Experiment 15 Chemical Kinetics Purpose The purpose of





![Rate = k[A]x[B]y • • • [A], [B]: molarities of A and B in Rate = k[A]x[B]y • • • [A], [B]: molarities of A and B in](https://slidetodoc.com/presentation_image_h2/0ed5a91fc5b42fa19aaced1f6eb3d1de/image-7.jpg)
















- Slides: 26

Experiment 15 Chemical Kinetics

Purpose • The purpose of this experiment is to determine the rate of a chemical reaction (potassium permanganate, KMn. O 4, + oxalic acid, H 2 C 2 O 4) as the concentrations are varied and to determine the rate law for the reaction.

Introduction • For a reaction a. A+b. B c. C+d. D


Different Rates • Average (this experiment) • Initial (Experiment 17) • Instantaneous

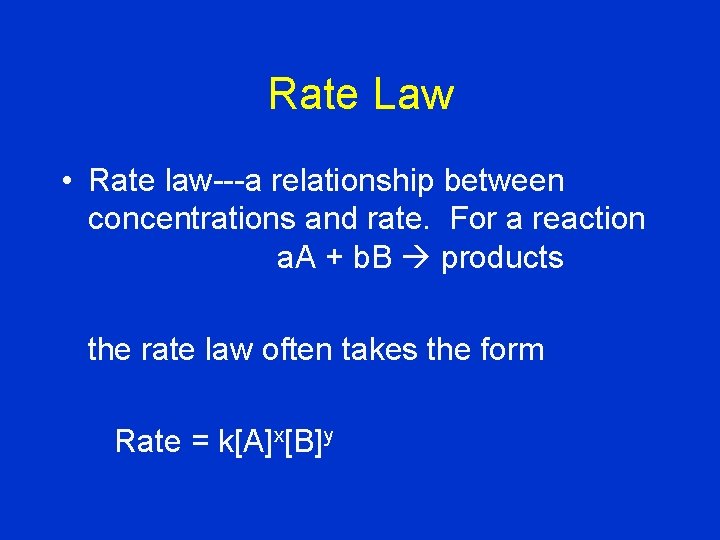
Rate Law • Rate law---a relationship between concentrations and rate. For a reaction a. A + b. B products the rate law often takes the form Rate = k[A]x[B]y
![Rate kAxBy A B molarities of A and B in Rate = k[A]x[B]y • • • [A], [B]: molarities of A and B in](https://slidetodoc.com/presentation_image_h2/0ed5a91fc5b42fa19aaced1f6eb3d1de/image-7.jpg)
Rate = k[A]x[B]y • • • [A], [B]: molarities of A and B in solution x, y: orders with respect to A and B, respectively. (These orders might not correspond to coefficients from the balanced equation!) k: rate constant

What We’re Running • 2 Mn. O 4 - + 5 H 2 C 2 O 4 + 6 H+ 2 Mn 2+ + 10 CO 2 + 8 H 2 O • We assume a rate law Rate = k [Mn. O 4 -]m[H 2 C 2 O 4]n • The rate law and rate constant are not affected by concentration.

Strategy • • • One pair of experiments (assignments 1 and 2): [Mn. O 4 -] is constant and [H 2 C 2 O 4] doubles. In another pair (1 and 3), [Mn. O 4 -] doubles and [H 2 C 2 O 4] is constant. For each of these pairs, divide the rate measured in one experiment by that from another.

• Comparing assignments 1 and 2: • This can be rewritten as • Since rates and concentrations are known, n (the order with respect to oxalic acid) is available.

• Similarly, comparing assignments 1 and 3 gives • And, therefore, From this, m (the order with respect to Mn. O 4 -) is available.

• Once the orders are known, we can calculate the rate constant from the rate law. • Since rates depend on temperature. we will also look at the effect of temperature on the rate of this reaction.

Safety • Aprons and glasses. • KMn. O 4 is a strong oxidant (and also stains skin and clothing); oxalic acid is poisonous. • Waste into waste bottles.

Safety 2 • If you use the Bunsen burner for heating water, keep hair, clothing, paper, and other flammable material away. • Shut off burner before mixing hightemperature samples.

Procedure • Work in pairs. • Check out pipettes and bulbs from stockroom. • Needed equipment: medium-sized test tubes; 250 - and 400 -m. L beakers. May also need ring stand, ring, wire gauze, and Bunsen burner.

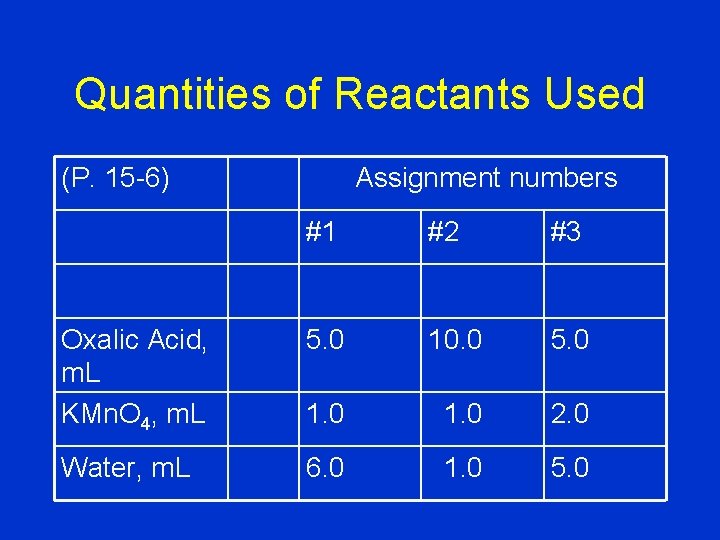
Quantities of Reactants Used Assignment numbers (P. 15 -6) #1 #2 #3 Oxalic Acid, m. L KMn. O 4, m. L 5. 0 10. 0 5. 0 1. 0 2. 0 Water, m. L 6. 0 1. 0 5. 0

• Mark an X on a piece of white paper. • Get 75 m. L oxalic acid and 15 m. L KMn. O 4 solutions; record concentrations. • Two tubes for each assignment; pipet desired volumes of oxalic acid and water into each tube. For use of volumetric pipets, review Expt. 11 from CHEM 1031.

• Prepare four additional tubes for Assignment #1; also pipet 1. 0 m. L of KMn. O 4 solution into each of four small test tubes. Save these for temperature study---last part of experiment. • Start with first oxalic acid-water tube for Assignment 1. Place the paper behind the test tube.

• Pipet KMn. O 4 solution into tube; begin timing when half the solution has been added. • When you can see the X through the test tube, record elapsed time on your data sheet. • Repeat the run you have just completed; then do duplicate runs for the other two assignments.

• Effect of temperature: Place two oxalic acid tubes and two KMn. O 4 tubes into a beaker containing warm water (10 o. C above room temperature). (If tap water is not sufficiently warm, use Bunsen burner. ) • After tubes have been in warm water for 5 minutes or so, add KMn. O 4 to an oxalic acid-water mix; record elapsed time for X to become visible. Repeat.

• Make cool water bath (ice in water) to get 10 o. C below room temperature. • Cool other two oxalic acid-water mixes and KMn. O 4 samples. Again mix, record elapsed time as before. Repeat.

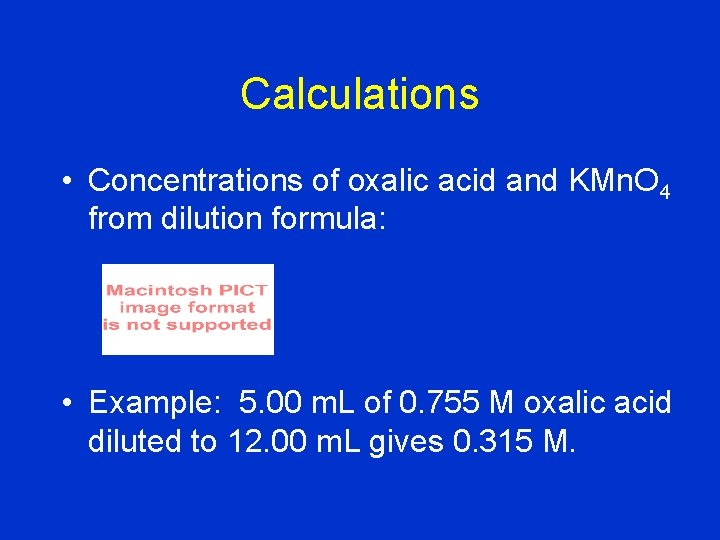
Calculations • Concentrations of oxalic acid and KMn. O 4 from dilution formula: • Example: 5. 00 m. L of 0. 755 M oxalic acid diluted to 12. 00 m. L gives 0. 315 M.

• For each assignment, average the times for the two runs. • D[Mn. O 4 -] = [Mn. O 4 -]f – [Mn. O 4 -]i = -[Mn. O 4 -]i

• From measured rates, determine orders: • Round the orders to the nearest integers.

• Go back to the rate law: Rate = k [Mn. O 4 -]m[H 2 C 2 O 4]n • You now know rates, concentrations, and orders. Calculate k for each assignment and average.

The effect of temperature • Rate is proportional to DConcentration/Dtime • ---if the time decreases by a factor of 3 (say), the rate correspondingly increases by a factor of 3.
 Chemical kinetics experiment
Chemical kinetics experiment Molekülerite nedir
Molekülerite nedir Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Order kinetics
Order kinetics Chemical kinetics definition
Chemical kinetics definition Applications of chemical kinetics
Applications of chemical kinetics Steady state approximation examples
Steady state approximation examples Blue bottle experiment chemical equation
Blue bottle experiment chemical equation Maksminkon
Maksminkon Kinetics and equilibrium
Kinetics and equilibrium What are the factors affecting drug distribution
What are the factors affecting drug distribution Plane kinetics of rigid bodies
Plane kinetics of rigid bodies Explain law of mass action
Explain law of mass action Difference between 1st order and zero order kinetics
Difference between 1st order and zero order kinetics Collision theory of kinetics
Collision theory of kinetics Kinetics flotation reagents
Kinetics flotation reagents Planar kinetics of a rigid body force and acceleration
Planar kinetics of a rigid body force and acceleration Kinetics of rigid body
Kinetics of rigid body Kinetics of a particle force and acceleration
Kinetics of a particle force and acceleration Ap chemistry kinetics
Ap chemistry kinetics Kinetics of crystal violet fading
Kinetics of crystal violet fading Octet kinetics
Octet kinetics Km in enzyme kinetics
Km in enzyme kinetics Lineweaver-burk equation
Lineweaver-burk equation Kinetics of particles newton's second law
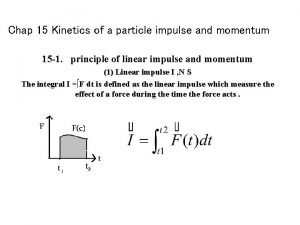
Kinetics of particles newton's second law Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Dynafit software
Dynafit software