Electrophoresis Separation according to migration of charged particles









- Slides: 41

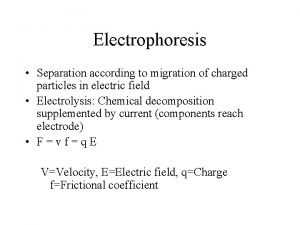
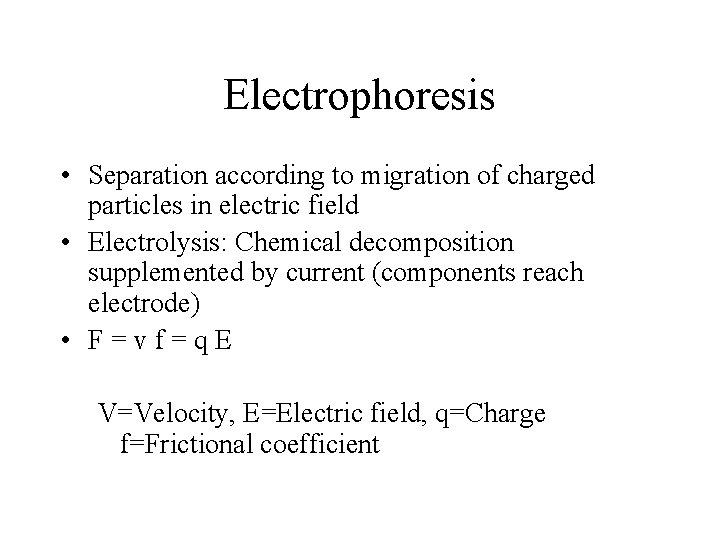
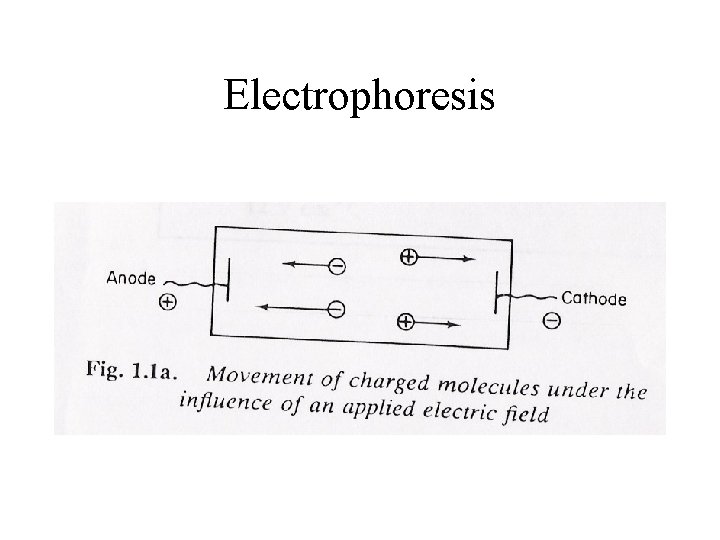
Electrophoresis • Separation according to migration of charged particles in electric field • Electrolysis: Chemical decomposition supplemented by current (components reach electrode) • F=vf=q. E V=Velocity, E=Electric field, q=Charge f=Frictional coefficient

Electrophoresis

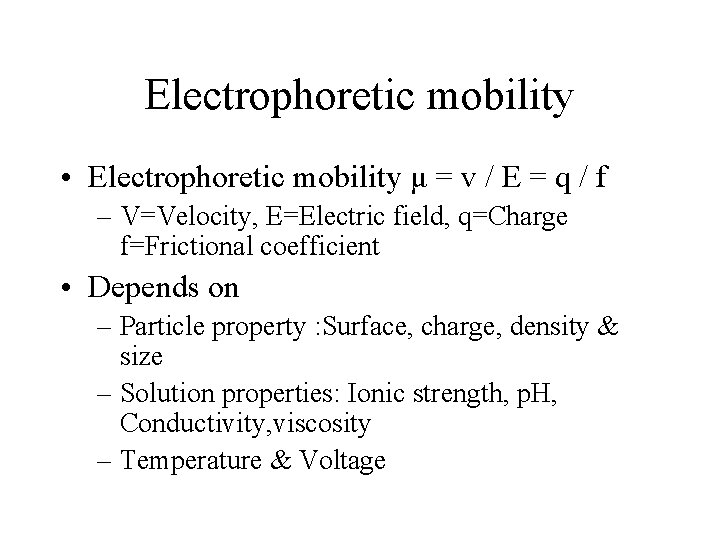
Electrophoretic mobility • Electrophoretic mobility μ = v / E = q / f – V=Velocity, E=Electric field, q=Charge f=Frictional coefficient • Depends on – Particle property : Surface, charge, density & size – Solution properties: Ionic strength, p. H, Conductivity, viscosity – Temperature & Voltage

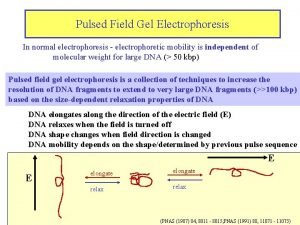
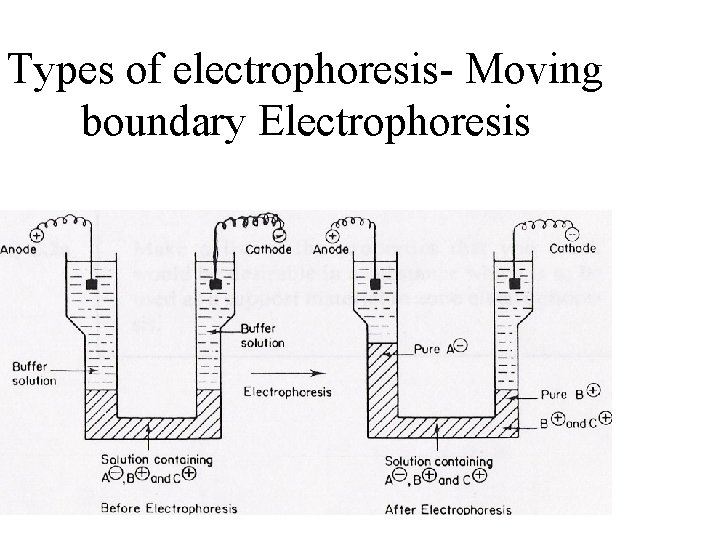
Types of electrophoresis- Moving boundary Electrophoresis

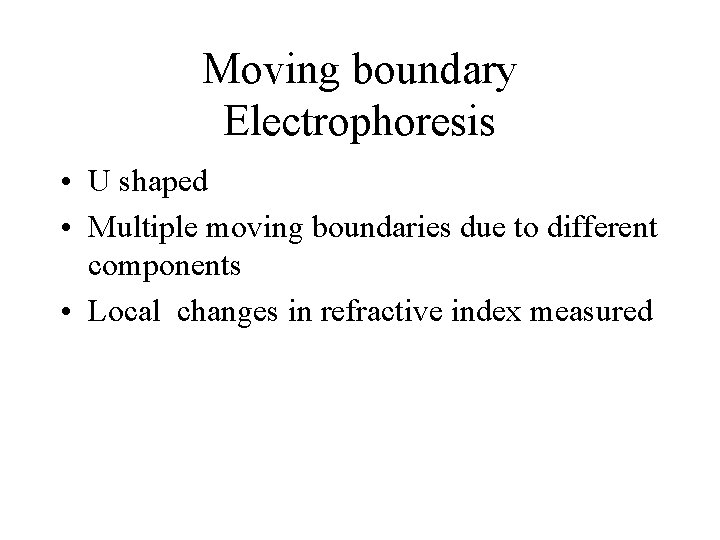
Moving boundary Electrophoresis • U shaped • Multiple moving boundaries due to different components • Local changes in refractive index measured

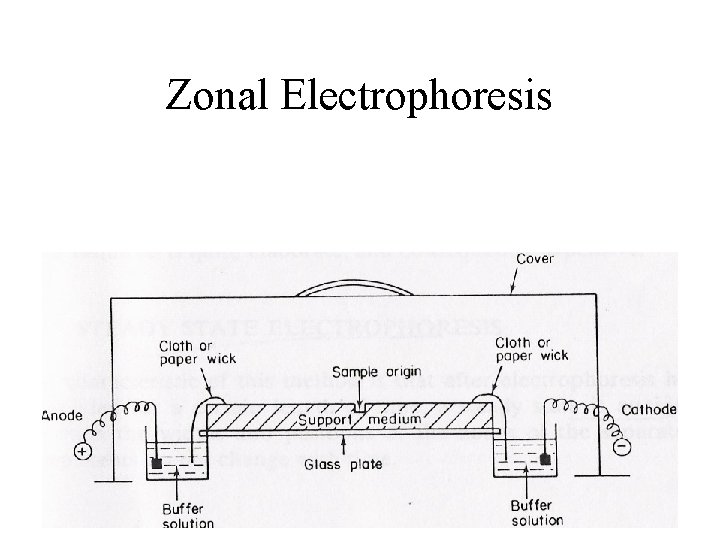
Zonal Electrophoresis

Zonal electrophoresis • Complete separation achieved • Discrete zones formed • 3 ways to stabilize components – (i)Use of support material: – Filter paper – Membrane – Gel

Zonal electrophoresis – (ii)Density gradient stabilization: - Sucrose, glycerol, ethylene glycerol - Prevents convection current (iii)Free zone electrophoresis • Rotation of vessel during run • Continuous film of buffer perpendicular to electric field

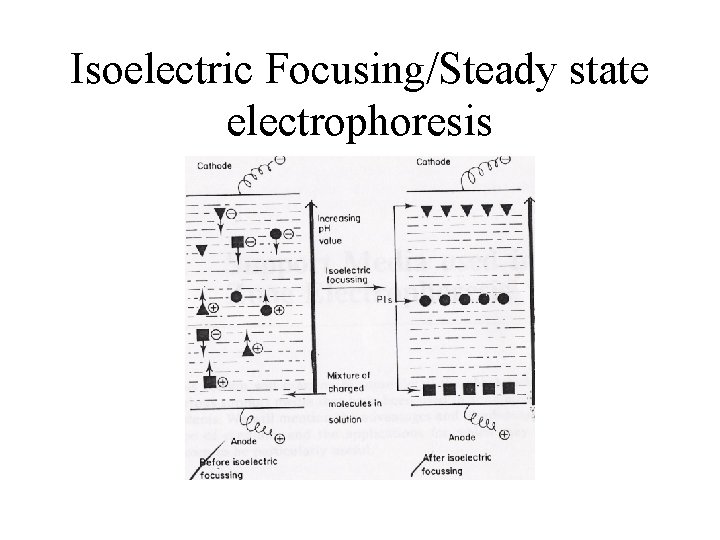
Isoelectric Focusing/Steady state electrophoresis

Steady State electrophoresis/ Isoelectric focusing • • Separates molecules by charge Performed in gel Distributed over medium that has p. H gradient Molecules move towards opposite polarity under current • Settle at Isoelectric point : Molecule no longer has a net electric charge (due to the protonation or deprotonation of the associated functional groups)

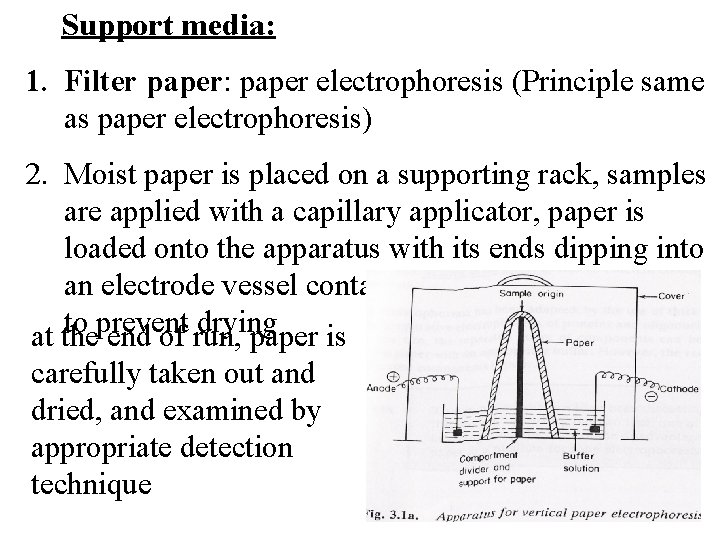
Support media: 1. Filter paper: paper electrophoresis (Principle same as paper electrophoresis) 2. Moist paper is placed on a supporting rack, samples are applied with a capillary applicator, paper is loaded onto the apparatus with its ends dipping into an electrode vessel containing buffer, and covered to prevent drying at the end of run, paper is carefully taken out and dried, and examined by appropriate detection technique

Disadvantage • Thin paper: Tear • Thick paper: Boundaries between the zones distort • OH group of cellulose interact with biomolecules and results in ‘tailing’ • water absorbing and electrical conductivity capacity of paper depends on its content (96% -cellulose) • Electro osmosis

Electro osmosis • dissociation of COOH group(cellulose matrix) generation of (H 3 O)+ ion Moves (electric field) • A correction factor for electrophoretic mobility: • distance between the sample origin and the position of the blue dextran (does not ionize) • 20 V cm-1 – zone diffusion of small molecules • 200 V cm-1 – High voltage EP

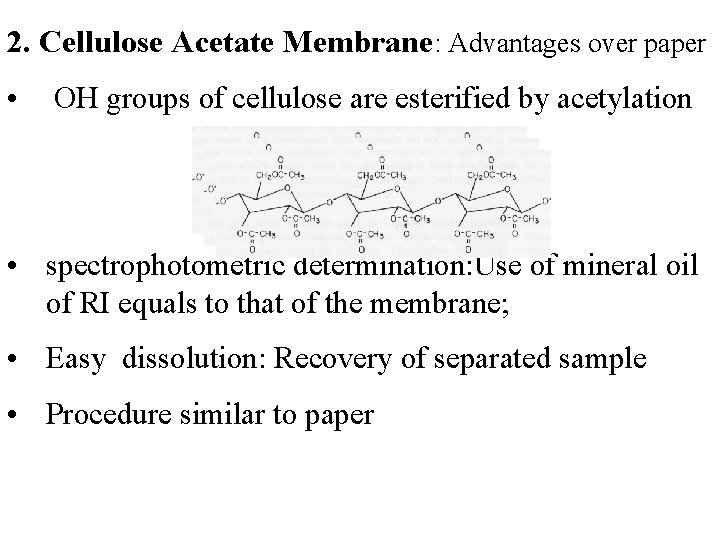
2. Cellulose Acetate Membrane: Advantages over paper • OH groups of cellulose are esterified by acetylation • spectrophotometric determination: Use of mineral oil of RI equals to that of the membrane; • Easy dissolution: Recovery of separated sample • Procedure similar to paper

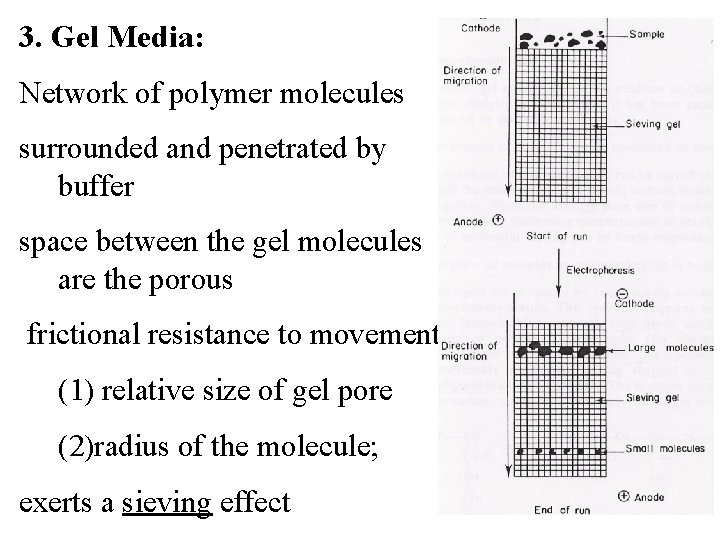
3. Gel Media: Network of polymer molecules surrounded and penetrated by buffer space between the gel molecules are the porous frictional resistance to movement (1) relative size of gel pore (2)radius of the molecule; exerts a sieving effect

a) Starch Gels: • earlier work with proteins used starch as support • cheap, easy and reasonably quick Disadvantage • pore size can vary within only a narrow range (determined by the starch conc) • contains some negatively charged side-chain: hinder separation

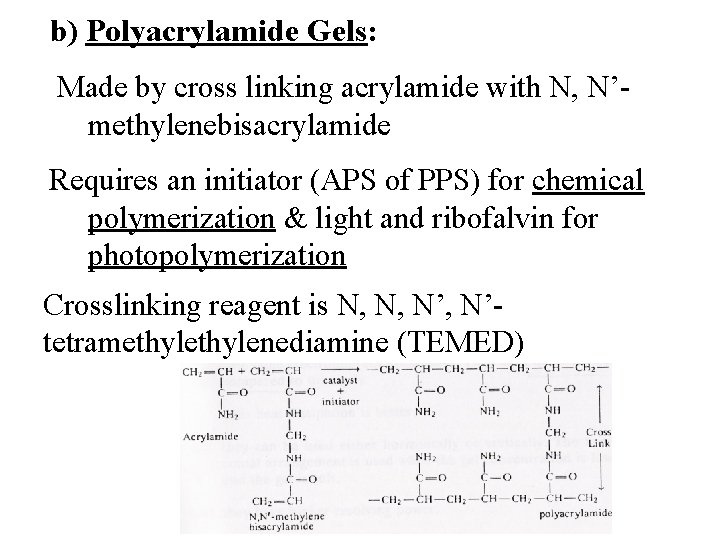
b) Polyacrylamide Gels: Made by cross linking acrylamide with N, N’methylenebisacrylamide Requires an initiator (APS of PPS) for chemical polymerization & light and ribofalvin for photopolymerization Crosslinking reagent is N, N, N’tetramethylenediamine (TEMED)

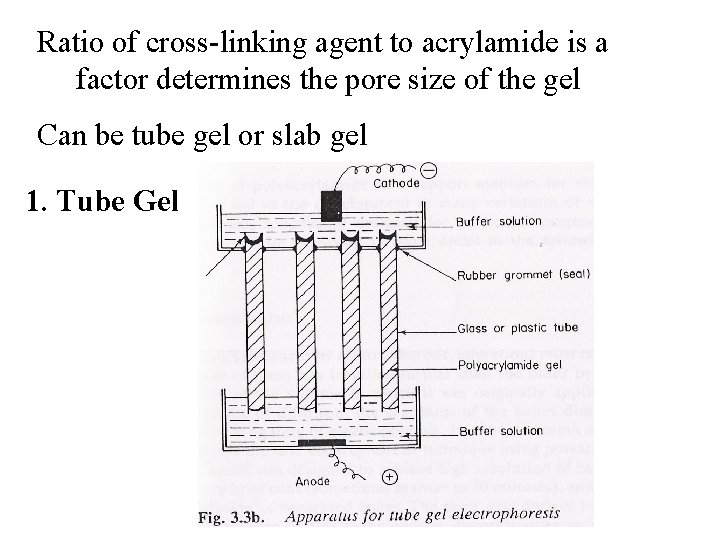
Ratio of cross-linking agent to acrylamide is a factor determines the pore size of the gel Can be tube gel or slab gel 1. Tube Gel

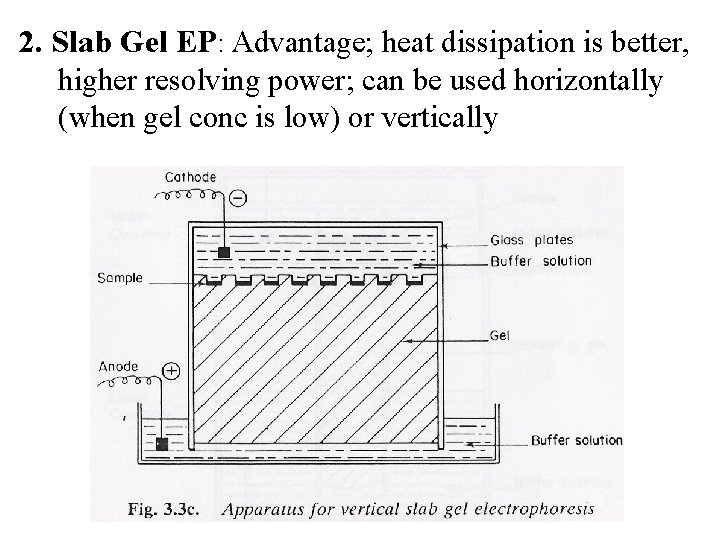
2. Slab Gel EP: Advantage; heat dissipation is better, higher resolving power; can be used horizontally (when gel conc is low) or vertically

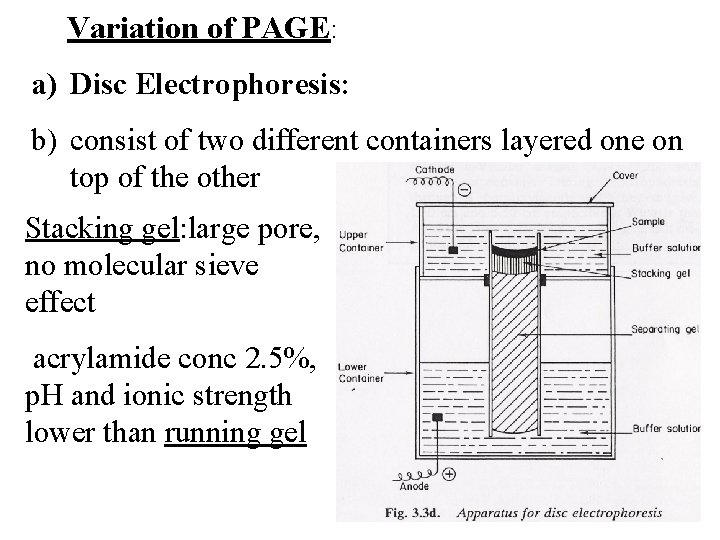
Variation of PAGE: a) Disc Electrophoresis: b) consist of two different containers layered one on top of the other Stacking gel: large pore, no molecular sieve effect acrylamide conc 2. 5%, p. H and ionic strength lower than running gel

b) SDS-PAGE: Separation depends on the net charge, size and shape SDS-PAGE: Difference in net charges eliminated by complexing with anionic detergent, SDS Identical charge-to-size ratio: Amount of SDS per unit mass of protein is constant (1. 4 g of SDS binds per gram of protein) Very power technique: Measures relative molecular masses of polypeptide / protein

c) Agarose Gel: Agarose, obtain from seaweeds, linear polymer of DGal and 3, 6 -anhydro. Gal agarose dissolved in boiling water when cooled forms a gel (held by H-bonds), pore size relatively large: large proteins or nucleic acids (too large to enter PAG) Contaminants charge groups (sulphate and carboxylic acid ) interfere with separation

Factors affecting electrophoretic mobility: Number of parameter of electrophoretic system: i) for zone EP, type of support medium chosen, and if it is a gel, its pore size ii) p. H of the electrophoresis buffer iii) ionic composition of buffer iv) applied voltage v) temperature

Support medium: selection is based on following consideration a) size of the molecules to be analyzed b) quantity of sample available c) cost of support medium to be used d) availability of suitable equipment e) purpose of the analysis f) time to run the analysis g) expertise of the operator

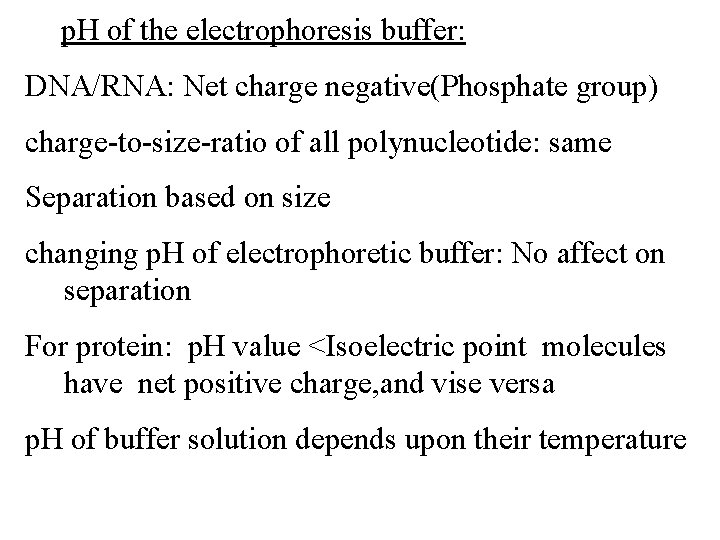
p. H of the electrophoresis buffer: DNA/RNA: Net charge negative(Phosphate group) charge-to-size-ratio of all polynucleotide: same Separation based on size changing p. H of electrophoretic buffer: No affect on separation For protein: p. H value <Isoelectric point molecules have net positive charge, and vise versa p. H of buffer solution depends upon their temperature

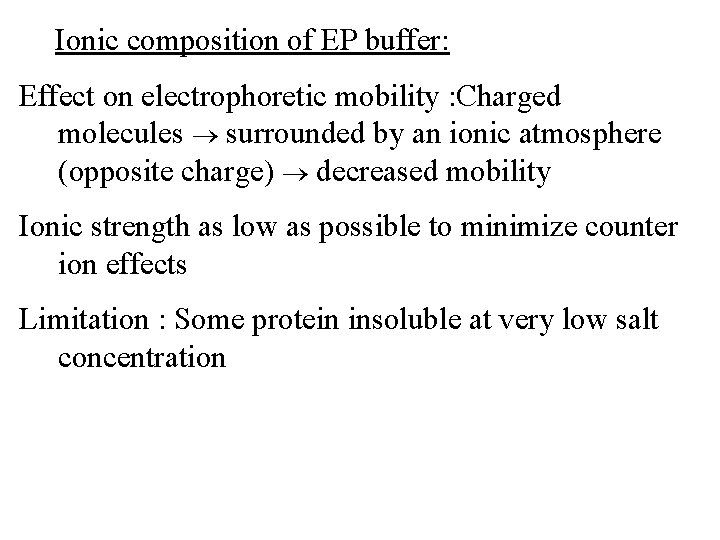
Ionic composition of EP buffer: Effect on electrophoretic mobility : Charged molecules surrounded by an ionic atmosphere (opposite charge) decreased mobility Ionic strength as low as possible to minimize counter ion effects Limitation : Some protein insoluble at very low salt concentration

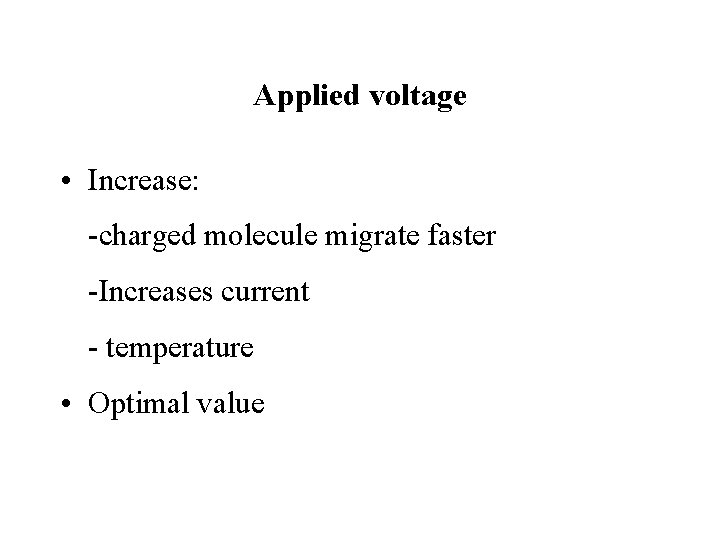
Applied voltage • Increase: -charged molecule migrate faster -Increases current - temperature • Optimal value

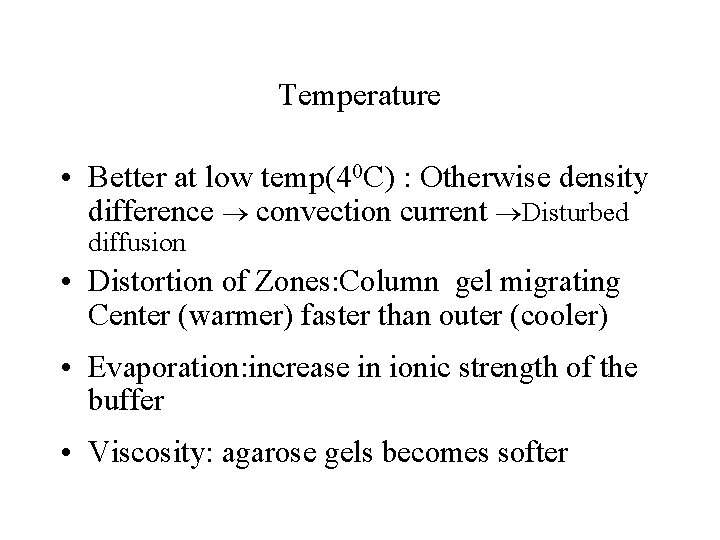
Temperature • Better at low temp(40 C) : Otherwise density difference convection current Disturbed diffusion • Distortion of Zones: Column gel migrating Center (warmer) faster than outer (cooler) • Evaporation: increase in ionic strength of the buffer • Viscosity: agarose gels becomes softer

Detection of sample Depends on • the nature of the molecule to be detected • type of electrophoresis system used • purpose of the analysis

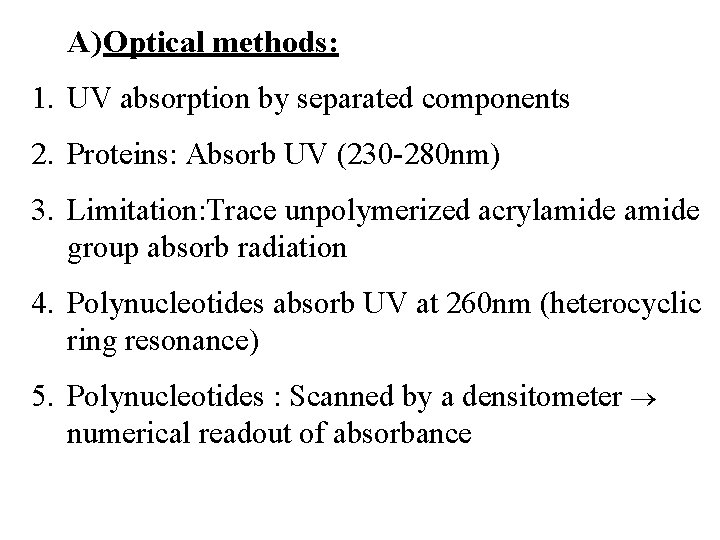
A) Optical methods: 1. UV absorption by separated components 2. Proteins: Absorb UV (230 -280 nm) 3. Limitation: Trace unpolymerized acrylamide group absorb radiation 4. Polynucleotides absorb UV at 260 nm (heterocyclic ring resonance) 5. Polynucleotides : Scanned by a densitometer numerical readout of absorbance

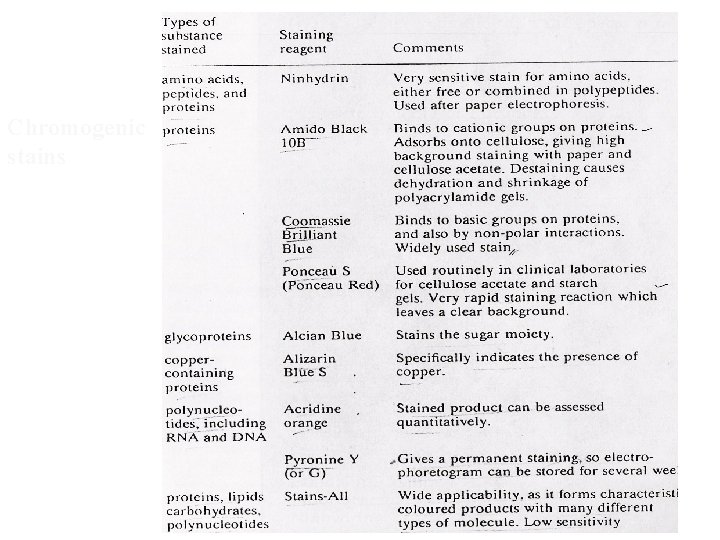
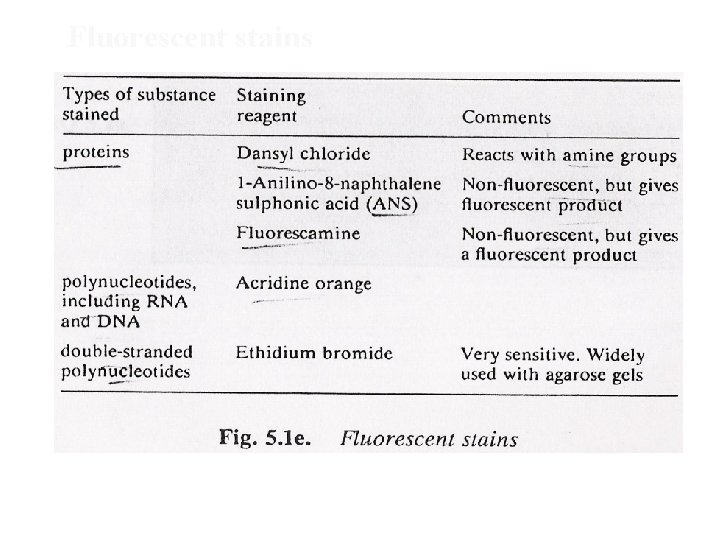
Staining • by reacting separated components with a stain or dye and detecting by absorption or fluorescence • stain should be - selective, react quickly, yield stable product • staining can be done A) before electrophoresis (rainbow markers), B) after electrophoresis- by immediately fixing the separated component (drying if paper or precipitating (by fixative) if gel, ) • Destaining: excess unbound stain removed


Chromogenic stains

Fluorescent stains

b) Radiochemical methods: • Labeling with a radioactive isotope(before) Support medium cut into pieces Measured by liquid scintillation counting/ read in autoradiography • high energy emitters are extremely useful labels for autoradiography (32 P) • Protein - 125 I(γ emitter) ; nucleic acid - 32 P (high energy β emitter) ; 14 C and 35 S are medium-energy β emitter and 3 H is a low energy β emitter – used as tracers

Liquid Scintillation counting: Support medium is dried, cut into strips or sections Samples in gel support media need to be solubilized before measurement i) elution from the slice by an appropriate buffer (solubiliser) ii) Gel structure may be destroyed using sonication or chemicals (formamide, H 2 O 2)

Autoradiography • Detecting radioactivity in labeled & separated compounds • Dried gel Specially designed photographic cassette (several days) Radiation passing through the photographic film Reflected as fluorescent emission (Intensifying screen) Enhanced image


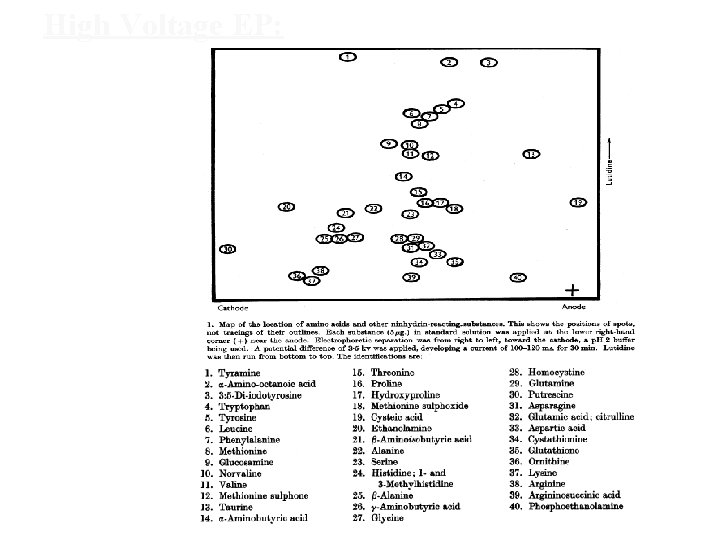
High Voltage EP:

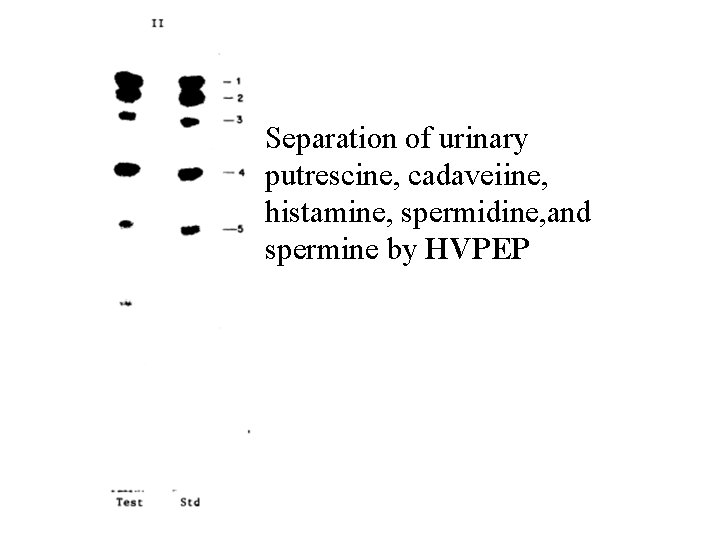
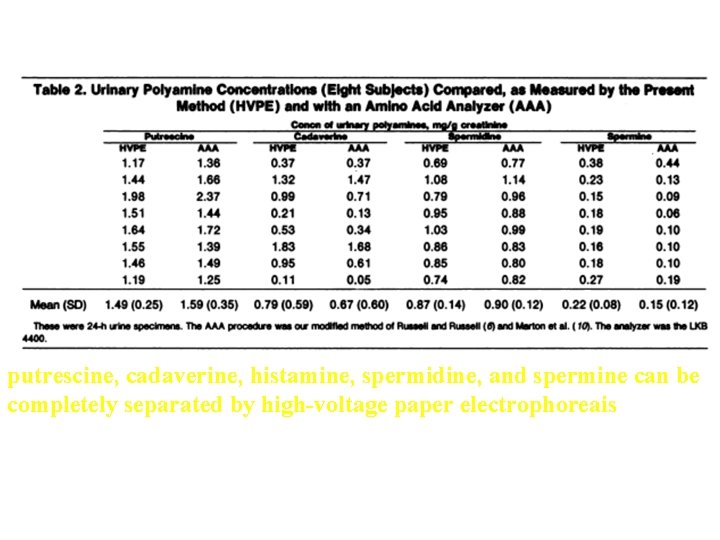
Separation of urinary putrescine, cadaveiine, histamine, spermidine, and spermine by HVPEP

putrescine, cadaverine, histamine, spermidine, and spermine can be completely separated by high-voltage paper electrophoreais
 Electrophoresis separation technique
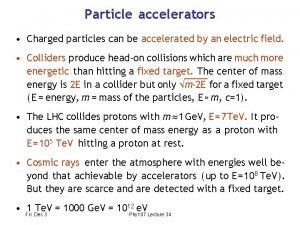
Electrophoresis separation technique Charged particles can be accelerated by
Charged particles can be accelerated by Ions charged particles in solution
Ions charged particles in solution For charged particles, what is the quantity q/m called?
For charged particles, what is the quantity q/m called? The search for fractionally charged particles has–
The search for fractionally charged particles has– Gas like mixture of charged particles
Gas like mixture of charged particles Polyacrylamide gel electrophoresis (page)
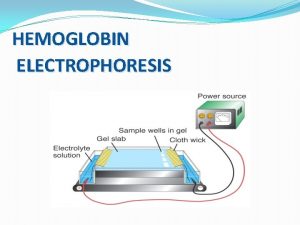
Polyacrylamide gel electrophoresis (page) Hemoglobin electrophoresis
Hemoglobin electrophoresis Gel electrophoresis result
Gel electrophoresis result Zone electrophoresis definition
Zone electrophoresis definition Zone electrophoresis definition
Zone electrophoresis definition Laser doppler electrophoresis
Laser doppler electrophoresis What is electrophoresis
What is electrophoresis Electrophoresis
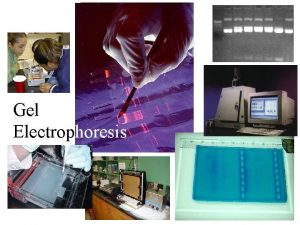
Electrophoresis Biology lab gel
Biology lab gel Stability of sols
Stability of sols Polyclonal gammopathy electrophoresis pattern
Polyclonal gammopathy electrophoresis pattern Temperature gradient
Temperature gradient Factors affecting electrophoresis
Factors affecting electrophoresis Gel electrophoresis definition
Gel electrophoresis definition Agarose vs acrylamide gel
Agarose vs acrylamide gel Process of gel electrophoresis
Process of gel electrophoresis Zonal electrophoresis
Zonal electrophoresis Procedure of sds page
Procedure of sds page Gel electrophoresis definition
Gel electrophoresis definition Hemoglobin electrophoresis
Hemoglobin electrophoresis Gel electrophoresis separates dna by
Gel electrophoresis separates dna by Automated+electrophoresis+system
Automated+electrophoresis+system Electrophoretic mobility equation
Electrophoretic mobility equation Disadvantages of agarose gel electrophoresis
Disadvantages of agarose gel electrophoresis Disadvantages of capillary electrophoresis
Disadvantages of capillary electrophoresis Open reading frame
Open reading frame Parts of electrophoresis apparatus
Parts of electrophoresis apparatus Paper electrophoresis
Paper electrophoresis Hemoglobin electrophoresis
Hemoglobin electrophoresis Faranoush
Faranoush How does dna move in gel electrophoresis
How does dna move in gel electrophoresis Thalassemia
Thalassemia Paper electrophoresis
Paper electrophoresis Pipetting exercises
Pipetting exercises Ashanthi desilva
Ashanthi desilva Gel electrophoresis advantages
Gel electrophoresis advantages