Analytical Chemistry Volumetric Analysis Volumetric Analysis 1 Volumetric











- Slides: 16

Analytical Chemistry Volumetric Analysis

Volumetric Analysis 1 Volumetric or titrimetric analysis are among the most useful and accurate analytical techniques, especially for milimole amounts of analyte. They are rapid and can be automated, and they can be applied to smaller amounts of analyte when combined with a sensitive instrumental technique for example, p. H measurement. Manual titration nowadays are used in solutions that require high accuracy for relatively small numbers of samples. Automated titrations are useful when large numbers of samples must be processed. For example, A + B 2 3 C In a titration the test substance (analyte) in a flask reacts with a reagent added from a buret as a solution of known concentration. This is referred to as a standard solution, and is called the titrant. The volume of the titrant required to just completely react with the analyte is measured. Since we know the concentration as well as the reaction between the analyte and the reagent, we can calculate the amount of analyte. The equivalence point is the point where the amount of both the titrant and the titrated solution are equivalent. The end point is the point where some of the solution’s characteristics change. The end point directly follows the equivalence point. More. .

Volumetric Analysis 3 A Cl- + Ag. Cl Since Ag+ and Cl- react on a 1: 1 molar basis, the number of millimoles of Clis equal to the number of millimoles of Ag+ needed for titration. We can calculate the millimoles of Na. Cl as follows: mmol. Na. Cl = m. LAg. NO 3 x MAg. NO 3 = 25. 0 m. L * 0. 100 (mmol/ m. L) = 2. 50 mmol mg. Na. Cl = mmol x f wt. Na. Cl = 2. 5 mmol x 58. 44 mg/ mmol =146 mg B Example: How many milliliters of 0. 25 M solution of H 2 SO 4 will react with 10 m. L of a 0. 25 M solution of Na. OH? Solution: The reaction is: H 2 SO 4+ 2 Na. OH Na 2 SO 4+ 2 H 2 O One- half as many millimoles of H 2 SO 4 as of Na. OH will react, or: M H 2 SO 4 x m. LH 2 SO 4= M Na. OH x m. LNa. OH x ½(mmol H 2 SO 4 / mmol Na. OH) Therefore, m. L H 2 SO 4= 0. 25 mmol Na. OH/ m. L x 10 m. L Na. OH x ½ (mmol H 2 SO 4/ mmol Na. OH) 0. 25 mmol H 2 SO 4/ m. L Note that, in this case, we multiplied the amount of titrant by the alt ratio (mmol analy mmol titrant).

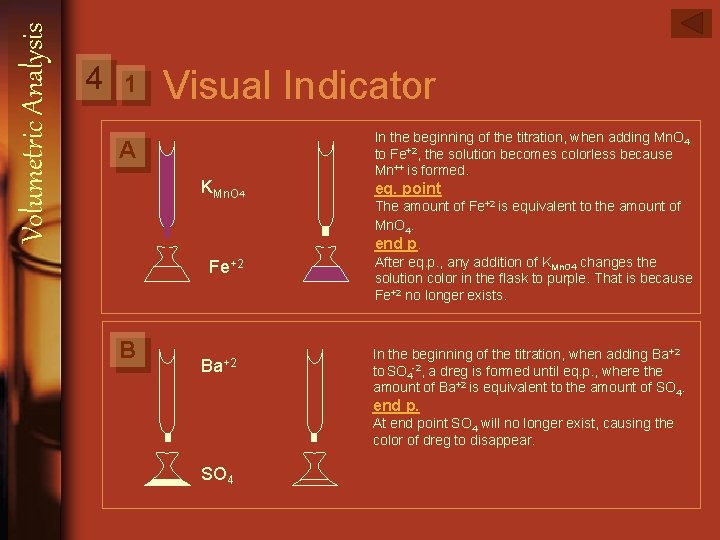
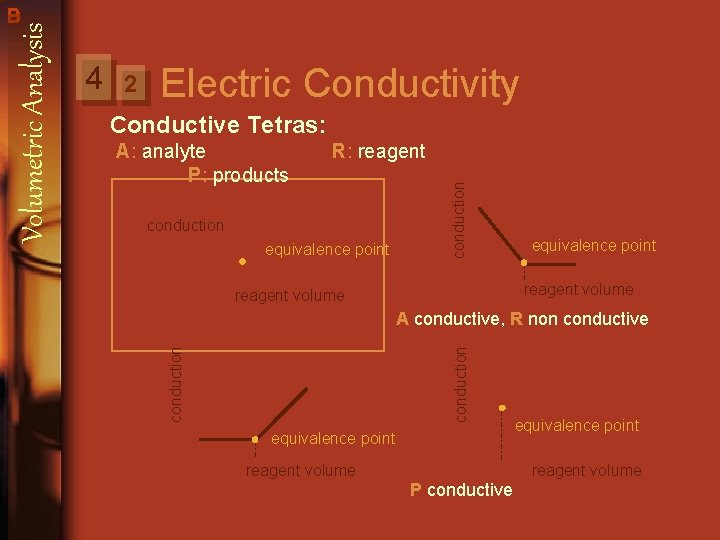
Volumetric Analysis 4 There are two parts to determine the end point: 1. Visual indicator: a. Change in color Mn. O-4 + 5 Fe 2+ + 8 H Mn 2+ + 5 Fe 3+ + 4 H 2 O That happens when either titrant or titrated solution is colored. If it is not colored we need the indicator. b. Formation of dregs (precipitation) Ba 2+ + SO 24 Ba. SO 4 2. Measured properties: They can’t be observed, so the are measured by instruments, for example: a. Electric conductivity b. Solution absorption for vis- uv ray: We can determine the end point by spertrophotometric titrations.

spertrophotometric

Volumetric Analysis 4 1 Visual Indicator A KMn. O 4 In the beginning of the titration, when adding Mn. O 4 to Fe+2, the solution becomes colorless because Mn++ is formed. eq. point The amount of Fe+2 is equivalent to the amount of Mn. O 4. end p. Fe+2 B Ba+2 After eq. p. , any addition of KMn. O 4 changes the solution color in the flask to purple. That is because Fe+2 no longer exists. In the beginning of the titration, when adding Ba+2 to SO 4 -2, a dreg is formed until eq. p. , where the amount of Ba+2 is equivalent to the amount of SO 4. end p. At end point SO 4 will no longer exist, causing the color of dreg to disappear. SO 4



2 Electric Conductivity Conductive Tetras: R: reagent conduction equivalence point conduction A: analyte P: products equivalence point reagent volume A conductive, R non conductive conduction 4 conduction Volumetric Analysis B D equivalence point reagent volume P conductive

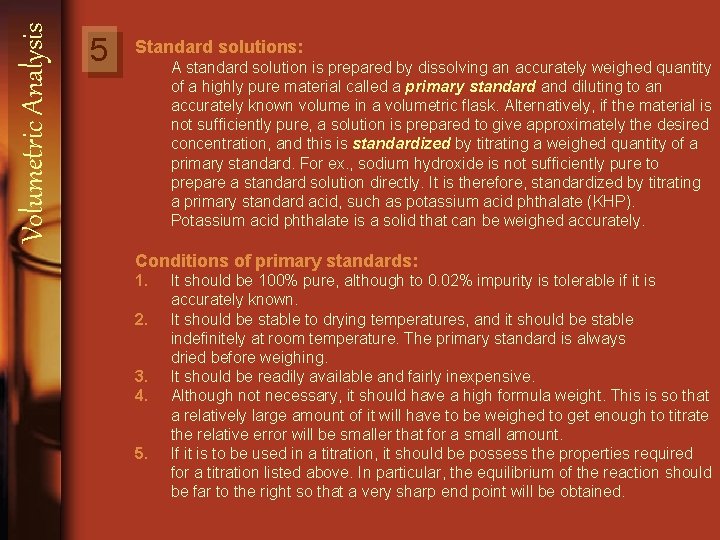
Volumetric Analysis 5 Standard solutions: A standard solution is prepared by dissolving an accurately weighed quantity of a highly pure material called a primary standard and diluting to an accurately known volume in a volumetric flask. Alternatively, if the material is not sufficiently pure, a solution is prepared to give approximately the desired concentration, and this is standardized by titrating a weighed quantity of a primary standard. For ex. , sodium hydroxide is not sufficiently pure to prepare a standard solution directly. It is therefore, standardized by titrating a primary standard acid, such as potassium acid phthalate (KHP). Potassium acid phthalate is a solid that can be weighed accurately. Conditions of primary standards: 1. 2. 3. 4. 5. It should be 100% pure, although to 0. 02% impurity is tolerable if it is accurately known. It should be stable to drying temperatures, and it should be stable indefinitely at room temperature. The primary standard is always dried before weighing. It should be readily available and fairly inexpensive. Although not necessary, it should have a high formula weight. This is so that a relatively large amount of it will have to be weighed to get enough to titrate the relative error will be smaller that for a small amount. If it is to be used in a titration, it should be possess the properties required for a titration listed above. In particular, the equilibrium of the reaction should be far to the right so that a very sharp end point will be obtained.

Volumetric Analysis 6 Conditions of the titration reaction: 1. The reaction must be stoichiometric. That is, there must be a well defined 2. 3. 4. and known reaction between the analyte and the titrant. In the titration of acetic in vinegar with sodium hydroxide. The reaction should be rapid. Most ionic reaction. There should be no side reaction, and the reaction should be specific. If there are interfering substances, these must be removed. The reaction should be quantitative. That is, the equilibrium of the reaction should be far to the right so that a sufficiently sharp change will occur at the end point to obtain the desired accuracy. If the equilibrium does not lie far to the right, then there will be gradual change in the property marking the end point (ex. , p. H) and this will difficult to detect precisely.

Volumetric Analysis 7 Classification of volumetric methods: There are four general classes of volumetric or titmetric methods. 1. 2. Acid- Base: Many compounds, both inorganic and organic, are either acids or bases and can be titrated with a standard solution of a strong base or a strong acid. The end points of these titrations are easy to detect, either by means of an indicator or by following the change in p. H with a p. H meter. The acidity and basicity of many organic acids or bases can be enhanced by titrating in a nonaqueous solvent. The result is a sharpen end point, and weaker acids and bases can be titrated in this manner. Precipitation: In the case precipitation, the titrant forms an insoluble product with the analyte. An example is the titration of chloride ion with silver nitrate solution to form silver chloride precipitate. Again, indicators can be used to detect the end point, or the potential of the solution can be monitored electrically. Continue. .

Volumetric Analysis 7 3. 4. Complexometric: In complexometric titrations, the titrant is a reagent that forms a water-soluble complex with the analyte, a metal ion. The titrant is often a chelating agent. The reverse titration maybe carried out also. Ethylenediaminetetraacetic acid (EDTA) is one of the most useful chelating agents used for titration. It will react with a large number of elements, and the reaction can be controlled by the adjustment of the p. H. Indicators can be used to form a highly colored complex with the metal ion. Reduction-Oxidation: These “redox” titrations involve the titration of an oxidizing agent with a reducing agent, or vice versa. An oxidizing agent gains electrons and a reducing agent loses electrons in a reaction between them. There must be a sufficiently large difference between the oxidizing and reducing capabilities of these agents for the reaction to go to completion and give a sharp end point; that is, one should be a fairly strong oxidizing agent (strong tendency to gain electrons) and the other a fairly strong reducing agent (strong tendency to lose electrons). You can use appropriate indicators for these titrations, or you may employ various electrometric means to detect the end point.

Volumetric Analysis 8 Types of titration Direct titration Indirect titration (Back titration) • The reaction is fast • The reaction is slow • The reagent is directly added to the sample

8 Back titration In conditions where the reaction between the titrant A and the titrated solution B is incomplete or when the proper evidence for the reaction is unavailable or when the solution B is unstable, we then turn to adding a fact to the titrant A, where a portion of A reacts with B and the remaining amount of A is titrated by a measured solution from the substance D: a. A + b. B C a. A + d. D E By knowing the remaining amount of A, we can know the amount of A reacted with B, and by that we can know the amount of B. mmoles B = mmoles A used in b a mmoles A used = mmoles A added – mmoles A remaining = mmoles D a d Mmoles B = (mmoles A added – mmoles D a ) b d a

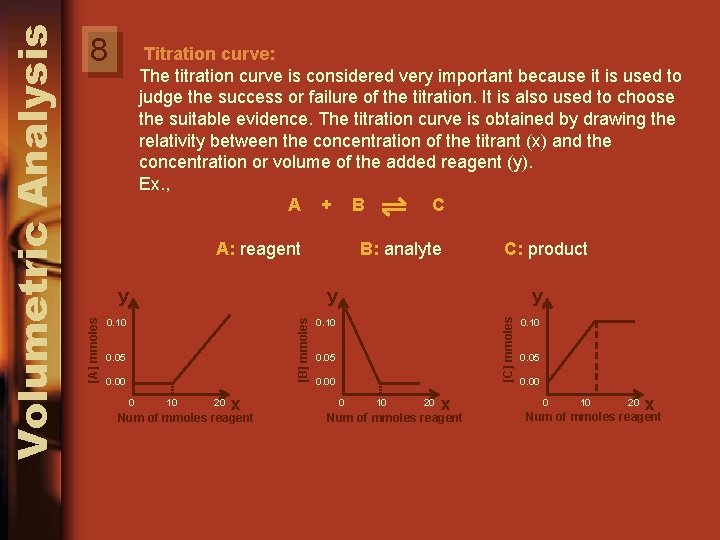
Titration curve: The titration curve is considered very important because it is used to judge the success or failure of the titration. It is also used to choose the suitable evidence. The titration curve is obtained by drawing the relativity between the concentration of the titrant (x) and the concentration or volume of the added reagent (y). Ex. , A + B C A: reagent B: analyte C: product 0. 10 0. 05 0. 00 0 10 20 x Num of mmoles reagent y [C] mmoles y [B] mmoles y [A] mmoles Volumetric Analysis 8 0. 10 0. 05 0. 00 0 10 20 x Num of mmoles reagent