Volumetric Analysis Apparatus The Volumetric Flask Volumetric Flask








- Slides: 13

Volumetric Analysis Apparatus

The Volumetric Flask

Volumetric Flask • Weigh out a specific mass of solute on a clean, dry clock glass. • Add the solute to a beaker containing a small volume of water. • Using a wash bottle wash all traces of the solute from the clock glass into the beaker. • Stir the mixture with a clean glass rod.

Volumetric Flask • Using a funnel transfer the contents of the beaker into a clean dry volumetric flask. • Wash all traces from the beaker, glass rod and funnel into the flask. • Continue to add deionised water into the flask until the level is just below the mark on the flask.

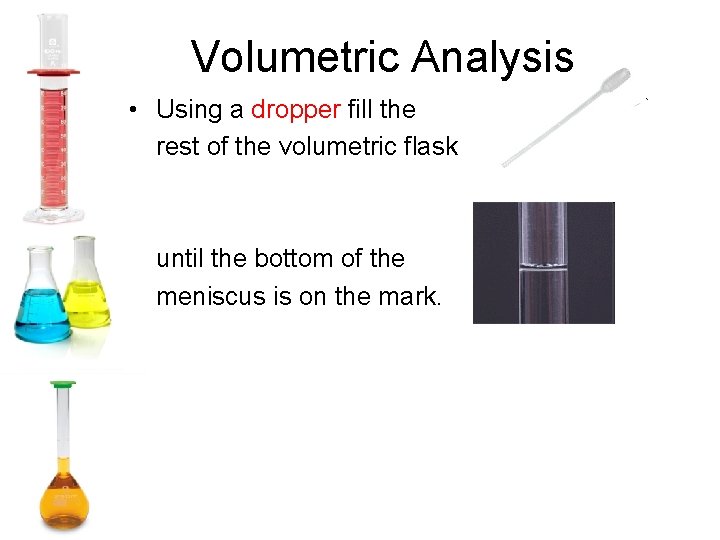
Volumetric Analysis • Using a dropper fill the rest of the volumetric flask until the bottom of the meniscus is on the mark.

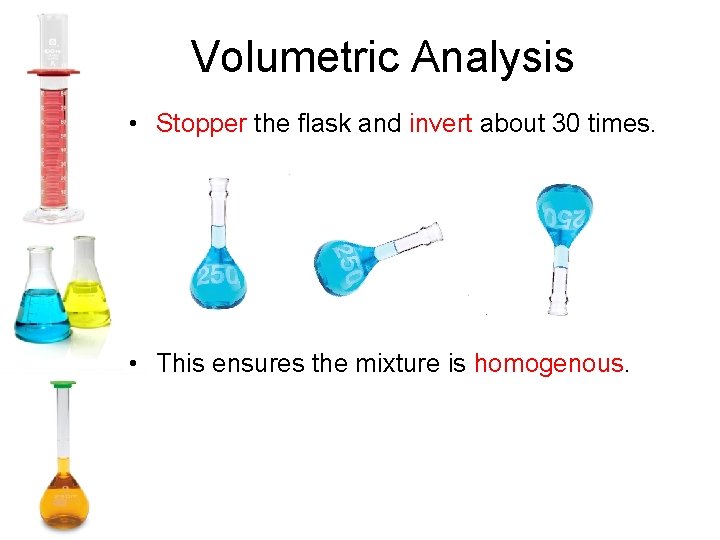
Volumetric Analysis • Stopper the flask and invert about 30 times. • This ensures the mixture is homogenous.

The Pipette

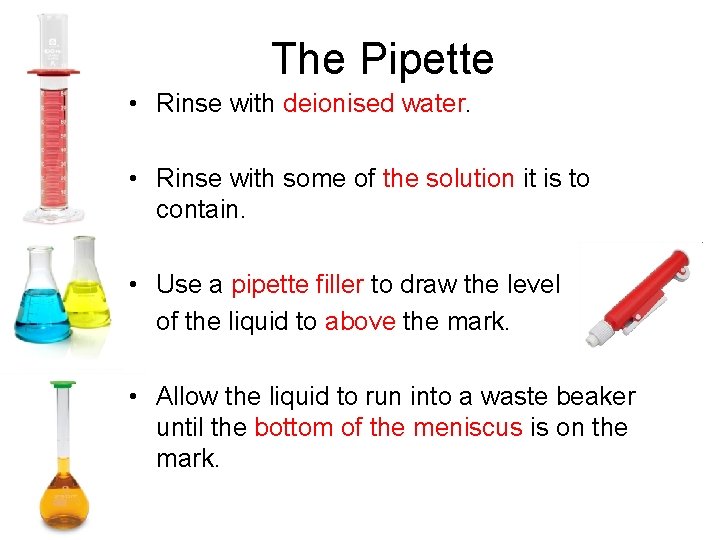
The Pipette • Rinse with deionised water. • Rinse with some of the solution it is to contain. • Use a pipette filler to draw the level of the liquid to above the mark. • Allow the liquid to run into a waste beaker until the bottom of the meniscus is on the mark.

The Pipette • Touch the pipette against the side of a glass beaker. This will remove any droplet of liquid that might be adhering to the pipette. • Allow the liquid to drain under gravity in to the conical flask. • Touch the tip of the pipette to the side of the flask but do not blow the last drop out.

The Burette

The Burette • Rinse with deionised water. • Rinse with some of the solution it is to contain. • Use a small funnel when filling the burette. • The burette is filled almost to the top and then the tap is opened to fill the part below the tap. • Re-fill the burette until the bottom of the meniscus is on the mark.

Conical Flask

Conical Flask • Its design allows it to be swirled without the solution splashing out. • Before use, wash out with deionised water. • Do not wash out with the solution it is to contain. • It will be holding an exact volume of a solution and so if it washed prior to this it would have droplets that would affect this volume.