ECE 802 604 Nanoelectronics Prof Virginia Ayres Electrical







- Slides: 52

ECE 802 -604: Nanoelectronics Prof. Virginia Ayres Electrical & Computer Engineering Michigan State University ayresv@msu. edu

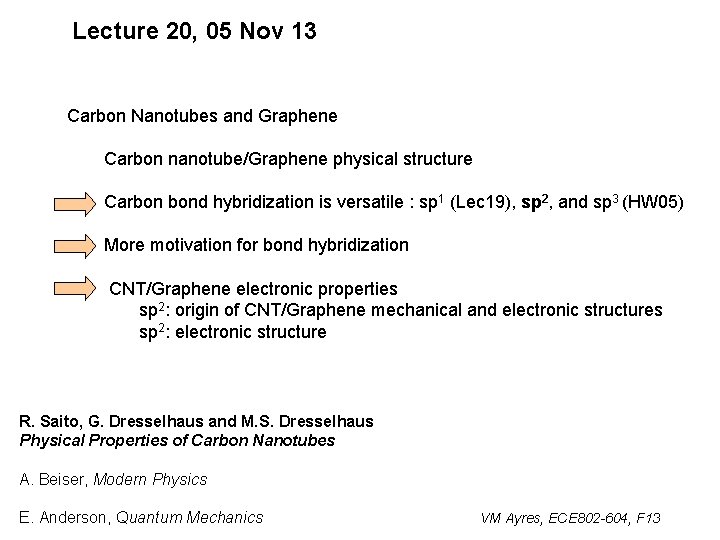
Lecture 20, 05 Nov 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure Carbon bond hybridization is versatile : sp 1 (Lec 19), sp 2, and sp 3 (HW 05) More motivation for bond hybridization CNT/Graphene electronic properties sp 2: origin of CNT/Graphene mechanical and electronic structures sp 2: electronic structure R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes A. Beiser, Modern Physics E. Anderson, Quantum Mechanics VM Ayres, ECE 802 -604, F 13

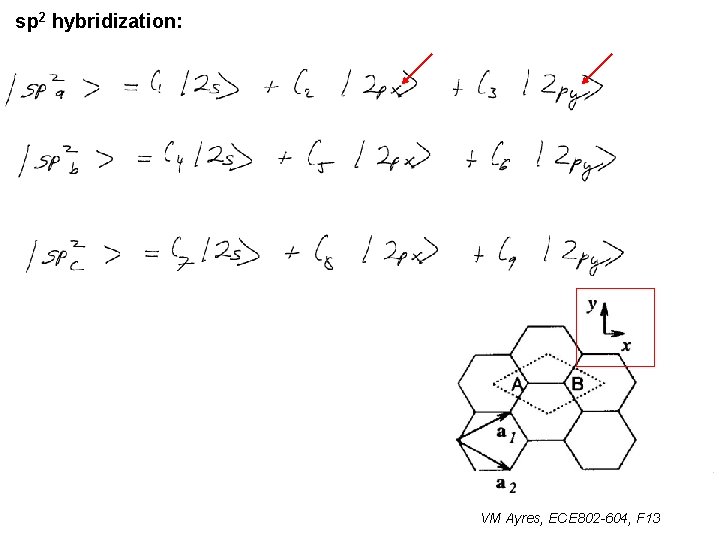
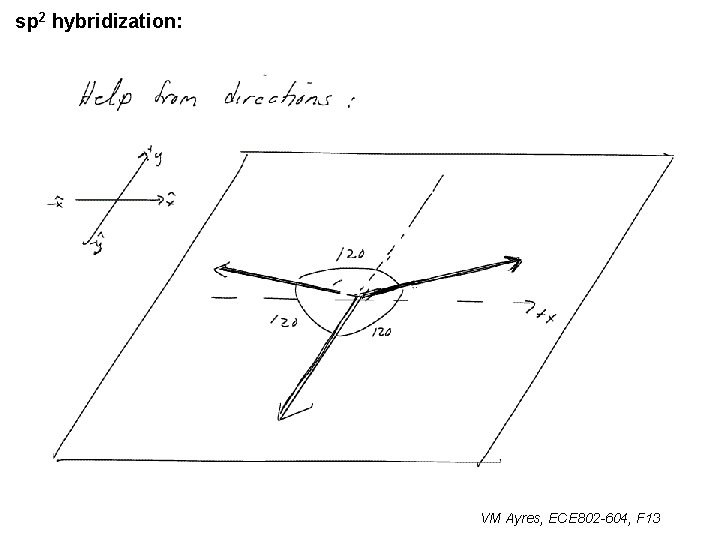
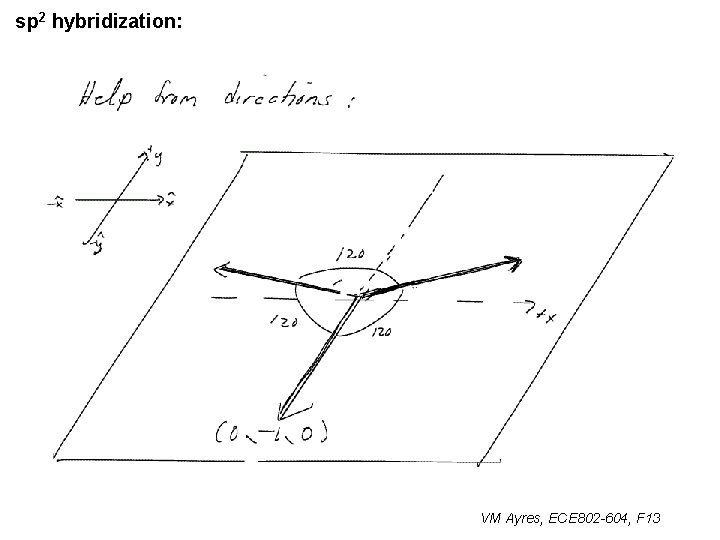
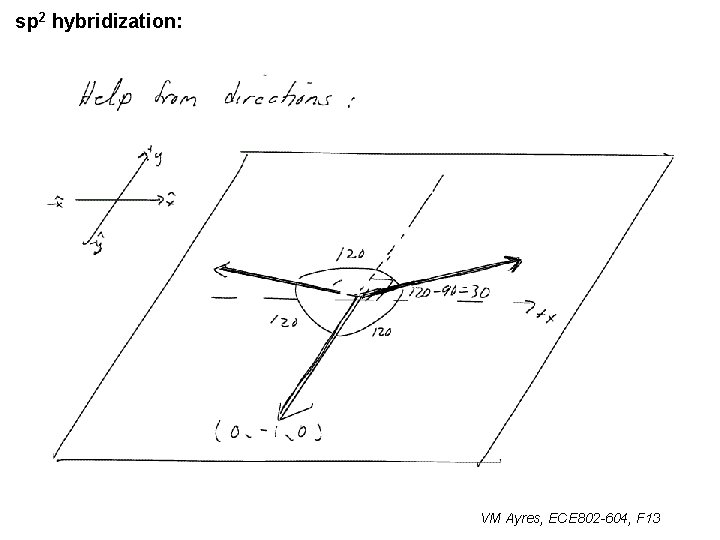
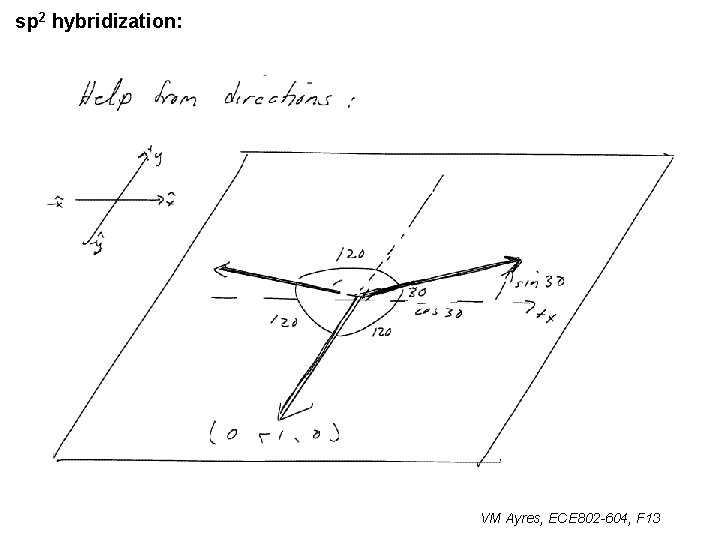
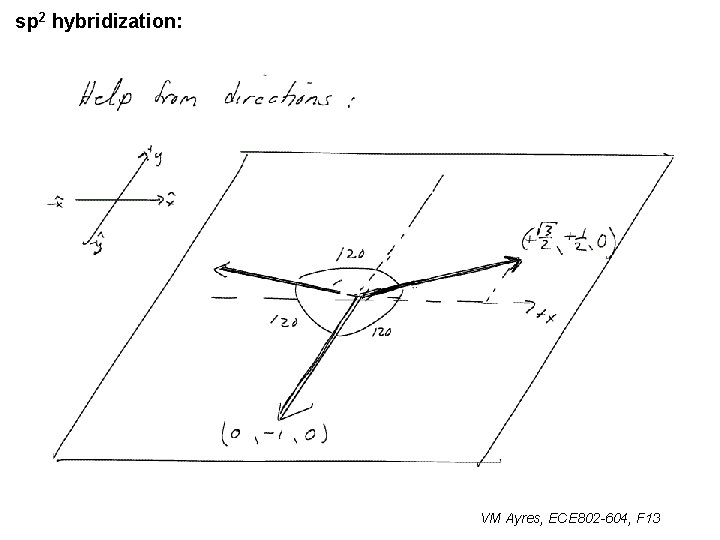
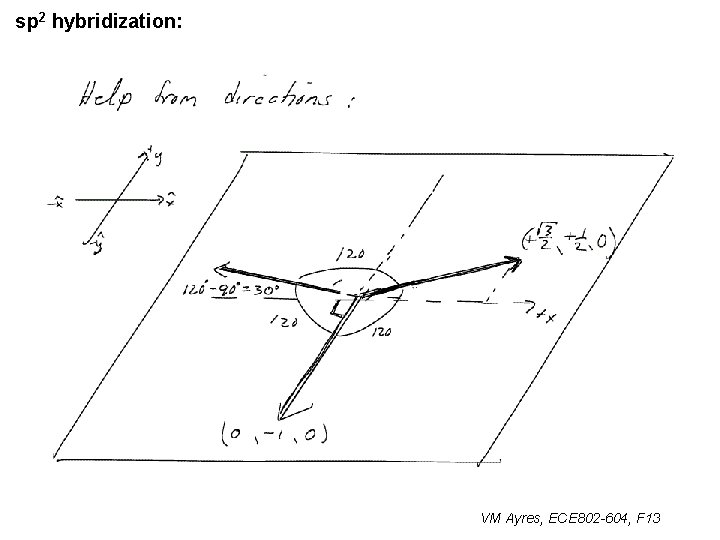
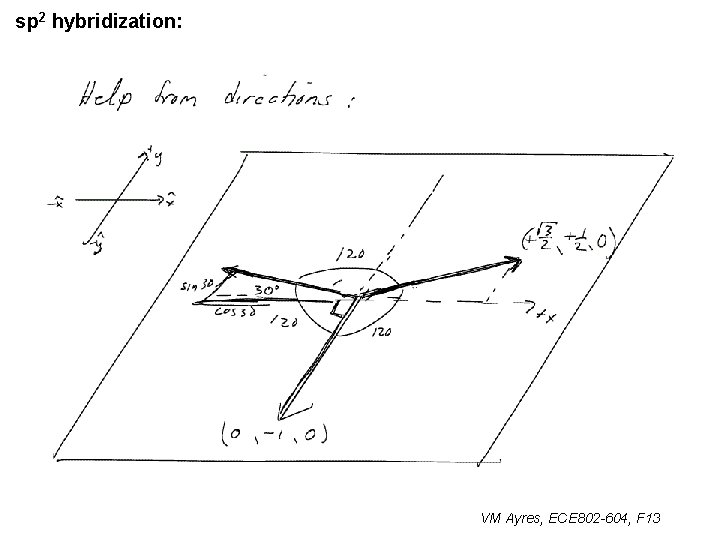
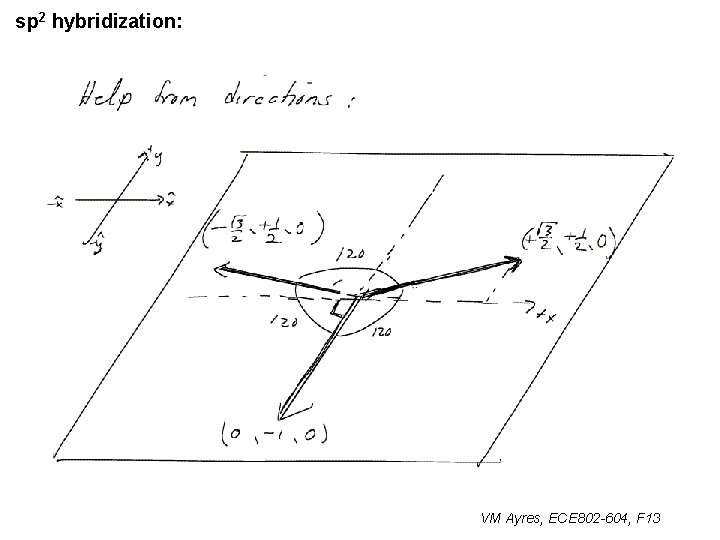
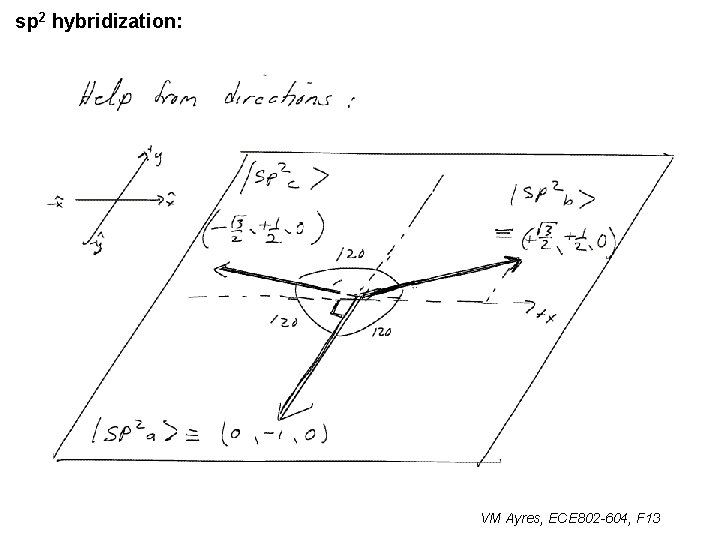
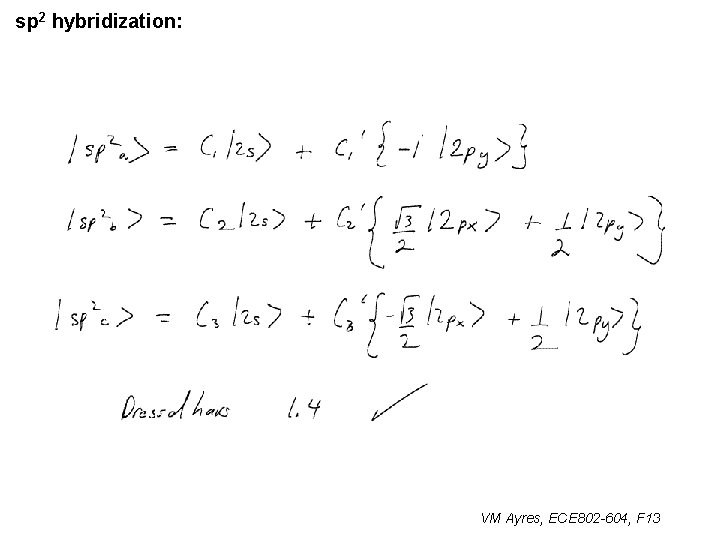
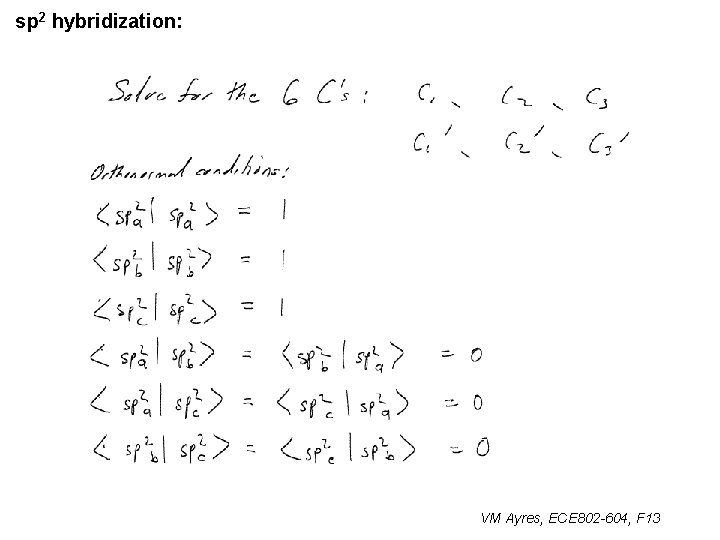
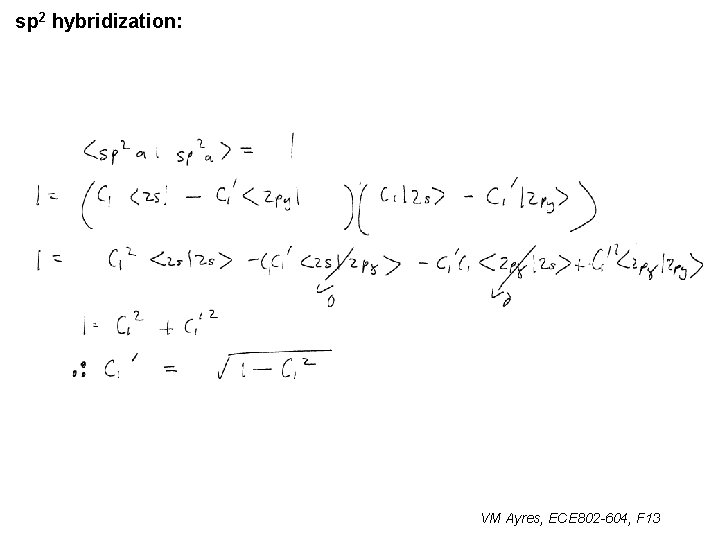
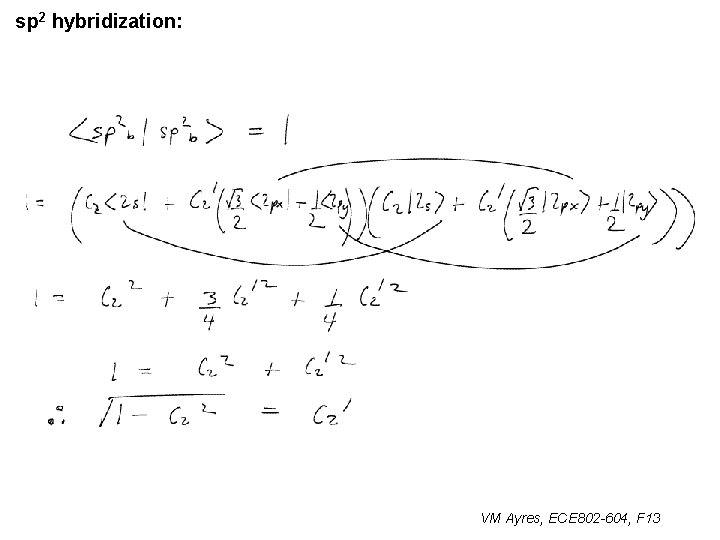
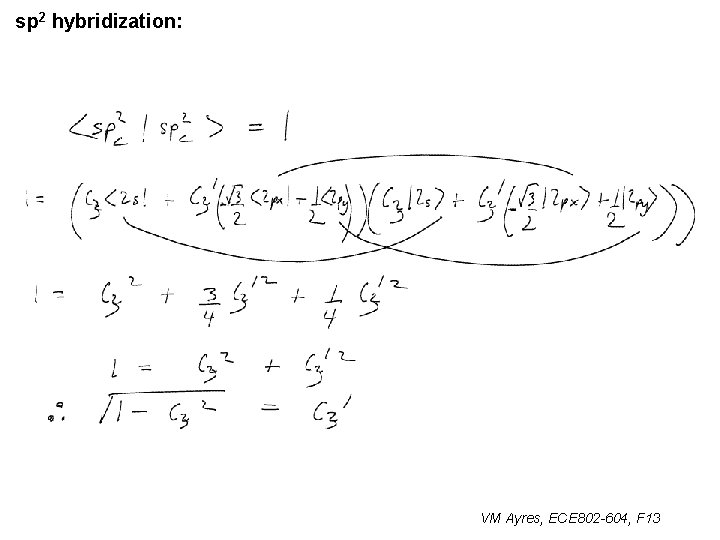
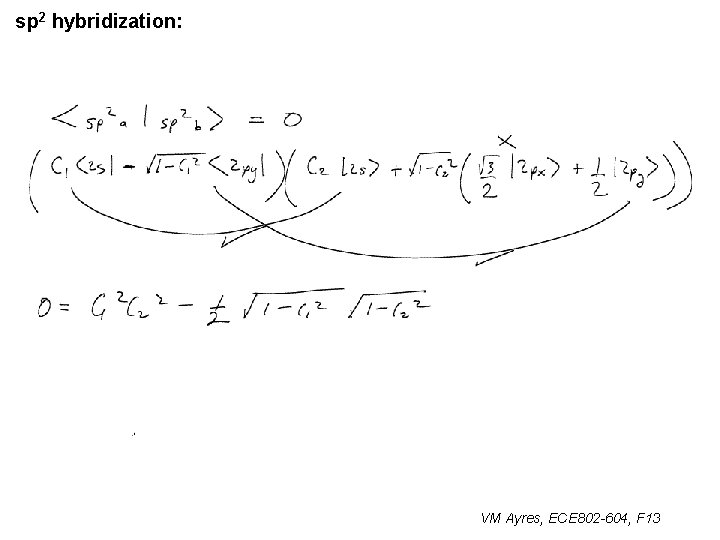
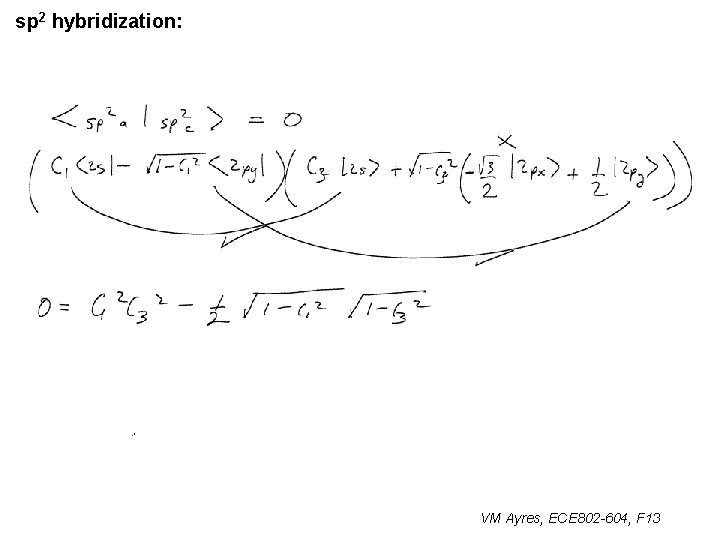
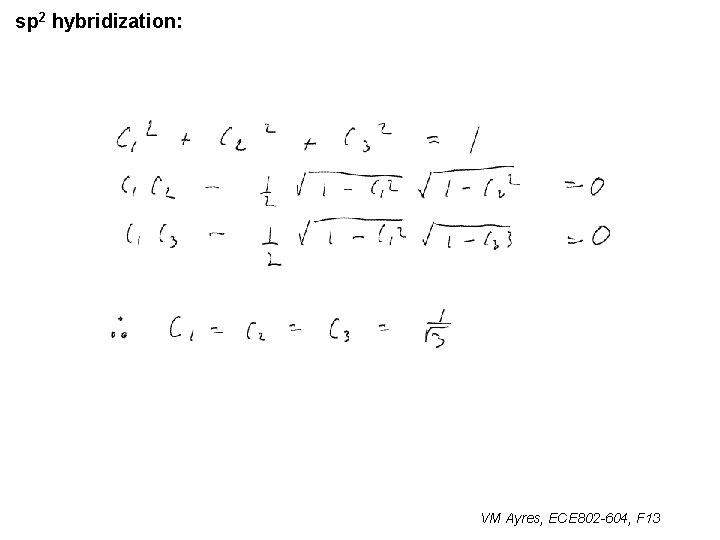
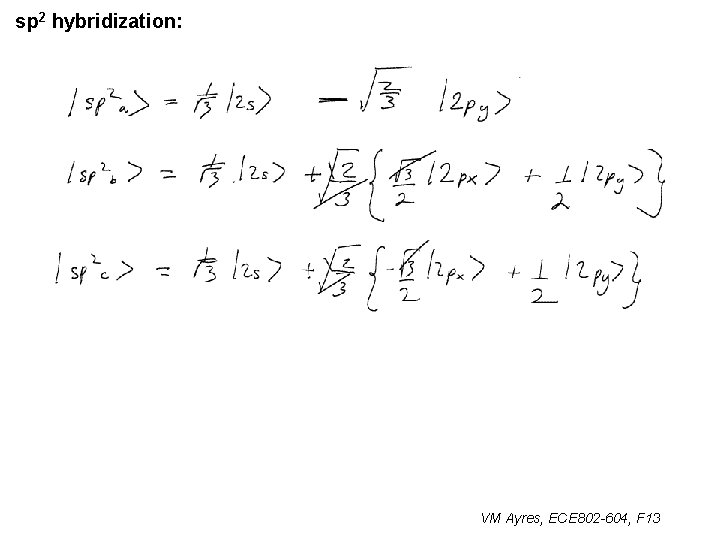
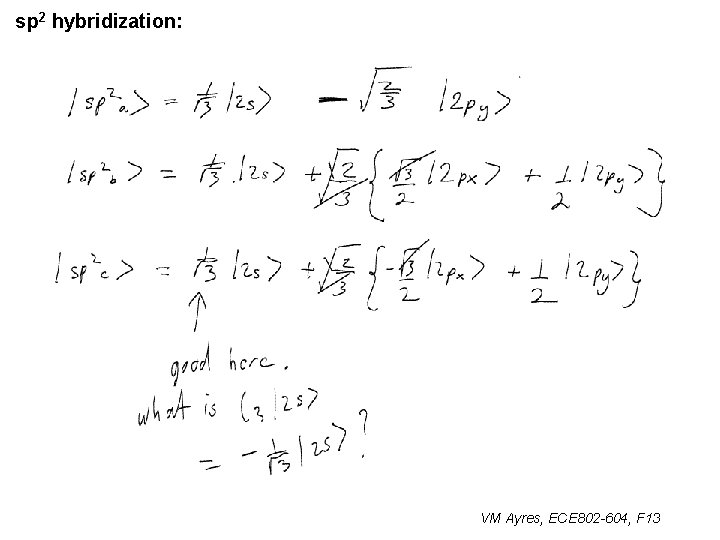
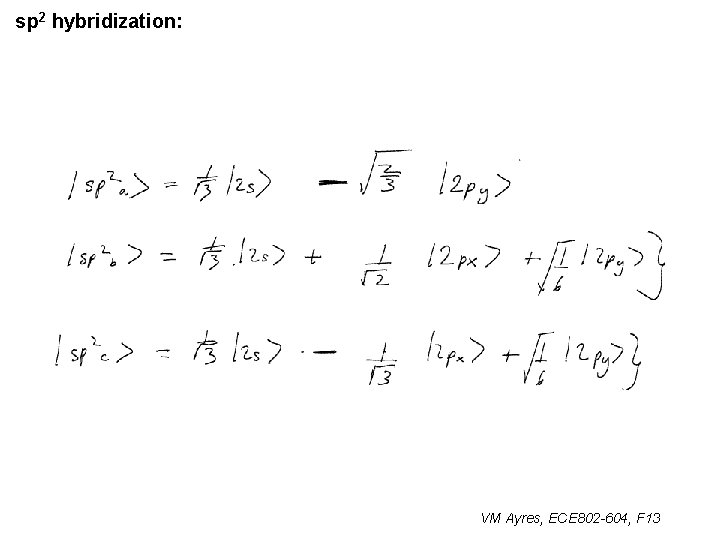
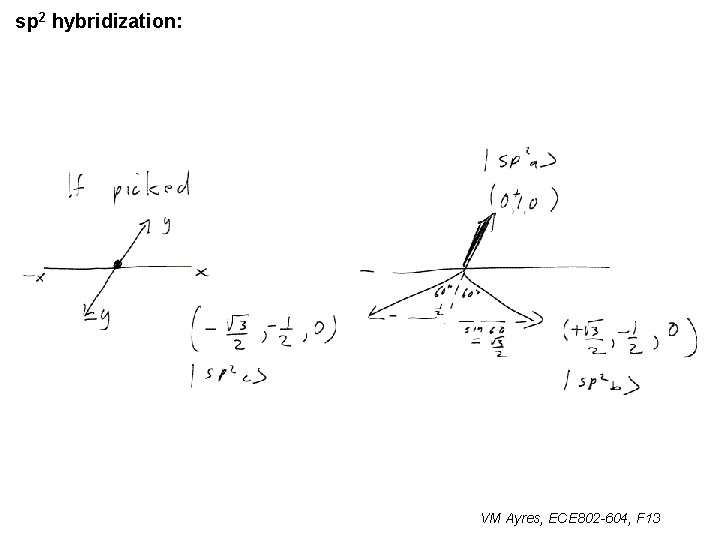
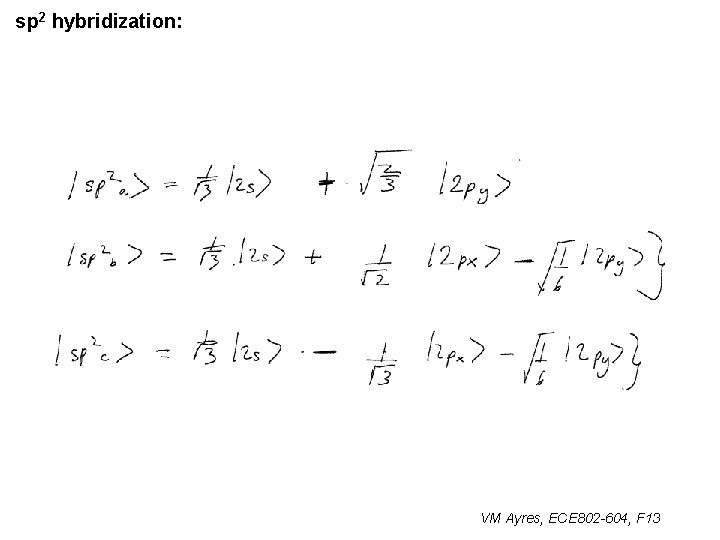
l sp 1 hybridization – Use orthonormality l sp 2 hybridization: – Get help from directions – Use orthonormality VM Ayres, ECE 802 -604, F 13

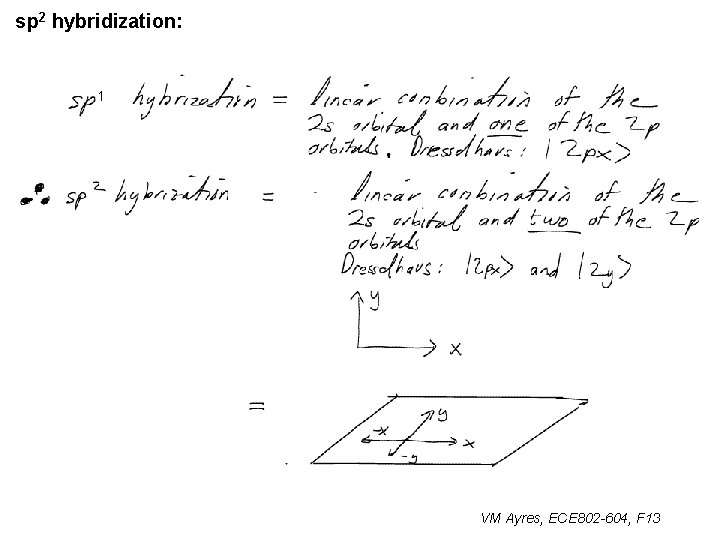
sp 2 hybridization: 1 VM Ayres, ECE 802 -604, F 13

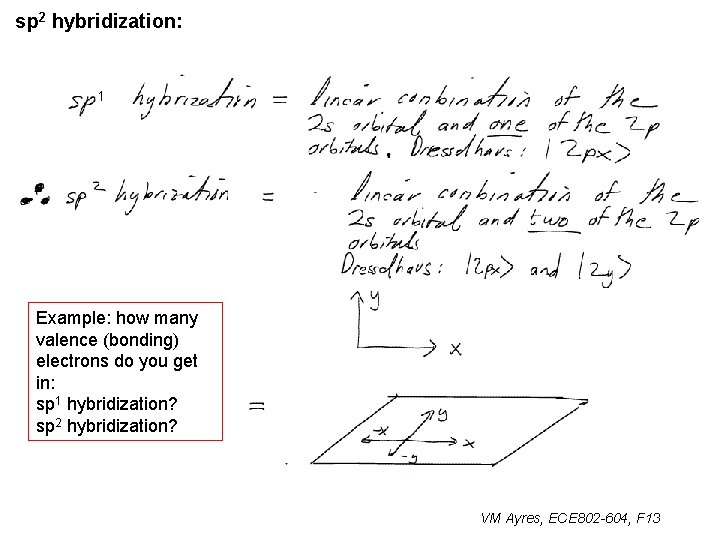
sp 2 hybridization: 1 Example: how many valence (bonding) electrons do you get in: sp 1 hybridization? sp 2 hybridization? VM Ayres, ECE 802 -604, F 13

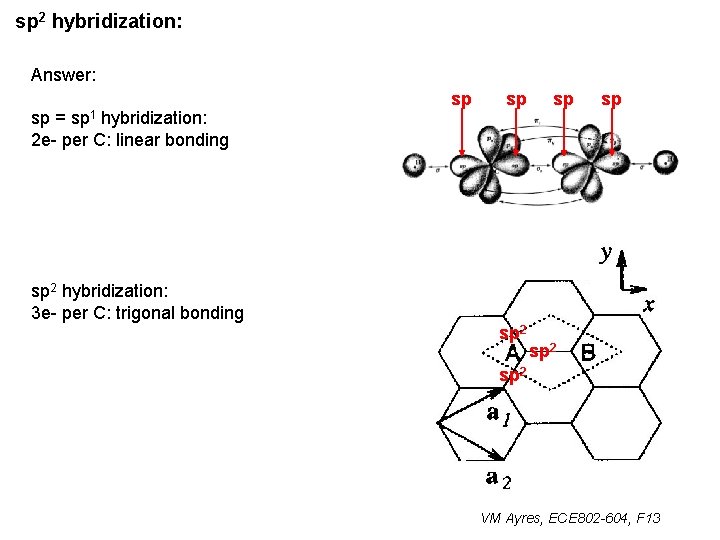
sp 2 hybridization: Answer: sp 1 sp = hybridization: 2 e- per C: linear bonding sp sp sp 2 hybridization: 3 e- per C: trigonal bonding sp 2 VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

sp 2 hybridization: VM Ayres, ECE 802 -604, F 13

Lecture 20, 05 Nov 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure Carbon bond hybridization is versatile : sp 1 (Lec 19), sp 2, and sp 3 (HW 05) More motivation for bond hybridization CNT/Graphene electronic properties sp 2: origin of CNT/Graphene mechanical and electronic structures sp 2: electronic structure R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes A. Beiser, Modern Physics E. Anderson, Quantum Mechanics VM Ayres, ECE 802 -604, F 13

VM Ayres, ECE 802 -604, F 13

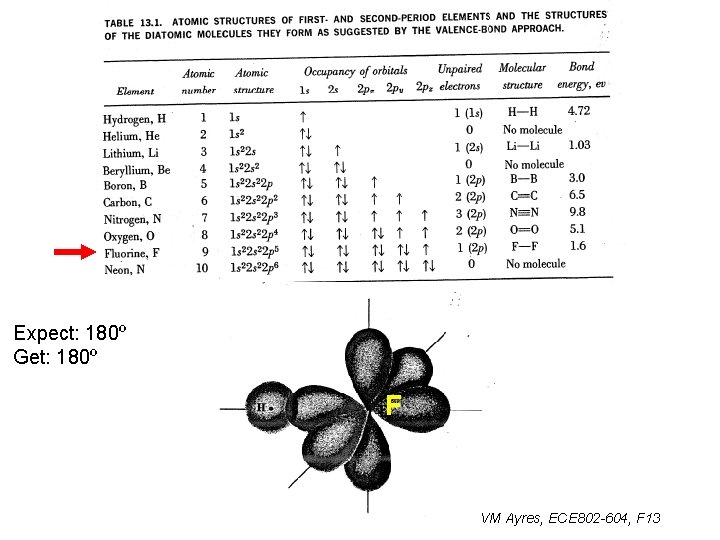
Expect: 180º Get: 180º F VM Ayres, ECE 802 -604, F 13

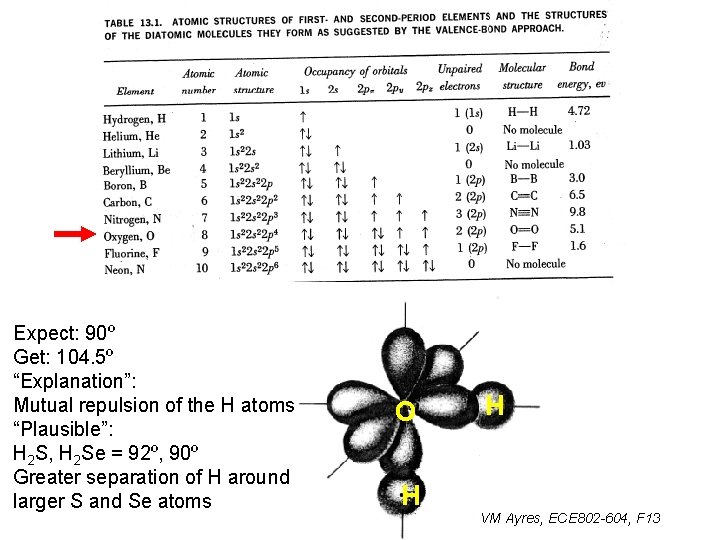
Expect: 90º Get: 104. 5º “Explanation”: Mutual repulsion of the H atoms “Plausible”: H 2 S, H 2 Se = 92º, 90º Greater separation of H around larger S and Se atoms O H H VM Ayres, ECE 802 -604, F 13

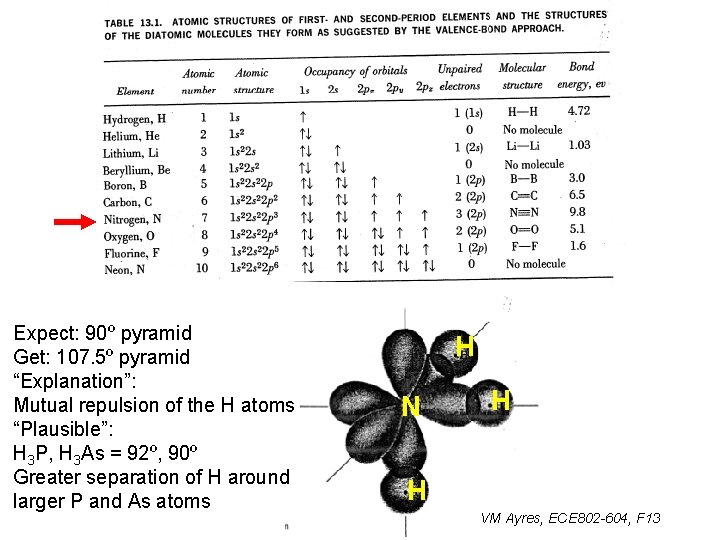
Expect: 90º pyramid Get: 107. 5º pyramid “Explanation”: Mutual repulsion of the H atoms “Plausible”: H 3 P, H 3 As = 92º, 90º Greater separation of H around larger P and As atoms H N H H VM Ayres, ECE 802 -604, F 13

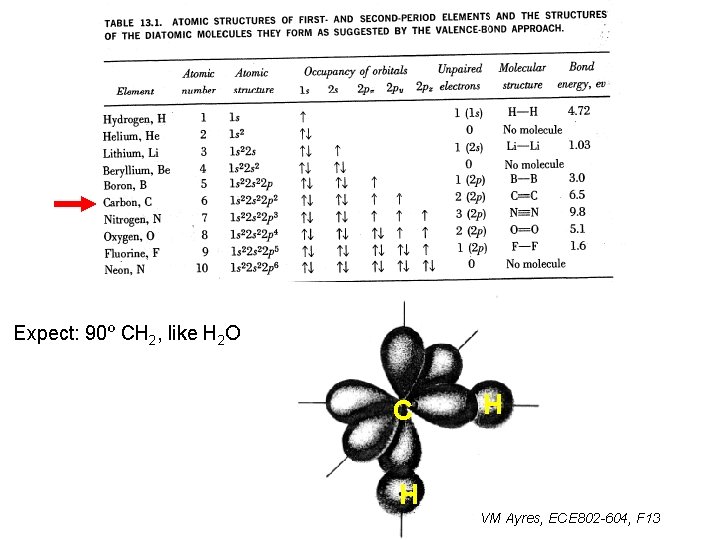
Expect: 90º CH 2, like H 2 O C H H VM Ayres, ECE 802 -604, F 13

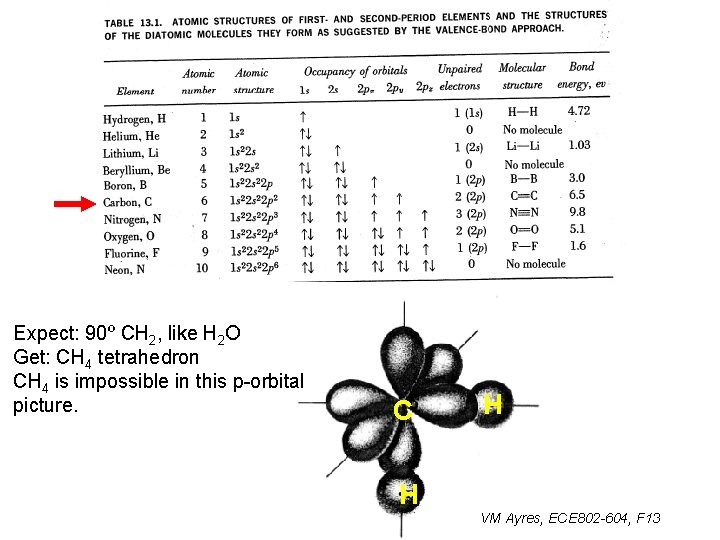
Expect: 90º CH 2, like H 2 O Get: CH 4 tetrahedron CH 4 is impossible in this p-orbital picture. C H H VM Ayres, ECE 802 -604, F 13

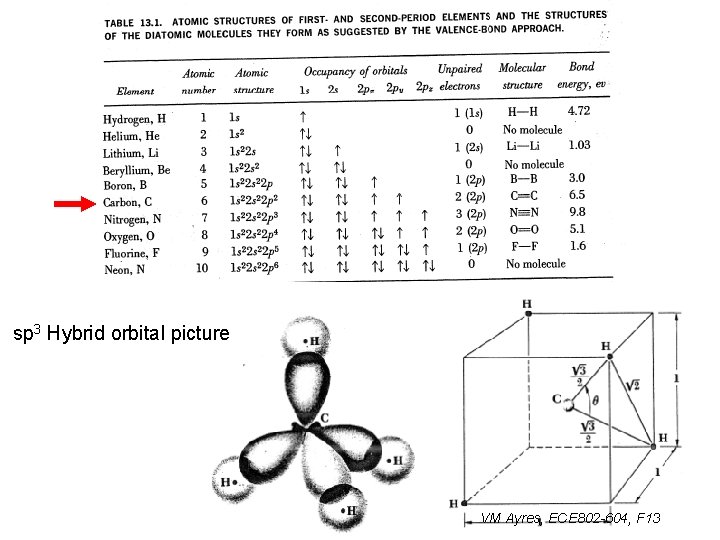
sp 3 Hybrid orbital picture VM Ayres, ECE 802 -604, F 13

Lecture 20, 05 Nov 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure Carbon bond hybridization is versatile : sp 1 (Lec 19), sp 2, and sp 3 (HW 05) More motivation for bond hybridization CNT/Graphene electronic properties sp 2: origin of CNT/Graphene mechanical and electronic structures sp 2: electronic structure R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes A. Beiser, Modern Physics E. Anderson, Quantum Mechanics VM Ayres, ECE 802 -604, F 13

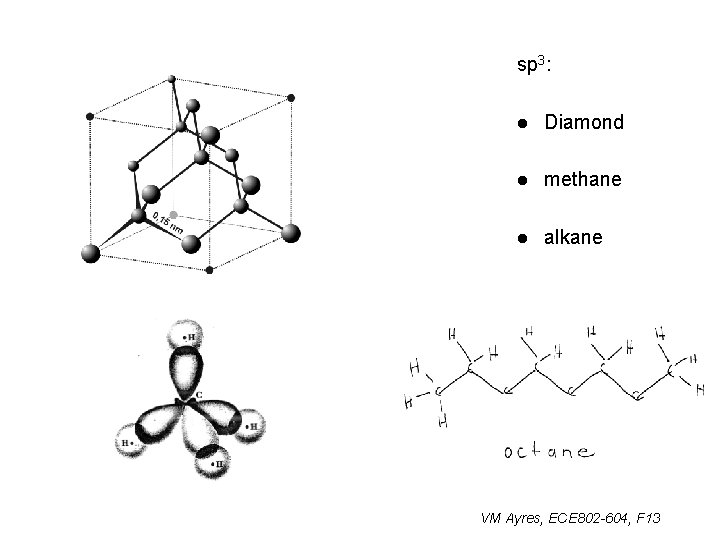
sp 3: l Diamond l methane l alkane VM Ayres, ECE 802 -604, F 13

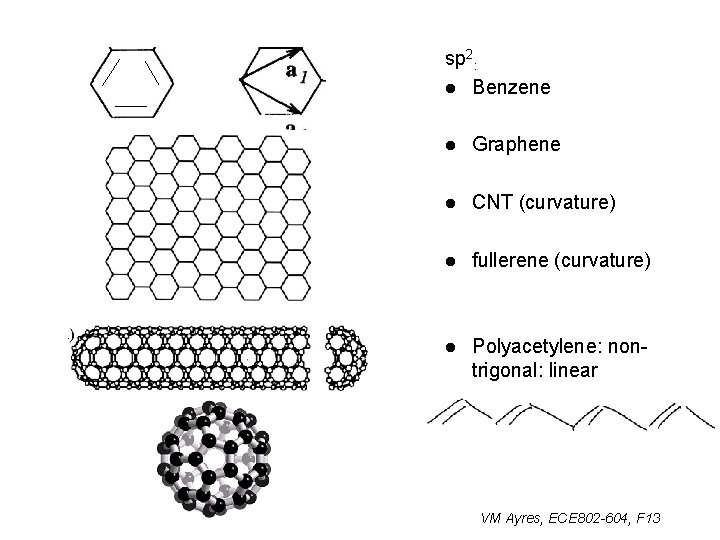
sp 2: l Benzene l Graphene l CNT (curvature) l fullerene (curvature) l Polyacetylene: nontrigonal: linear VM Ayres, ECE 802 -604, F 13

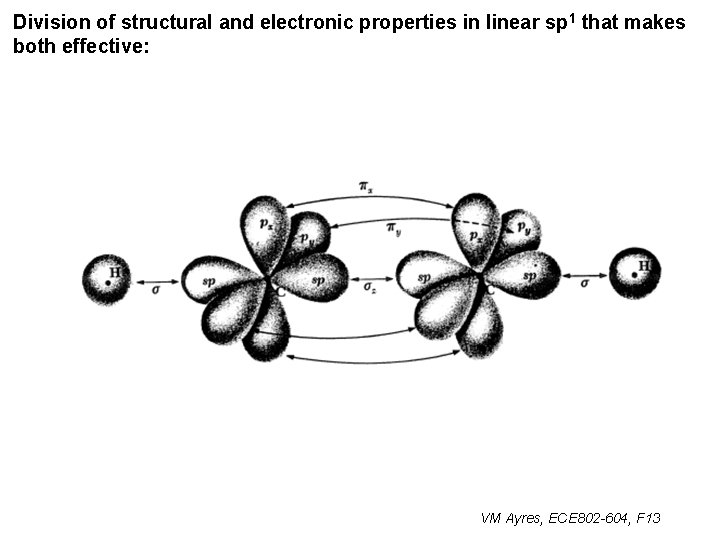
Division of structural and electronic properties in linear sp 1 that makes both effective: VM Ayres, ECE 802 -604, F 13

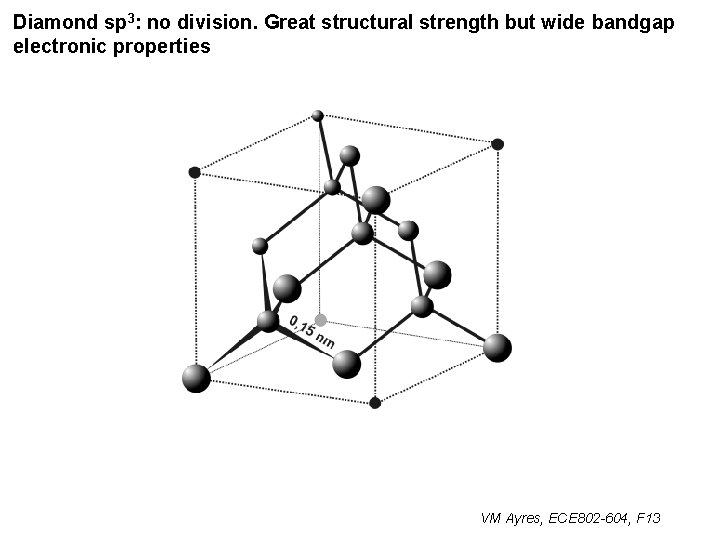
Diamond sp 3: no division. Great structural strength but wide bandgap electronic properties VM Ayres, ECE 802 -604, F 13

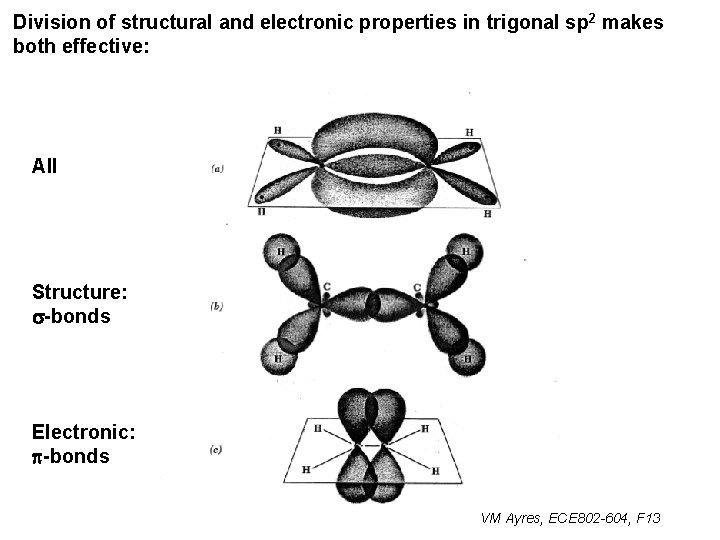
Division of structural and electronic properties in trigonal sp 2 makes both effective: All Structure: s-bonds Electronic: p-bonds VM Ayres, ECE 802 -604, F 13

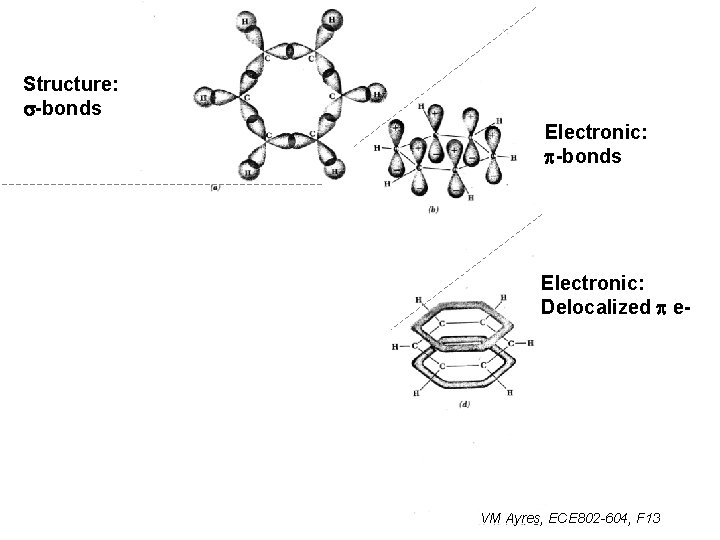
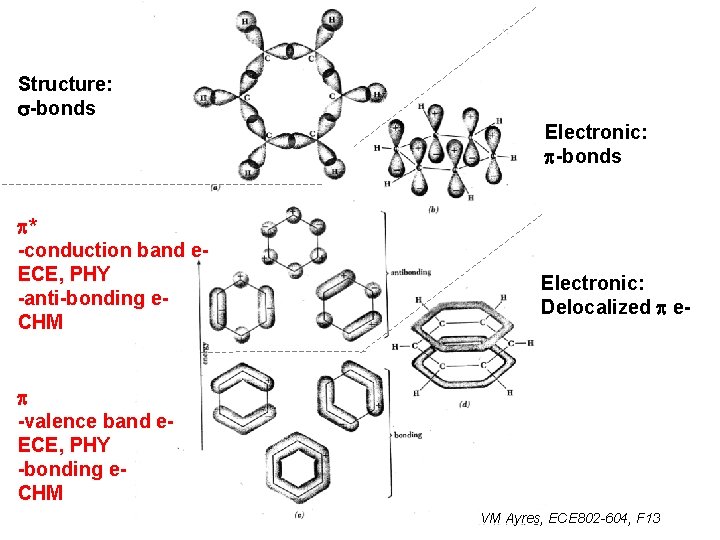
Structure: s-bonds Electronic: p-bonds p* -conduction band e. ECE, PHY -anti-bonding e. CHM Electronic: Delocalized p e- p -valence band e. ECE, PHY -bonding e. CHM VM Ayres, ECE 802 -604, F 13

Structure: s-bonds Electronic: p-bonds p* -conduction band e. ECE, PHY -anti-bonding e. CHM Electronic: Delocalized p e- p -valence band e. ECE, PHY -bonding e. CHM VM Ayres, ECE 802 -604, F 13

Lecture 20, 05 Nov 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure Carbon bond hybridization is versatile : sp 1 (Lec 19), sp 2, and sp 3 (HW 05) More motivation for bond hybridization CNT/Graphene electronic properties sp 2: origin of CNT/Graphene mechanical and electronic structures sp 2: electronic structure: 1 st: polyacetylene 2 nd: graphene: R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes A. Beiser, Modern Physics E. Anderson, Quantum Mechanics VM Ayres, ECE 802 -604, F 13

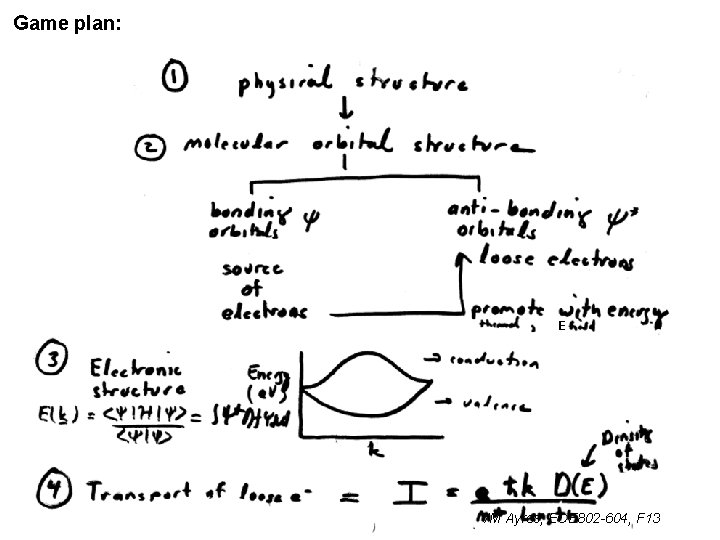
Game plan: E VM Ayres, ECE 802 -604, F 13

Rules for finding the electronic structure (p. 21): 1 Find Unit cell “a” 2 Find k: 3 Find H and S elements Det [H – SI] =0 4 Solve for E(k) VM Ayres, ECE 802 -604, F 13

Question: We’ve been doing E(k) versus k diagrams since Datta Chp 01. Why all this now? What happened to conservation of Energy as the starting point? What has changed? VM Ayres, ECE 802 -604, F 13

Question: We’ve been doing E(k) versus k diagrams since Datta Chp 01. Why all this now? What happened to conservation of Energy as the starting point? What has changed? Answer: Dresselhaus uses correct Bloch wave functions to describe the electrons. Datta uses travelling waves. This is discussed in Datta page 11. Therefore we must include the symmetry of the polyacetlyene, graphene, etc. lattice in our wave functions. That’s what finding the reciprocal space k is about. VM Ayres, ECE 802 -604, F 13