Environmental Chemistry Visual Dictionary Nutrient elements and compounds






















- Slides: 30

Environmental Chemistry Visual Dictionary

Nutrient: elements and compounds that organisms need for living, growing and reproducing

Enzyme: catalyst involved in chemical reactions in living things. �

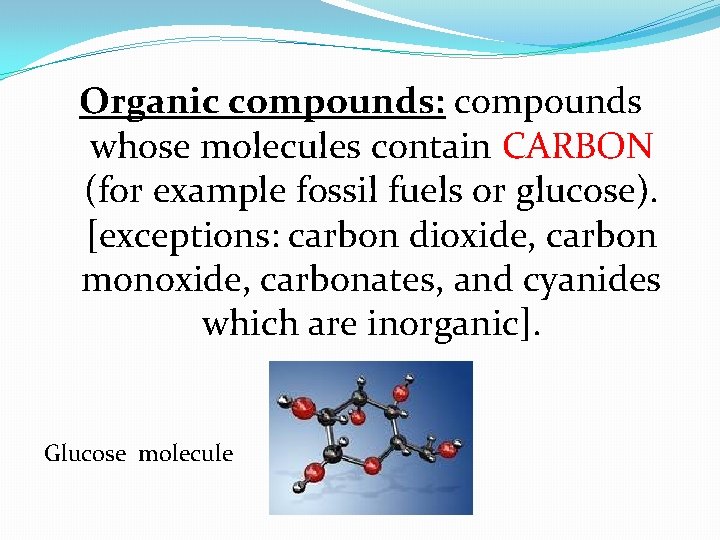
Organic compounds: compounds whose molecules contain CARBON (for example fossil fuels or glucose). [exceptions: carbon dioxide, carbon monoxide, carbonates, and cyanides which are inorganic]. Glucose molecule

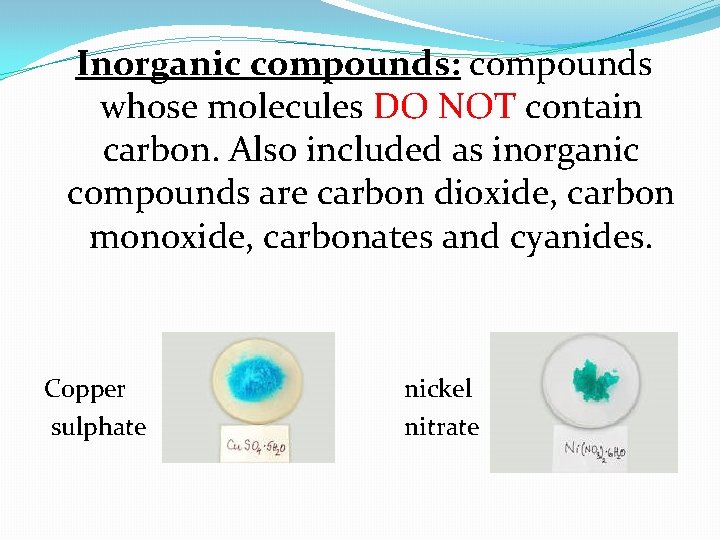
Inorganic compounds: compounds whose molecules DO NOT contain carbon. Also included as inorganic compounds are carbon dioxide, carbon monoxide, carbonates and cyanides. Copper sulphate nickel nitrate

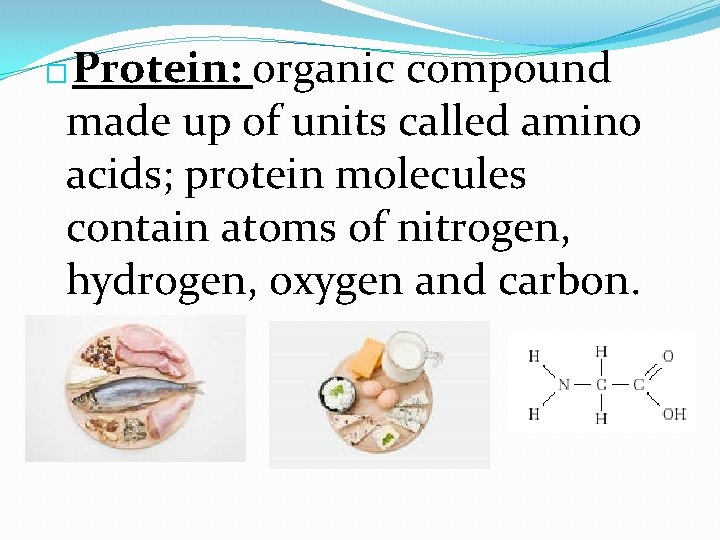
Protein: organic compound made up of units called amino acids; protein molecules contain atoms of nitrogen, hydrogen, oxygen and carbon. �


Lipid : organic molecules made up of atoms of carbon, hydrogen, and oxygen (example, fats, oils and waxes). Lipids are not soluble in water. �


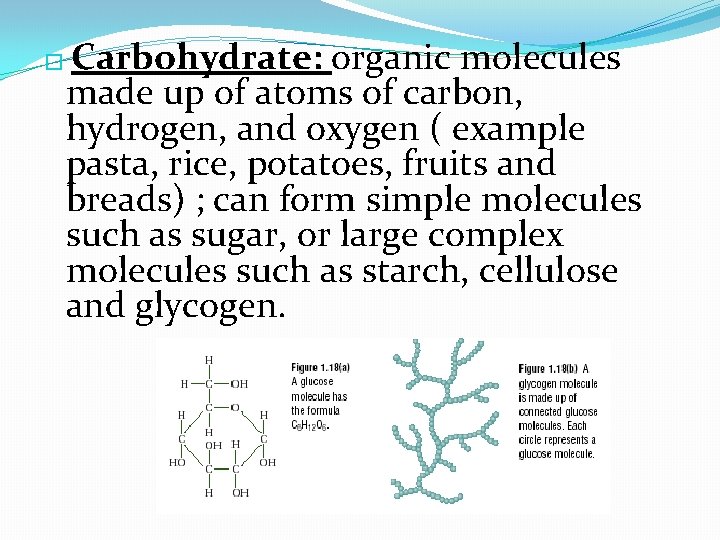
Carbohydrate: organic molecules made up of atoms of carbon, hydrogen, and oxygen ( example pasta, rice, potatoes, fruits and breads) ; can form simple molecules such as sugar, or large complex molecules such as starch, cellulose and glycogen. �


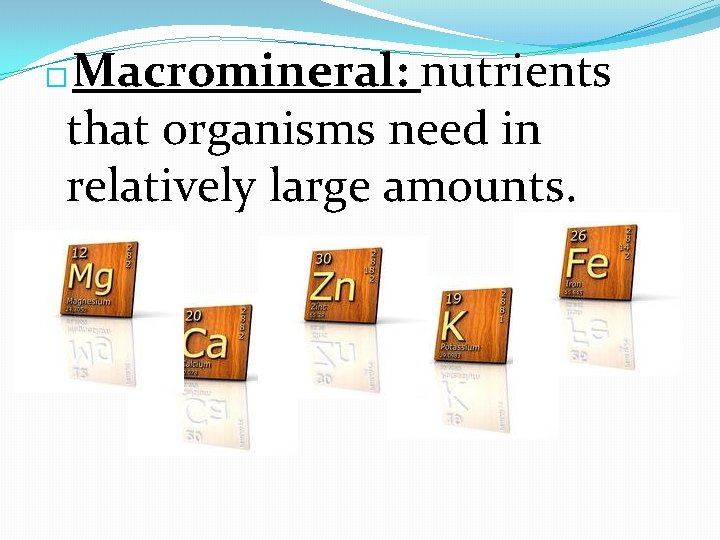
Macromineral: nutrients that organisms need in relatively large amounts. �

Trace element: nutrients that are essential for plant and animal growth and development, but are only needed in very small amounts. Also called microminerals. Ex. Selenium. �

Vitamin: Any of a group of organic compounds that are essential for normal growth and nutrition and are required in small quantities in the diet. �

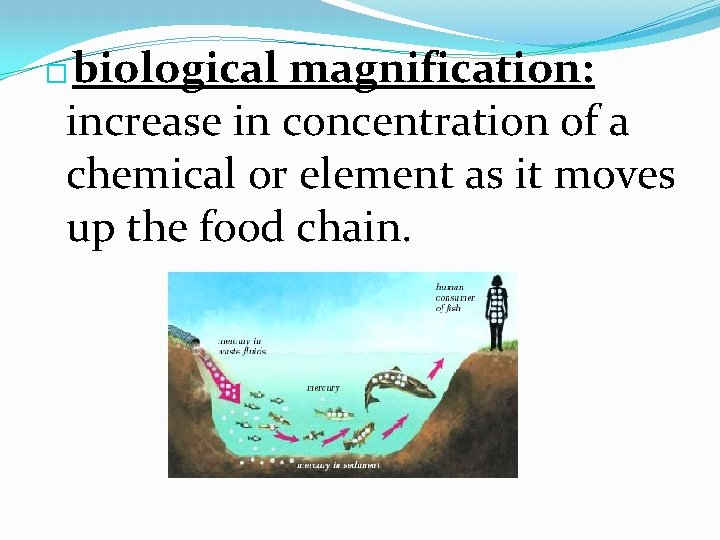
biological magnification: increase in concentration of a chemical or element as it moves up the food chain. �

Herbicide: chemical used to kill or control weeds. �

Fungicide: chemical used to kill fungi. �

Pesticide: chemical used to kill pests. Pests are organisms that harm people, crops or structures. �

Acid: compound that dissolves in water to form a solution with a p. H lower than 7. Ex. Sulphuric acid, formic acid, nitric acid

Base: compound that dissolves in water to form a solution with a p. H higher than 7. Ex. Baking soda, soap, lye, toothpaste. �

acid precipitation (acid rain): precipitation with a p. H lower than 5. 6 is considered acid rain. �

Indicator: to identify a substance as an acid or base or neutral, an indicator is used ( litmus paper, phenol red for example). �

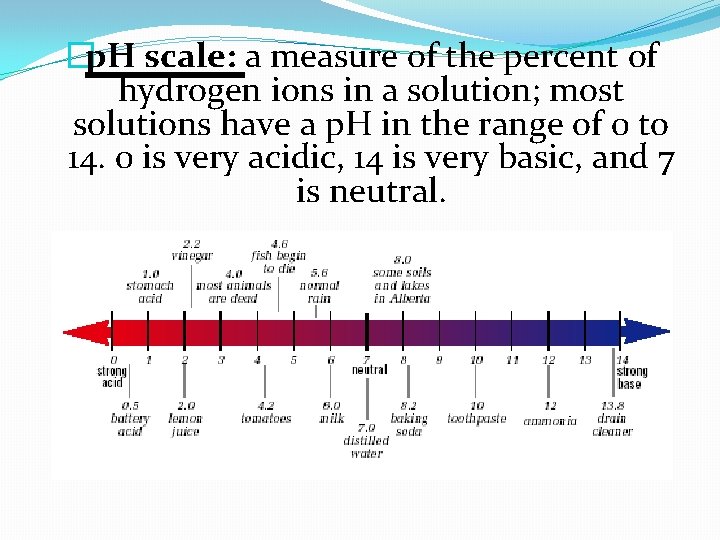
�p. H scale: a measure of the percent of hydrogen ions in a solution; most solutions have a p. H in the range of 0 to 14. 0 is very acidic, 14 is very basic, and 7 is neutral.

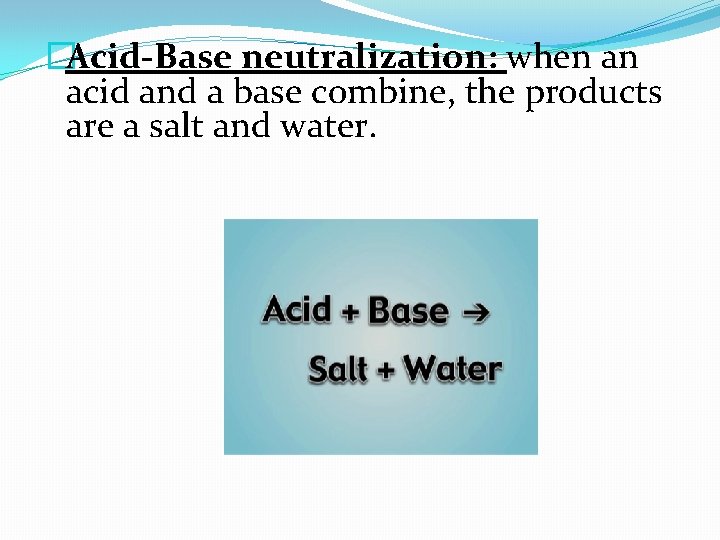
�Acid-Base neutralization: when an acid and a base combine, the products are a salt and water.

�leachate: water that passes through soil and carries dissolved substances with it.

Heavy metal: metals that have a density of 5 or higher ( copper, zinc, lead, mercury, cadmium, nickel); heavy metals are one type of substance monitored to determine water quality.

pollution: any change in the environment that produces a condition that is harmful to living things.

Toxicity: how poisonous a substance is. �acute toxicity: toxicity that is harmful after one exposure. �chronic toxicity: toxicity that is harmful after long term exposure.

LD 50: lethal dose 50; amount of a substance that causes 50% of a group of test animals to die if they are given a specified dose of the substance all at once.

Biological indicator: example – monitoring the number of crayfish in a lake. If the population starts to decrease, it is an indicator that the lake is being poisoned. Scientists use organisms that live in water to help determine water quality.