CHAPTER 3 VOLUMETRIC PROPERTIES OF PURE FLUIDS PURE



- Slides: 13

CHAPTER 3 VOLUMETRIC PROPERTIES OF PURE FLUIDS

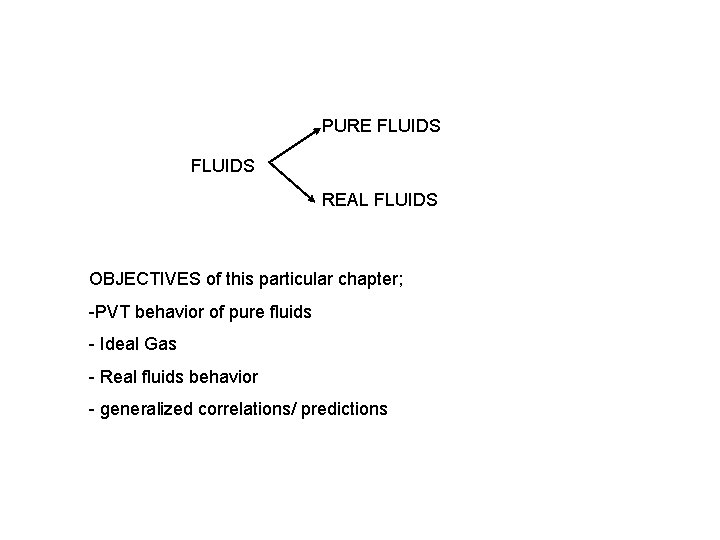
PURE FLUIDS REAL FLUIDS OBJECTIVES of this particular chapter; -PVT behavior of pure fluids - Ideal Gas - Real fluids behavior - generalized correlations/ predictions

Pure Fluids/ Substance § A substance that has a fixed chemical composition throughout is called a Pure Substance. § Pure Substance: - N 2, O 2, gaseous Air -A mixture of liquid and gaseous water is a pure substance

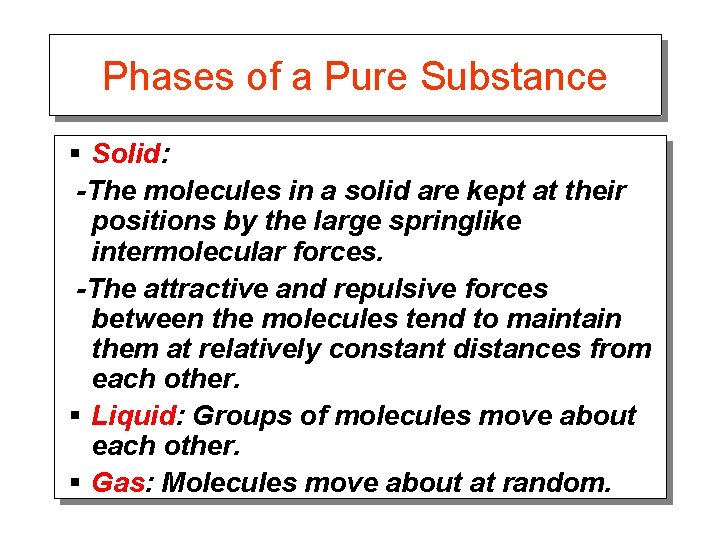
Phases of a Pure Substance § Solid: -The molecules in a solid are kept at their positions by the large springlike intermolecular forces. -The attractive and repulsive forces between the molecules tend to maintain them at relatively constant distances from each other. § Liquid: Groups of molecules move about each other. § Gas: Molecules move about at random.

Phase-Change Processes of Pure Substance § Compressed liquid or a subcooled liquid: A liquid that is not about to vaporize. § Saturated liquid: A liquid that is about to vaporize. § Saturated vapor: A vapor that is about to condense. § Saturated liquid-vapor mixture: the liquid and vapor phases coexist in equilibrium. § Superheated vapor: A vapor that is not about to condense

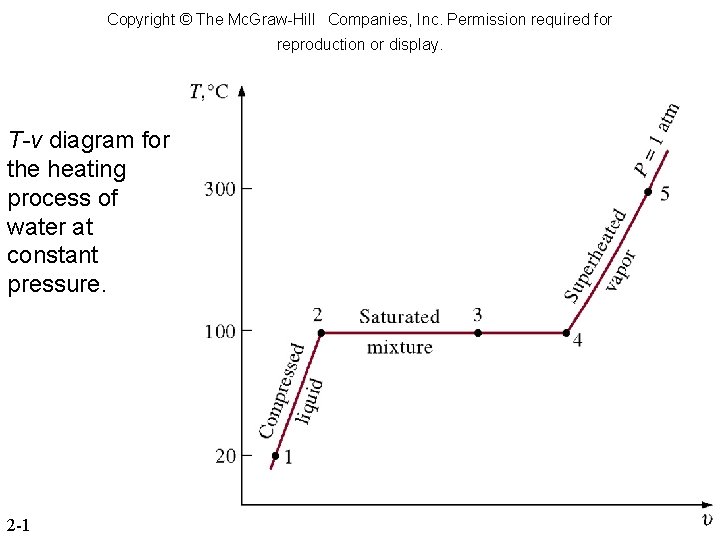
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. T-v diagram for the heating process of water at constant pressure. 2 -1

Phase-Change Processes of Pure Substance § Saturated temperature, Tsat: At a given pressure, the temperature at which a pure substance changes phase. § Saturated pressure, Psat: At a given temperature, the pressure at which a pure substance changes phase. § Latent heat: the amount of energy absorbed or released during a phase-change process. § Latent heat of fusion: the amount of energy absorbed during melting. § Latent heat of vaporization: the amount of energy absorbed during vaporization.

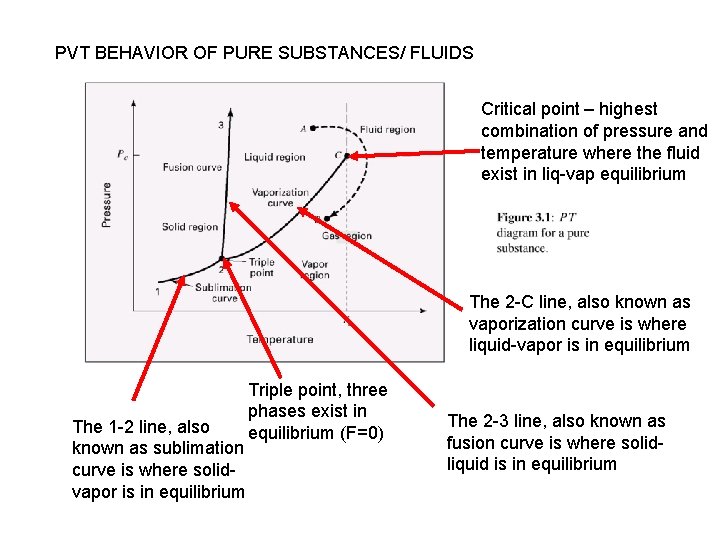
PVT BEHAVIOR OF PURE SUBSTANCES/ FLUIDS Critical point – highest combination of pressure and temperature where the fluid exist in liq-vap equilibrium The 2 -C line, also known as vaporization curve is where liquid-vapor is in equilibrium The 1 -2 line, also known as sublimation curve is where solidvapor is in equilibrium Triple point, three phases exist in equilibrium (F=0) The 2 -3 line, also known as fusion curve is where solidliquid is in equilibrium

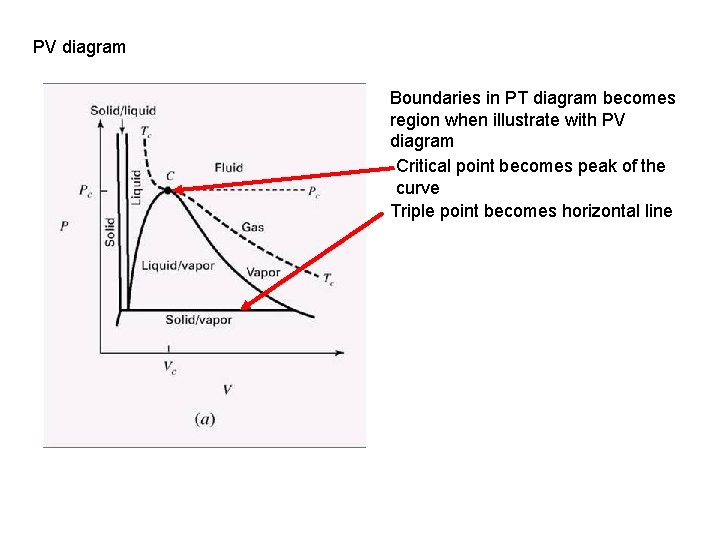
PV diagram Boundaries in PT diagram becomes region when illustrate with PV diagram Critical point becomes peak of the curve Triple point becomes horizontal line

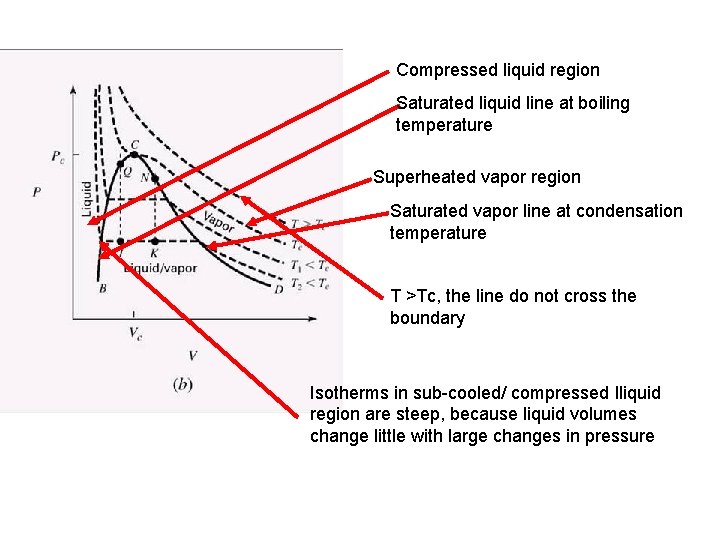
Compressed liquid region Saturated liquid line at boiling temperature Superheated vapor region Saturated vapor line at condensation temperature T >Tc, the line do not cross the boundary Isotherms in sub-cooled/ compressed lliquid region are steep, because liquid volumes change little with large changes in pressure

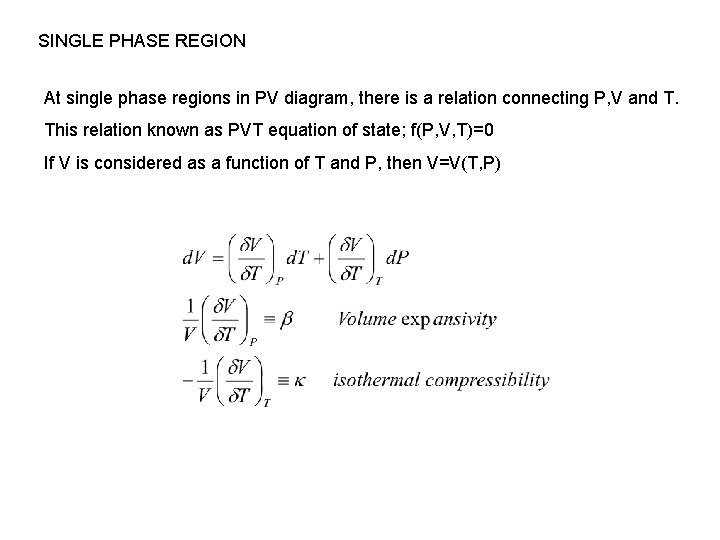
SINGLE PHASE REGION At single phase regions in PV diagram, there is a relation connecting P, V and T. This relation known as PVT equation of state; f(P, V, T)=0 If V is considered as a function of T and P, then V=V(T, P)

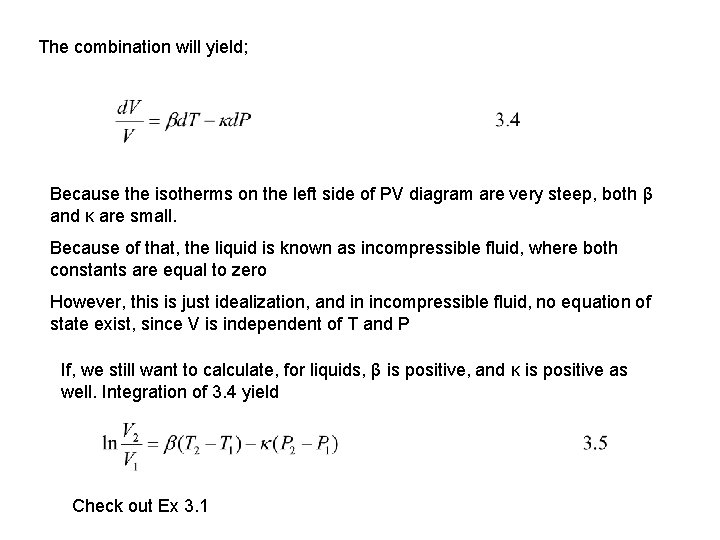
The combination will yield; Because the isotherms on the left side of PV diagram are very steep, both β and κ are small. Because of that, the liquid is known as incompressible fluid, where both constants are equal to zero However, this is just idealization, and in incompressible fluid, no equation of state exist, since V is independent of T and P If, we still want to calculate, for liquids, β is positive, and κ is positive as well. Integration of 3. 4 yield Check out Ex 3. 1

 Volumetric properties of pure fluids
Volumetric properties of pure fluids Buoyancyability
Buoyancyability Properties of pure substances
Properties of pure substances Chapter 18 fluids and electrolytes
Chapter 18 fluids and electrolytes Volumetric analysis problems
Volumetric analysis problems Types of volumetric analysis
Types of volumetric analysis Apparatus used in volumetric analysis
Apparatus used in volumetric analysis Titration definition
Titration definition Teen power pmv
Teen power pmv Gravimetric analysis assignment
Gravimetric analysis assignment Volumetric efficiency
Volumetric efficiency Graduated cylinder vs volumetric flask
Graduated cylinder vs volumetric flask Volumetric glassware examples
Volumetric glassware examples Precipitation titration questions
Precipitation titration questions