases too slowly Decrease sodium concentration of IV fluid or Increase rate of IV fluid Replace excessive ongoing losses as they occur

Sadium Disorders • HYPONATREMIA • HYPERNATREMIA

• Pseudohyponatremia • Hyperosmolality, resulting from mannitol infusion or hyperglycemi • For every 100 mg/dl-increment of the serum glucose, the serum sodium decreases by 1. 6 m. Eq/L.

• In hypovolemic hyponatremia, the child has lost sodium from the body. • Water balance may be positive or negative, but there has been a higher net sodium loss than water loss; this is due to oral or intravenous (IV) water intake, with water retention bv the kidneys to compensate for the intravascular volume depletion. • If the sodium loss is due to a nonrenal disease the urine sodium concentration is very low, as the kidneys attempt to preserve the intravascular volume by conserving sodium. • In renal diseases the urine sodium is inappropriately elevated.

• Patients with hyponatremia and no evidence of volume overload or volume depletion have euvolemic hyponatremia. • These patients typically have an excess of total body water and a slight decrease in total body sodium. • Some of these patients have an increase in weight, implying that they are volume overloaded. • Nevertheless, they usually appear normal or have only subtle signs of fluid overload. • In syndrome of inappropriate ADH (SIADH), there is secretion of ADH that is not inhibited by either low serum osmolality or expanded intravascular volume.

• Hyponatremia in hospitalized patients is often due to inappropriately produced ADH secondary to stress in the presence of hypotonic fluids. • SIADH is associated with pneumonia, mechanical ventilation, meningitis, and other central nervous system disorders (trauma). • Ectopic (tumor) producrion of ADH is rare in children. • Infants also can develop euvolemic hvponatremia as a result of excessive water consumption or • inappropriately diluted formula.

• In hypervolemic hyponatremia, there is an excess of total body water and sodium, although the increase in water is greater than the increase in sodium. • In renal failure, there is an inability to excrete sodium or water; the urine sodium may be low or high, depending on the cause of the renal insufficiency. • In other causes of hypervolemic hyponatremia, there is a decrease in the effective blood volume because of either third space fluid loss or poor cardiac output.

Clinical Manifestation • Brain cell swelling is responsible for most of the symptoms of hyponatremia. • Neurologic symptoms of hyponatremia include anorexia, nausea, emesis, malaise, lethargy, confusion, agitation, headache, seizures, coma and decreased reflexes. • Patients may develop hypothermia and Cheyne-Stokes respirations. • Hyponatremia can cause muscle cramps and weakness. • Symptoms are more severe when hyponatremia develops rapidly; chronic hyponatremia can be asymptomatic because of a compensatory decrease in brain cell osmolality, which limits cerebral swelling.

Treatment • Rapid correction of hyponatremia can produce central pontine myelinolysis. • Avoiding more than a I 2 -m. Eq/L increase in the serum sodium every 24 hours is prudent, especially if the hyponatremia developed gradually.

• • Treatment of hypovolemic hyponatremia Treatment of children with SIADH Treatment of acute water intoxication Treatment of hypervolemic hyponatremia

• Emergency treatment of symptomatic hyponatremia, such as seizures, uses IV hypertonic saline to increase the serum sodium concentration rapidly, which leads to a decrease in brain edema. • One milliliter per kilogram of 3% sodium chloride increases the serum sodium by approximately 1 m. Eq/L. A child often improves after receiving 4 ro 5 m. L/kg of 3% sodium chloride.

HYPERNATREMIA

Etiology • • • Sodium intoxication Hyperaldosteronism Diabetes insipidus Water losses Diarrhea Some renal diseases including obstructive uropathy, renal dysplasia, and juvenile nephronophrhisis, can cause losses of sodium and water

Clinical Manifestations • Probably because of intracellular water loss, the pinched abdominal skin of a dehydrated, hypernatremic infant has a doughy fee. I. • Patients are irritable, restless, weak, and lethargic. • Some infants have a high-pitched cry and hyperpnea. • Alert patients arc very thirsty, although nausea may be present. • Hypernatremia causes fever, although many patients have • an underlying process that contributes to the fever. • Hypernatremia is associated with hyperglycemia and mild hypocalcemia; the mechanisms are unknown.

• Brain hemorrhage is the most devastating consequence of hypernatremia. • As the extracellular osmolaliry increases, water moves out of brain cells, resulting in a decrease in brain volume. • This decrease in volume can result in tearing of intracerebral veins and bridging blood vessels as the brain moves away from the skull and the meninges. • Patients may have subarachnoid, subdural, and parenchymal hemorrhage. • Seizures and coma are possible sequelae of the hemorrhage.

Potassium Disorder • HYPOKALEMIA • HYPERKALEMIA

HYPOKALEMIA

Etiology • Spurious High white blood cell counr • Transcellular shifts Alkalemia Insulin Beta-Adrenergic agonists Drugs/toxins (theophylline, barium, toluene) Hypokalemic periodic paralysis • Decreased intake • Extrarenal losses Diarrhea Laxative abuse Sweating

• Renal losses With metabolic acidosis Distal RTA Proximal RTA Ureterosigmoidostomy Diabetic ketoacidosis Without specific acid-base disturbance Tubular roxins (amphorericin, cisplacin, aminoglycosides) Intersririd nephritis Diuretic phase of acuce rubular necrosis Postobstrucdve diuresrs Hypomagnesemia High urine anions (e. g: penicillin or penicillin derivatives)

With merabolic alkalosis • • • Low urine chloride Emesis/nasogastric suction Pyoric stenosis Chloride-losing diarrhea Cystic fibrosis Low-chloride formula Posthypercapnia Previous loop or thiazide diuretic use High urine chloride and normal blood pressure Gitelman syndrome Bartter syndrome Loop and thiazide diurerics High urine chloride and high blood pressure Adrenal adenoma or hyperplasia Glucocorricoid-remediable aldosreronism Renovascular disease Renin-secreting tumor 17 a-Hydroxylase deficiency 1 1 B-Hydroxylase deficiency Cushing syndrome 1 1 B-Hydroxysteroid dehydrogenase deficiency Licorice ingescion Liddle syndrome

clinical Manifestations • The heart and skeletal muscle are especially vulnerable to hypokalemia. • Electrocardiographic (ECG) changes include a flattened T wave, a depressed ST segment, and the appearance of a U wave. • The clinical consequences in skeletal muscle include muscle weakness and cramps. • Paralysis is a possible complication (generally only at potassium levels <2. 5 m. Eq/L). • Paralysis usually starts with the legs, followed by the arms. • Respiratory paralysis may require mechanical ventilation.

• Some patients develop rhabdomyolysis; the risk increases with exercise. • Hypokalemia slows gastrointestinal motility; potassium levels less rhan 2. 5 rm. Eql. L may cause an ileus. • Hypokalemia impairs bladder function, potentially Ieading to urinary retention. • Hypokalemia causes polyuria by producing secondary nephrogenic diabetes insipidus. • Chronic hypokalemia may cause kidney damage, including interstitial nephritis and renal cysts. • In children, chronic hypokalemia, as in Bartter syndrome, leads to poor linear growth.

Diagnosis • It is important to review the child's diet, history of gastrointestinal losses, and medications. • Emesis and diuretic use can be surreptitious. • The presence of hypertension suggests excess mineralocorticoids. • Concomitant electrolyte abnormalities are useful clues. • The combination of hvpokalemia and metabolic acidosis is characteristic of diarrhea, distal renal tubular acidosis, and proximal renal tubular acidosis. • A concurrent metabolic alkalosis is characteristic of gastric losses, aldosterone excess, diuretics, and Bartter syndrome or Gitelman syndrome. • Alkalosis also causes a transcellular shift of potassium and increased urinary losses of potassium.

HYPERKALEMIA

• • • • • • Causes of Hyperkalemia Spurious laboratory value Hemolysis Tissue ischemia during blood drawing Thrombocytosis Leukocytosis Increased incake IV or PO Blood transfusions Transcellular shifts Acidemia Rhabdomyolysis Tumor lysis syndrome Tissue necrosrs Hemolysis/hematomas/gastrointestinabl leeding Succinylcholine Digitalis intoxication Fluoride intoxication B-Adrenergic blockers Exercrse Hyperosmolaiiry Insulin deficiency Malignanc hyperchermra Hyperkalemic periodic Paral. Ysis

Decreased excretion • • • • Renal failure Primary adrenal disease Acquired Addison disease 2 l-Hydroxylase deficiency 3 Beta-Hydroxysteroid-dehydrogenase deficiency Lipoid congenital adrenal hyperplasia Adrenal hypoplasia congenira Aldoscerone synthase deficienc. Y Adrenoleukodystrophy Hyporeninemic hypoaldosteronism Urinary tract obscruction Sickle cell disease Kidney transplant Lupus nephritis

Decreased excretion • Renal tubular disease • • • Pseudohypoaldosteronismr type 1 Pseudohypoaldosteronismr type 2 Urinary tract obstruction Sickle cell disease Kidney transplant • Medications • • • ACE inhibitors Angiotensin II blockers Potassium-sparing diuretics Cyclosporine NSAIDS Trimethoprim

Clinical manifestations • The most important effects of hyperkalemia are due to the role of potassium in membrane polarization. • The cardiac conduction system is usually the dominant concern. • ECG changes begin with peaking of the T waves. • As the potassium level increases, an increase P-R interval, flattening of the P wave, and widening of the QRS complex occur, this eventually can progress to ventricular fibrillation.

Treatment • The first action in a child with a concerning elevation of plasma potassium is to stop all sources of additional potassium (oral and IV). • If the potassium level is greater than 6 to 6. 5 m. Eq/L, an ECG should be obtained to help assess the urgency of the situation. • Therapy of hyperkalemia has two basic goals: • 1. Prevent life-threatening arrhythmias. • 2. Remove potassium from the body

Treatment of hyperkalemia • Rapidly decrease the risk of life-threatening arrhythmias Shift pocassium inrracellularly Sodium bicarbonare administration (IV) Insulin and glucose (IV) B-Agonist (albuterol via nebulizer) Cardiac membrane stabilization IV calcium • Remove potassium from the body Loop diurecic (IV or PO) Sodium polystyrene (PO or rectal) Dialysis