ELECTROPHORESIS Electrophoresis is a separation technique used to








- Slides: 10

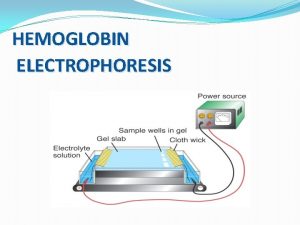
ELECTROPHORESIS Electrophoresis is a separation technique used to separate charged molecules according to size. It is often used to separate, DNA, RNA and Protein molecules.

Equipment Power pack Gel Tank Pipettes and pipette tips(20 ul) Tube Racks Agar Powder Dyes Buffer

Safety Considerations The power supply and the gel use high voltage and should be handled with caution. Do not operate the power supply in damp conditions. Keep water away from power supply. Gloves and eye protection should be worn at all times during procedure. Observe position of bands during electrophoresis, and stop once sufficient separation has occurred. Measurements and photographs to be taken soon after stopping electrophoresis as molecules will continue to diffuse through gel after electrophoresis has stopped. All used gels to be placed in a plastic bag and disposed of in the general waste bin. Buffer solution can be disposed of down the sink. As always depending on the chemicals in use for the electrophoresis procedure, the SDS should be consulted in assessing risks associated with the chemicals

Preparation of Buffer Prepare a 1% W/V stock Solution of Sodium Bicarbonate by dissolving 1 g of powder into 100 ml of distilled water. The working solution is prepared by dissolving 100 ml of the stock solution with 900 ml of distilled water Other types of buffer that can be used to separate dyes and stains include TAE and TBE, but these are quite expensive, and the cheaper sodium bicarbonate alternative is safer for use in schools.

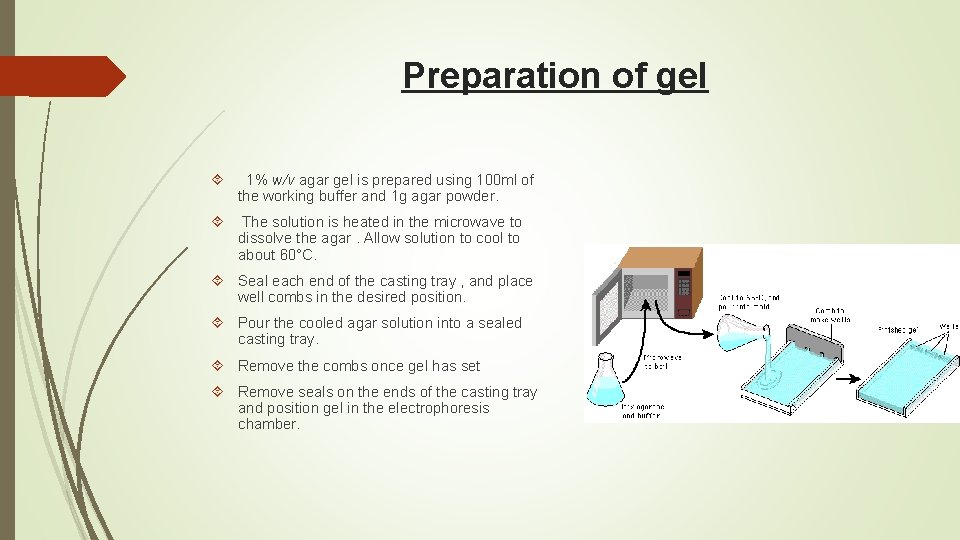
Preparation of gel 1% w/v agar gel is prepared using 100 ml of the working buffer and 1 g agar powder. The solution is heated in the microwave to dissolve the agar. Allow solution to cool to about 60°C. Seal each end of the casting tray , and place well combs in the desired position. Pour the cooled agar solution into a sealed casting tray. Remove the combs once gel has set Remove seals on the ends of the casting tray and position gel in the electrophoresis chamber.

Prepapration of Dyes Bromophenol Blue Methyl Green Orange G Ingigo Carmine, Rose Bengal, tartarazine Xylene Cyanol These stains can be made up from powder to a concentration of 0. 2%w/v in 20% glycerol/distilled water. Ladder can be prepared by mixing stains to get a reference or “control’. These stains produce distinct bands of colour when electrophoresed. Most dyes are negatively charged like DNA except for Methyl green which is positively charged and therefore migrates towards the negative electrode Gloves mu. Most of these dyes are classified as irritants. Gloves must be worn.

Loading The Gel Place gel in the electrophoresis chamber. Ensure that the wells are closer to the negative terminal. Flood chamber with working buffer solution until reservoirs on both ends of the chamber are full and the gel is submerged in the solution. Using a clean micropipette, transfer 10 ul of sample into the appropriate well. Ensure you do not puncture the gel by guiding the pipette with your forefinger. Once the sample has been ejected into the well, withdraw pipette tip before releasing piston to avoid drawing up the sample again.

When the wells have been loaded, fit the lid of the chamber onto the tank. Do not force the lid to close. Running the Gel Plug the leads into the power supply , choose a voltage setting (100 v) and turn the power on. This causes the samples to quickly move into the gel where they cannot be disturbed. At this voltage the entire run will take approx. 30 40 minutes. To ensure the unit is receiving power , check for the presence of small bubbles on both electrodes, this occurs due to electrolysis. Monitor progress by observing the position of the dyes. Switch of power once sufficiently separated. Make sure to take photo of gel shortly after the run is complete. * NEVER REMOVE THE LID FROM THE GEL TANK WHILE THE UNIT IS STILL CONNECTED TO THE POWER SUPPLY*.

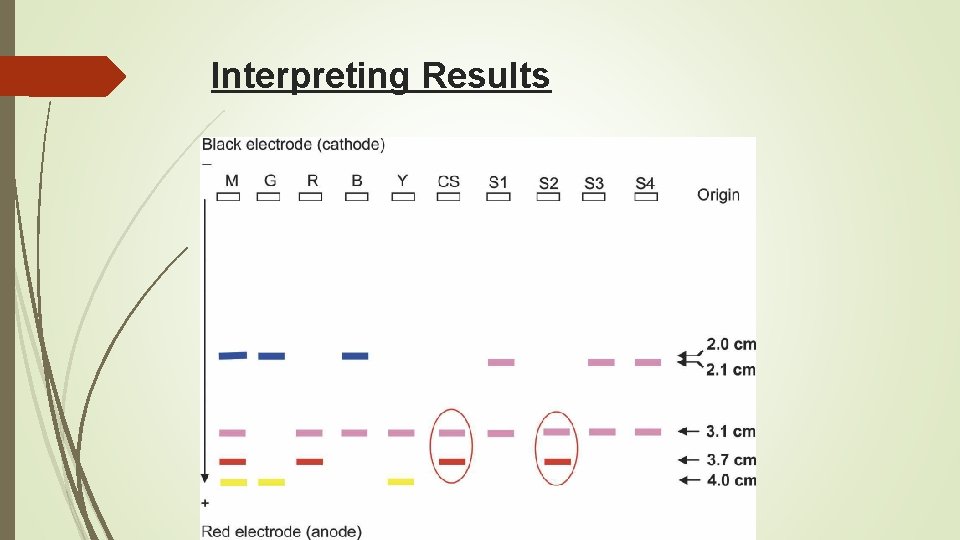
Interpreting Results

References ii Murdoch University. n. d. Biotech out of the box, Murdoch University website, http: //www. murdoch. edu. au/Biotech-out-of-the-box/_document/Kit-Handout-Sheets/Teacher-and-Technician- Handout. pdf (Accessed December 2015) ‘Activity 2: Gel Electrophoresis of Dyes’, The American Phytopathological Society (APS) website, http: //www. apsnet. org/EDCENTER/K 12/TEACHERSGUIDE/PLANTBIOTECHNOLOGY/Pages/Activity 2. aspx (Accessed December 2015) Bio-Tek Services Pty Ltd. n. d. Agarose Gel Electrophoresis using dyes – Instruction manual, Biotechnology Education website, https: //web. archive. org/web/20120321125959/http: //www. biotechnologyeduca. . . (Internet Archived Version, April 2018) Carolina Biological Supply Company, 2005. Introductory Gel Electrophoresis – Teacher’s Manual with Student Guide, Centre for Precollegiate Education and Training, University of Florida website https: //www. cpet. ufl. edu/wp-content/uploads/2013/10/Intro-Gel-Electrophoresis-manual. pdf Flinn Scientific Inc. 2008. Dyeing for Electrophoresis, Flinn Scientific Inc. website, http: //www. flinnsci. com/media/452802/bf 10901. pdf Queensland Museum. 2006. Food dye electrophoresis – Student task sheet, Queensland Museum website, http: //www. qm. qld. gov. au/microsites/qx/pdf/dd 002_food_dye_electrophoresis. pdf Scharlau, 2013. Safety data sheet Agarose Low EEO, electrophoresis grade, Chemsupply website https: //www. chemsupply. com. au/documents/AG 0030_AU. pdf (11 July 2013) Chem-supply. 2014. Bromophenol Blue Safety data sheet, Chemsupply website, https: //www. chemsupply. com. au/documents/BL 0361 CH 1 F. pdf (March 2014) Chem-Supply. 2011. Indigo Carmine Safety Data Sheet, Chemsupply website, https: //www. chemsupply. com. au/documents/IL 0041 CH 36. pdf (May 2011) Scharlau, 2013. Safety data sheet Methyl Green, C. I. 42585, for microscopy , Chemsupply website https: //www. chemsupply. com. au/documents/VE 0120_AU. pdf (11 July 2013) Scharlau, 2013. Safety data sheet Orange G, C. I. 16230, for microscopy , Chemsupply website https: //www. chemsupply. com. au/documents/AN 0030_AU. pdf (11 July 2013) Sigma-Aldrich. 2013. Safety Data Sheet 330000 - Rose Bengal, Sigma-Aldrich website, http: //www. sigmaaldrich. com/MSDS/Display. MSDSPage. do? country=AU&lang. . . (15 April 2013) Chem-supply. 2013. Tartrazine Yellow (C. I. 19140) Safety data sheet, Chemsupply website, https: //www. chemsupply. com. au/documents/TL 0011 CHCB. pdf (January 2013) Sigma-Aldrich. 2013. Safety Data Sheet X 4126 - Xylene Cyanol FF, Sigma-Aldrich website, http: //www. sigmaaldrich. com/MSDS/Display. MSDSPage. do? country=AU&lang. . . (23 December 2013) Southern Biological, n. d. Gel Electrophoresis of Dyes, Southern Biological website, http: //file. southernbiological. com/Assets/Products/Kits_and_Equipment/Ge. . . (Accessed December 2015)
 Electrophoresis separation technique
Electrophoresis separation technique Separating funnel labelled diagram
Separating funnel labelled diagram Red and blue marbles of same size and mass
Red and blue marbles of same size and mass Risk separation
Risk separation Modes of motion in size separation is
Modes of motion in size separation is Vocal style of india
Vocal style of india Equipment used in angiography
Equipment used in angiography Which technique is used almost exclusively for sighting
Which technique is used almost exclusively for sighting Bandwagon propaganda images
Bandwagon propaganda images Persuasive writing
Persuasive writing Fear propaganda in animal farm
Fear propaganda in animal farm