Electrical Properties of Dispersed Systems Ph D Halina

![Zeta potential [m. V] from 0 to ± 5, Stability behavior of the colloid Zeta potential [m. V] from 0 to ± 5, Stability behavior of the colloid](https://slidetodoc.com/presentation_image_h/b0384864454d8db8e0f6d94ab4961b29/image-12.jpg)


- Slides: 34

Electrical Properties of Dispersed Systems Ph. D Halina Falfushynska

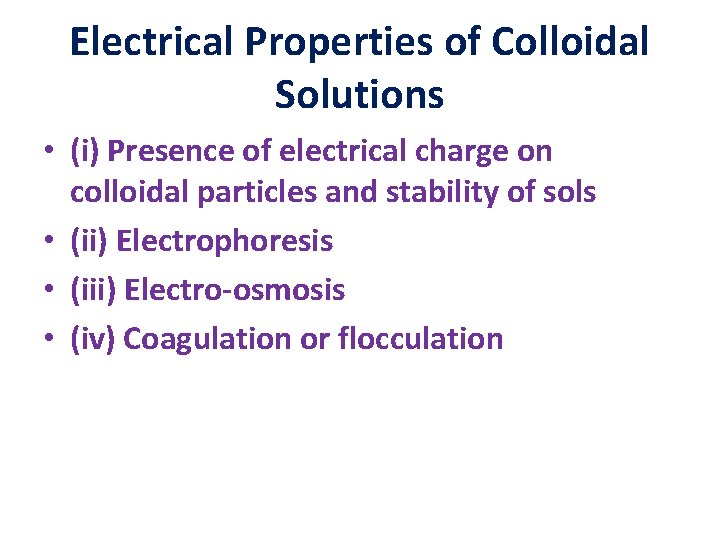
Electrical Properties of Colloidal Solutions • (i) Presence of electrical charge on colloidal particles and stability of sols • (ii) Electrophoresis • (iii) Electro-osmosis • (iv) Coagulation or flocculation

Electrical double layer theory • A double layer of ions appear at the surface of solid. • The ion preferentially adsorbed is held in fixed part and imparts charge to colloidal particles. • The second part consists of a diffuse mobile layer of ions. This second layer consists of both the type of charges. The net charge on the second layer is exactly equal to that on the fixed part. • The existence of opposite sign on fixed and diffuse parts of double layer leads to appearance of a difference of potential, known as zeta potential


Gouy-Chapman Double Layer • Gouy suggested that • The kinetic energy of interfacial potential at the counter ions will, in the charged surface part, affect the could be attributed to thickness of the presence of a resulting diffuse double number of ions of given layer. sign attached to its • n = noexp(-ze. Y/k. T) surface, and to an equal • where n = bulk concentration o number of ions of z = charge on the ion e = charge on a proton opposite charge in the k = boltzman constant solution.

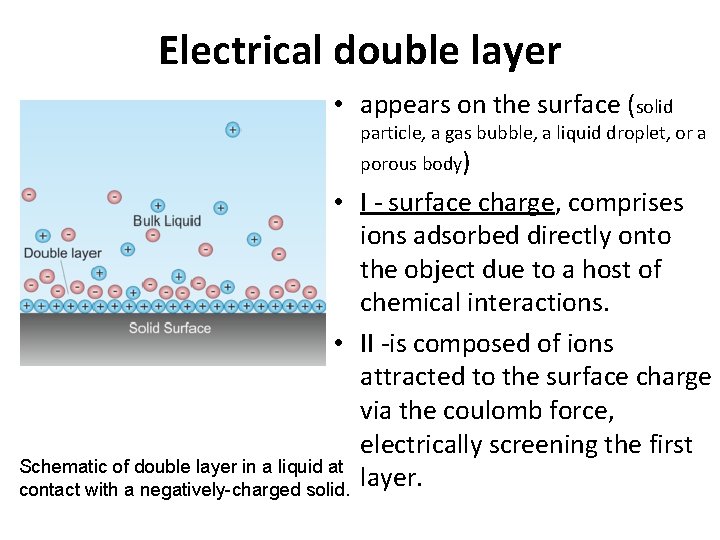
Electrical double layer • appears on the surface (solid particle, a gas bubble, a liquid droplet, or a porous body) • I - surface charge, comprises ions adsorbed directly onto the object due to a host of chemical interactions. • II -is composed of ions attracted to the surface charge via the coulomb force, electrically screening the first Schematic of double layer in a liquid at layer. contact with a negatively-charged solid.

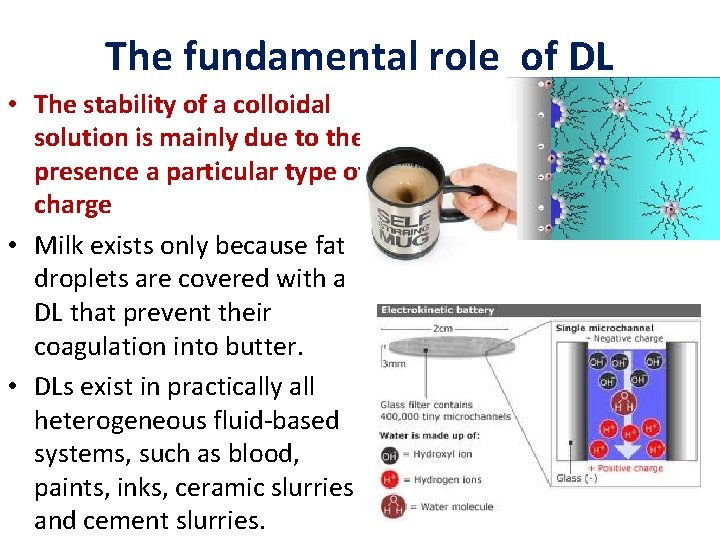
The fundamental role of DL • The stability of a colloidal solution is mainly due to the presence a particular type of charge • Milk exists only because fat droplets are covered with a DL that prevent their coagulation into butter. • DLs exist in practically all heterogeneous fluid-based systems, such as blood, paints, inks, ceramic slurries and cement slurries.

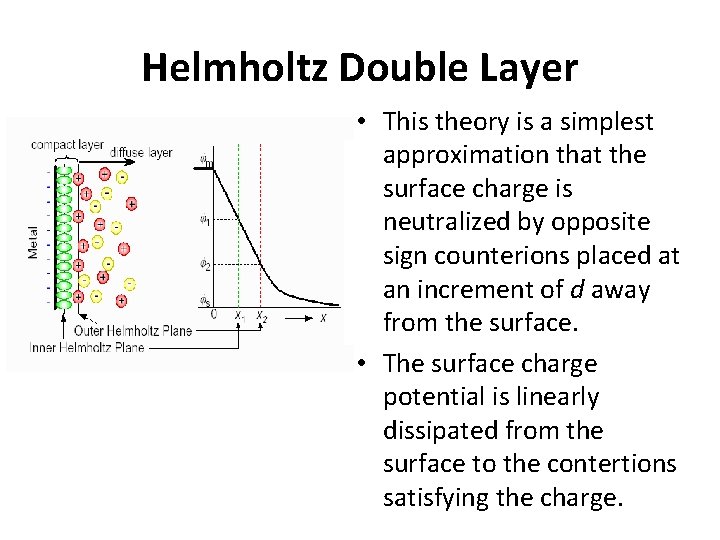
Helmholtz Double Layer • This theory is a simplest approximation that the surface charge is neutralized by opposite sign counterions placed at an increment of d away from the surface. • The surface charge potential is linearly dissipated from the surface to the contertions satisfying the charge.

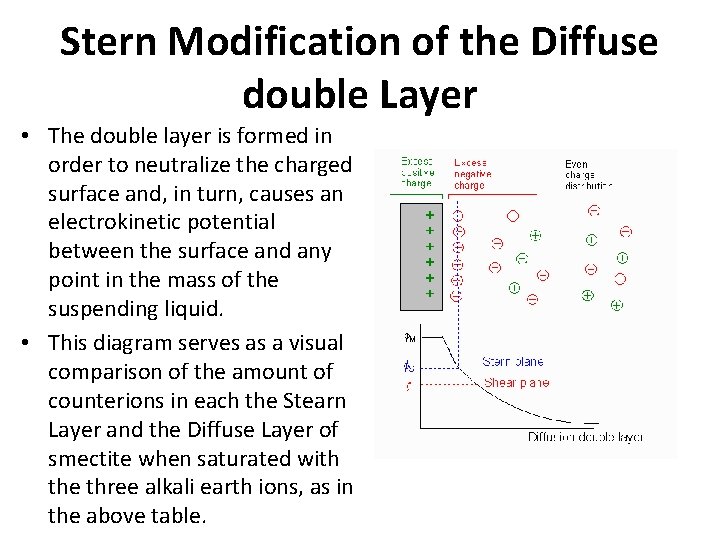
Stern Modification of the Diffuse double Layer • The double layer is formed in order to neutralize the charged surface and, in turn, causes an electrokinetic potential between the surface and any point in the mass of the suspending liquid. • This diagram serves as a visual comparison of the amount of counterions in each the Stearn Layer and the Diffuse Layer of smectite when saturated with the three alkali earth ions, as in the above table.

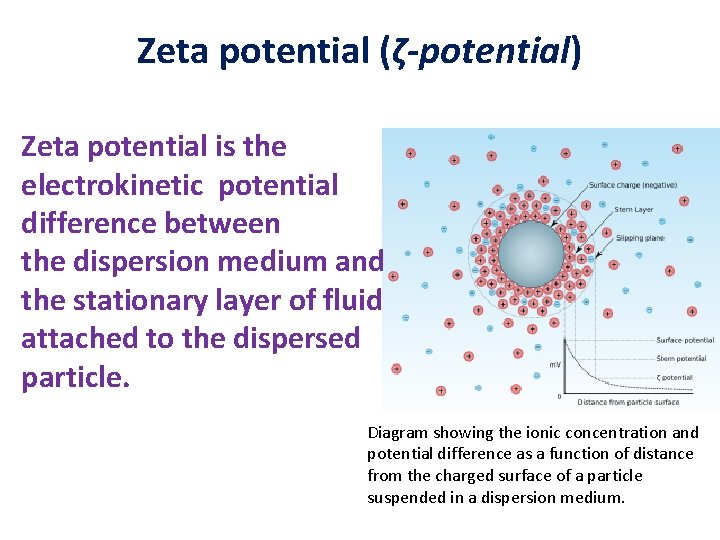
Zeta potential (ζ-potential) Zeta potential is the electrokinetic potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. Diagram showing the ionic concentration and potential difference as a function of distance from the charged surface of a particle suspended in a dispersion medium.

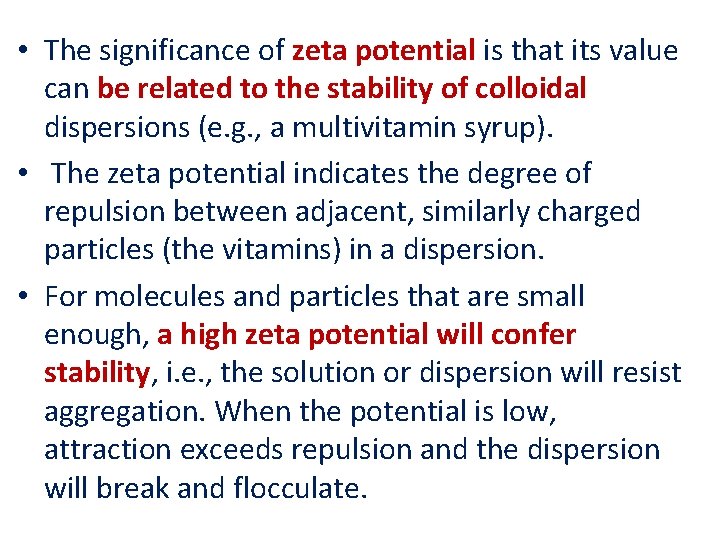
• The significance of zeta potential is that its value can be related to the stability of colloidal dispersions (e. g. , a multivitamin syrup). • The zeta potential indicates the degree of repulsion between adjacent, similarly charged particles (the vitamins) in a dispersion. • For molecules and particles that are small enough, a high zeta potential will confer stability, i. e. , the solution or dispersion will resist aggregation. When the potential is low, attraction exceeds repulsion and the dispersion will break and flocculate.
![Zeta potential m V from 0 to 5 Stability behavior of the colloid Zeta potential [m. V] from 0 to ± 5, Stability behavior of the colloid](https://slidetodoc.com/presentation_image_h/b0384864454d8db8e0f6d94ab4961b29/image-12.jpg)
Zeta potential [m. V] from 0 to ± 5, Stability behavior of the colloid Rapid coagulation or flocculation from ± 10 to ± 30 Incipient instability from ± 30 to ± 40 Moderate stability from ± 40 to ± 60 Good stability more than ± 61 Excellent stability

Presence of electrical charge on colloidal particles and stability of sols • In a particular colloidal solution, all the colloidal particles carry the same type of charge, while the dispersion medium has an equal but opposite charge. Thus, the charge on colloidal particles is balanced by that of the dispersion medium and the colloidal solution as a whole is electrically neutral.

Colloidal sols may be classified • Positively charged sols: Metallic hydroxide sols e. g. , Fe(OH)3, Al(OH)3, Cr(OH)3, etc. , Ti. O 2 sol, haemoglobin, sols of basic dyes such as methylene blue Negatively charged sols: Metal sols e. g. , Au, Ag, Cu, Pt etc. sols, metal sulphide sols e. g. , As 2 S 3, Cd. S etc. sols; starch sol, sols of acid dyes such as Congo red etc.

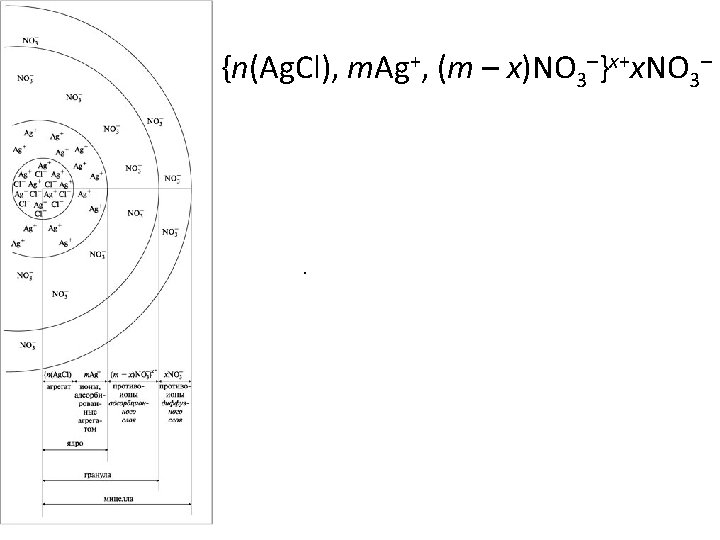
{n(Ag. Cl), m. Ag+, (m – x)NO 3–}x+x. NO 3– .

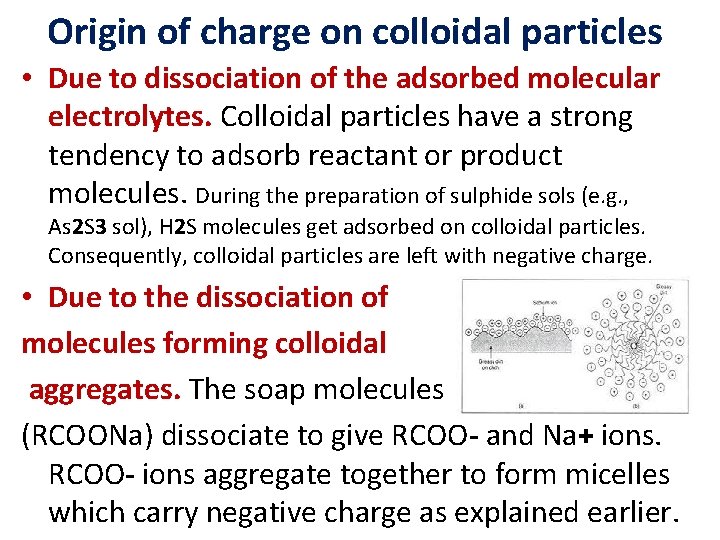
Origin of charge on colloidal particles • Due to dissociation of the adsorbed molecular electrolytes. Colloidal particles have a strong tendency to adsorb reactant or product molecules. During the preparation of sulphide sols (e. g. , As 2 S 3 sol), H 2 S molecules get adsorbed on colloidal particles. Consequently, colloidal particles are left with negative charge. • Due to the dissociation of molecules forming colloidal aggregates. The soap molecules (RCOONa) dissociate to give RCOO- and Na+ ions. RCOO- ions aggregate together to form micelles which carry negative charge as explained earlier.

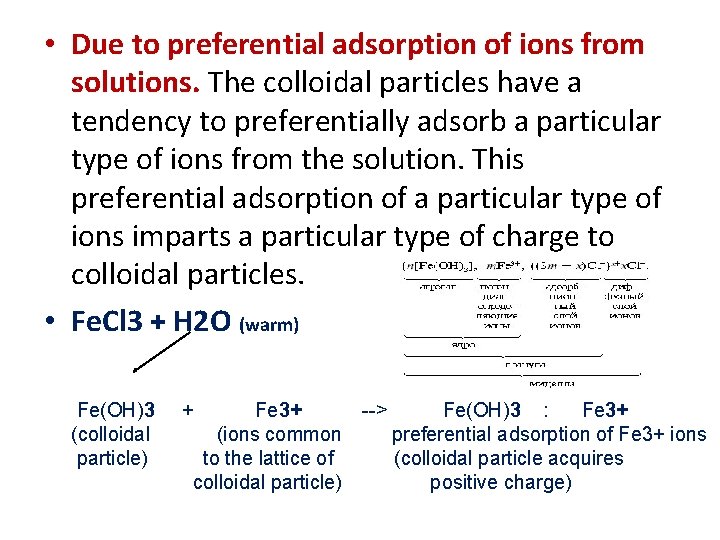
• Due to preferential adsorption of ions from solutions. The colloidal particles have a tendency to preferentially adsorb a particular type of ions from the solution. This preferential adsorption of a particular type of ions imparts a particular type of charge to colloidal particles. • Fe. Cl 3 + H 2 O (warm) Fe(OH)3 + Fe 3+ --> Fe(OH)3 : Fe 3+ (colloidal (ions common preferential adsorption of Fe 3+ ions particle) to the lattice of (colloidal particle acquires colloidal particle) positive charge)

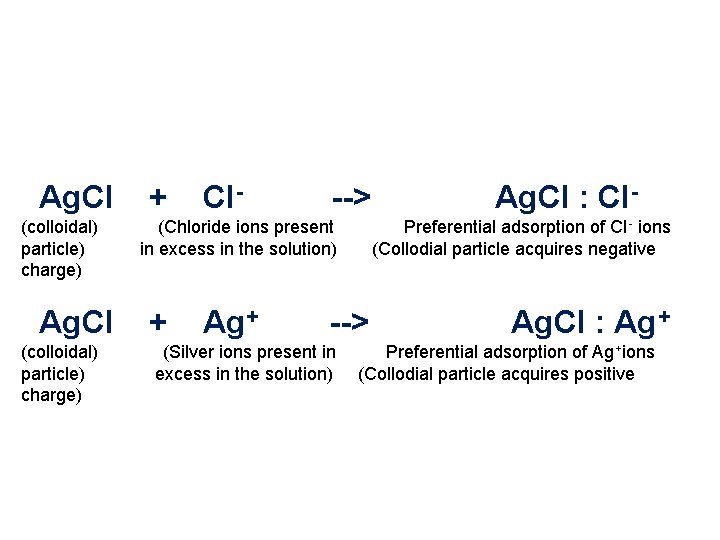
Ag. CI + CI- --> Ag. CI : CI- (colloidal) (Chloride ions present Preferential adsorption of CI - ions particle) in excess in the solution) (Collodial particle acquires negative charge) Ag. CI + Ag+ --> Ag. CI : Ag+ (colloidal) (Silver ions present in Preferential adsorption of Ag +ions particle) excess in the solution) (Collodial particle acquires positive charge)

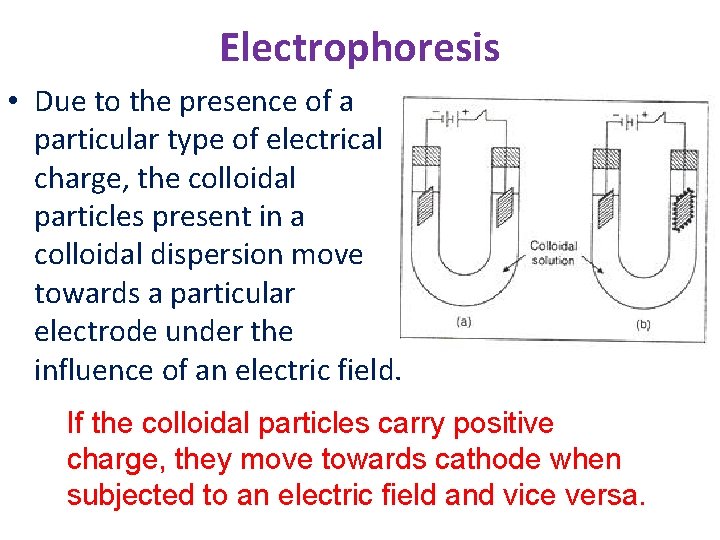
Electrophoresis • Due to the presence of a particular type of electrical charge, the colloidal particles present in a colloidal dispersion move towards a particular electrode under the influence of an electric field. If the colloidal particles carry positive charge, they move towards cathode when subjected to an electric field and vice versa.

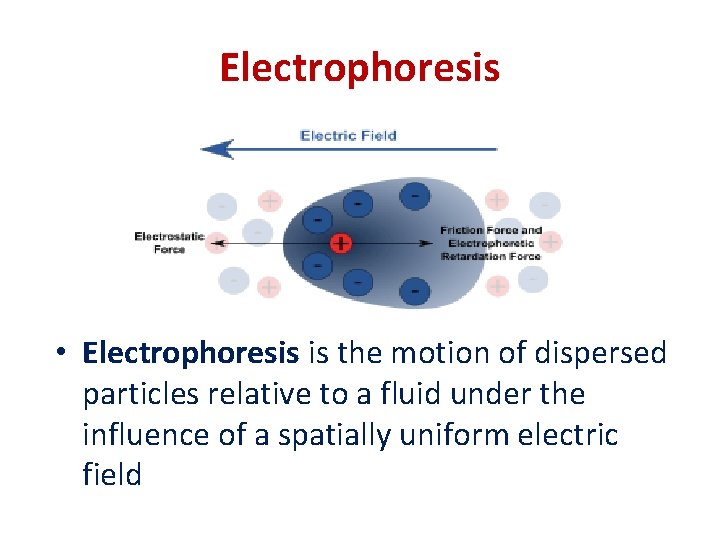
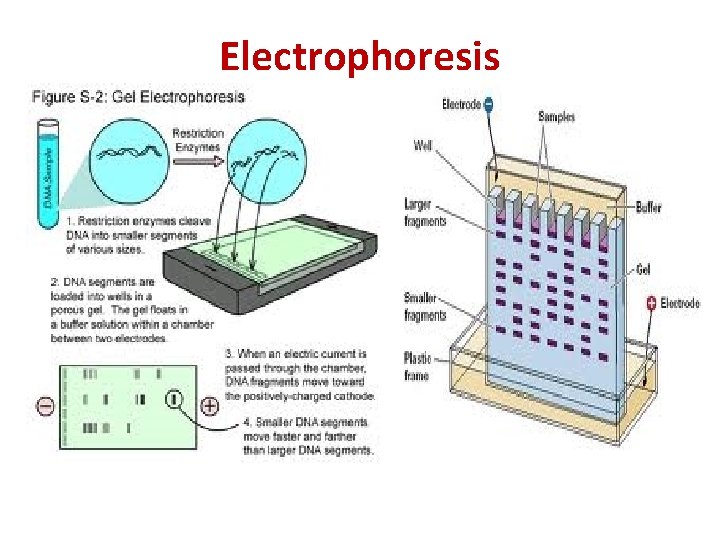
Electrophoresis • Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field

Electrophoresis • If the particles accumulate near the negative electrode, the charge on the particles is positive. • When electrophoresis of a sol is carried out with out stirring, the bottom layer gradually becomes more concentrated while the top layer which contain pure and concentrated colloidal solution may be decanted. This is called electro decanation and is used for the purification as well as for concentrating the sol. • The reverse of electrophoresis is called Sedimentation potential or Dorn effect. The sedimentation potential is setup when a particle is forced to move in a resting liquid. This phenomenon was discovered by Dorn and is also called Dorn effect.

• is the electrophoretic mobility, • epsilon is the electric permittivity of the liquid • ξ the zeta potential, • eta is the viscosity

Electrophoresis

Practical implementation of Electrophoresis in medicine

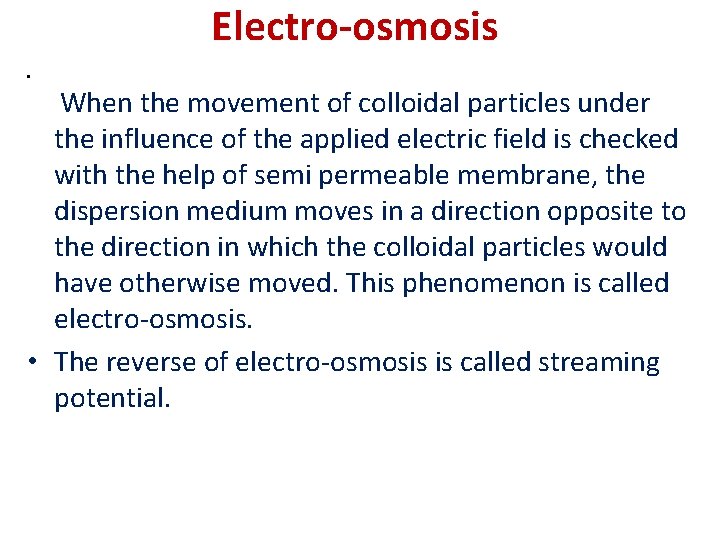
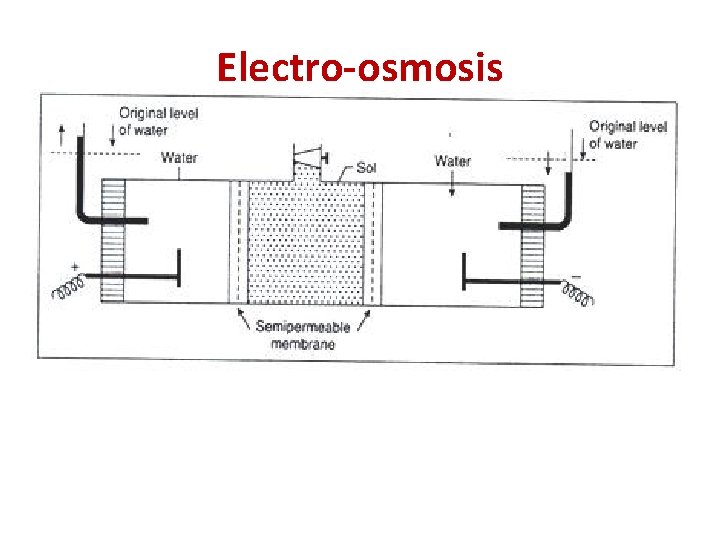
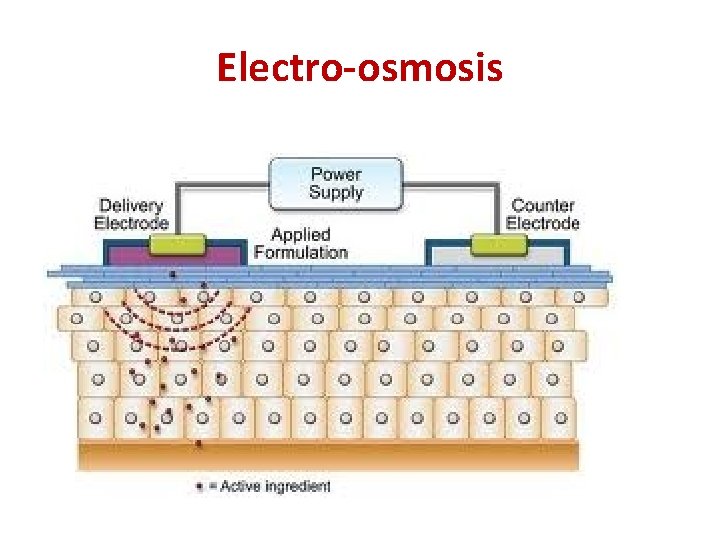
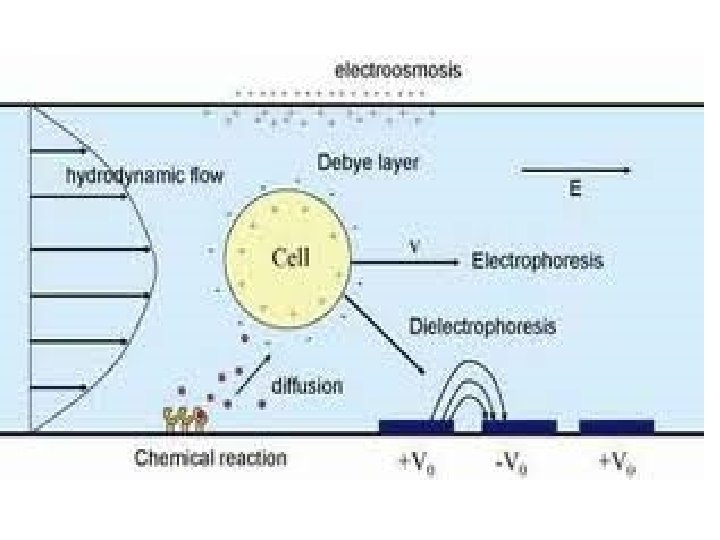
Electro-osmosis • When the movement of colloidal particles under the influence of the applied electric field is checked with the help of semi permeable membrane, the dispersion medium moves in a direction opposite to the direction in which the colloidal particles would have otherwise moved. This phenomenon is called electro-osmosis. • The reverse of electro-osmosis is called streaming potential.

Electro-osmosis

Electro-osmosis


Coagulation or flocculation • The stability of a sol is due to the charge present on the colloidal particles. However, if the charge on colloidal particles is destroyed, they are free to come nearer and grow in size and get precipitated. This phenomenon is termed as coagulation or flocculation. • The coagulation of colloidal solution can be achieved by the addition of an electrolyte. It is to be noted that a small amount of electrolyte is necessary for the stability of a sol because the ions of the electrolyte get adsorbed on colloidal particles and impart them some charge.

Hardy-Schulze rule • The coagulation capacity of an electrolyte depends upon the valence of ion responsible for causing coagulation. The ion responsible for causing coagulation is the one which carries charge opposite to that present on colloidal particles. • The greater is the valence of the oppositely charged ion of the electrolyte added to a colloidal solution, the faster is the coagulation of the colloidal solution.

Coagulation power of different cations • Negatively charged sol of As 2 S 3 Al 3+ > Ba 2+ > Na+ Positively charged sol Fe(OH)3 [Fe(CN)6]4 - > PO 43 - > SO 42 - > Cl-

Flocculation value The coagulating power of an electrolyte is usually expressed in terms of its flocculation value. The minimum concentration (in millimoles per litre) of an electrolyte required to cause the coagulation of a sol is called the flocculation value of the electrolyte.

Additional methods for causing coagulation (a) By electrophoresis: In electrophoresis, the charged colloidal particles migrate to the oppositely charged electrode and get discharged. This results in the coagulation of the colloidal solution. (b) By mixing two oppositely sols: When two sols carrying opposite charges are mixed together in suitable proportions, the colloidal particles of one sol neutralize the charge present on the particles of the other sol and both get coagulated. (c) By persistent dialysis: We have already seen that a small amount of electrolyte is essential to make a sol stable. When a sol is subjected to persistent dialysis, the traces of electrolyte also pass out through the membrane. In the absence of electrolyte, sol becomes unstable and gets coagulated.

 Aix smit
Aix smit Ibm geographically dispersed resiliency for power systems
Ibm geographically dispersed resiliency for power systems Halina abramczyk
Halina abramczyk Halina abramowicz
Halina abramowicz Halina koczyk
Halina koczyk Bars halina kalemba
Bars halina kalemba Dr halina flisiak-antonijczuk kontakt
Dr halina flisiak-antonijczuk kontakt Halina baran
Halina baran Halina sitko
Halina sitko Halina
Halina Halina baran
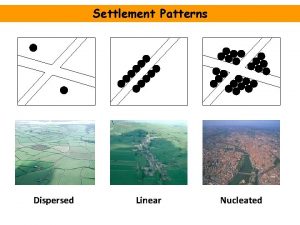
Halina baran Disadvantage of nucleated settlement
Disadvantage of nucleated settlement Dispersed nucleated and linear settlements
Dispersed nucleated and linear settlements Nucleated settlement pattern
Nucleated settlement pattern This type of dispersion forms compact cake
This type of dispersion forms compact cake Dispersion phase and dispersion medium
Dispersion phase and dispersion medium Define dispersed system
Define dispersed system Dispersed definition ap human geography
Dispersed definition ap human geography Beggar ticks seed dispersal
Beggar ticks seed dispersal Guava seed dispersal
Guava seed dispersal Dispersed
Dispersed Geographically dispersed parallel sysplex
Geographically dispersed parallel sysplex Dispersed rural settlement definition
Dispersed rural settlement definition The basic systems and services of a city
The basic systems and services of a city Dispersed phase
Dispersed phase Dispersed collective behavior
Dispersed collective behavior Kinetic properties of colloids
Kinetic properties of colloids Properties of nerve fibres
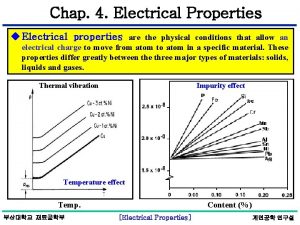
Properties of nerve fibres Electrical properties of matter
Electrical properties of matter Electrical properties of matter
Electrical properties of matter Properties of nerve fibre
Properties of nerve fibre Rising mains wiring system
Rising mains wiring system Mathematical modelling of mechanical systems
Mathematical modelling of mechanical systems Introduction to electrical power systems
Introduction to electrical power systems One electrical systems
One electrical systems