ECE 802 604 Nanoelectronics Prof Virginia Ayres Electrical






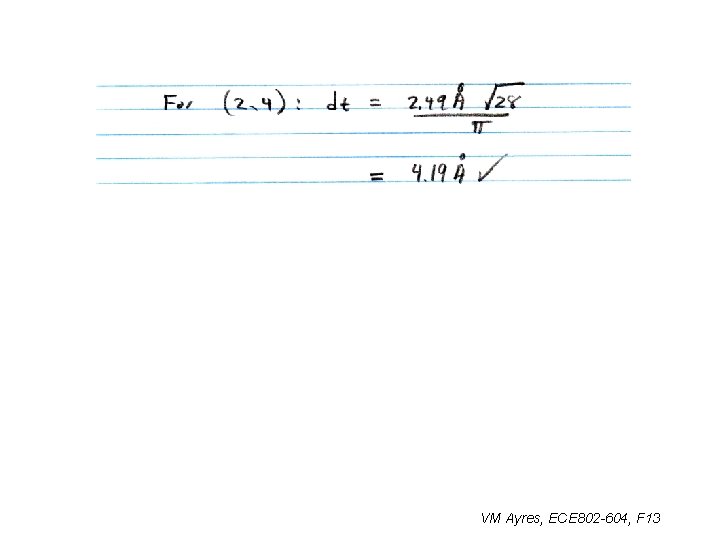

- Slides: 43

ECE 802 -604: Nanoelectronics Prof. Virginia Ayres Electrical & Computer Engineering Michigan State University ayresv@msu. edu

Lecture 19, 31 Oct 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure Carbon bond hybridization is versatile : sp 1, sp 2, and sp 3 sp 2: origin of CNT/Graphene mechanical and electronic structures CNT/Graphene electronic properties R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes Imperial College Press, London, 1998. VM Ayres, ECE 802 -604, F 13

Lecture 19, 31 Oct 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure SWCNT endcaps Carbon bond hybridization is versatile : sp 1, sp 2, and sp 3 sp 2: origin of mechanical and electronic structures Carbon nanotube/Graphene electronic ‘structure’ R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes Imperial College Press, London, 1998. VM Ayres, ECE 802 -604, F 13

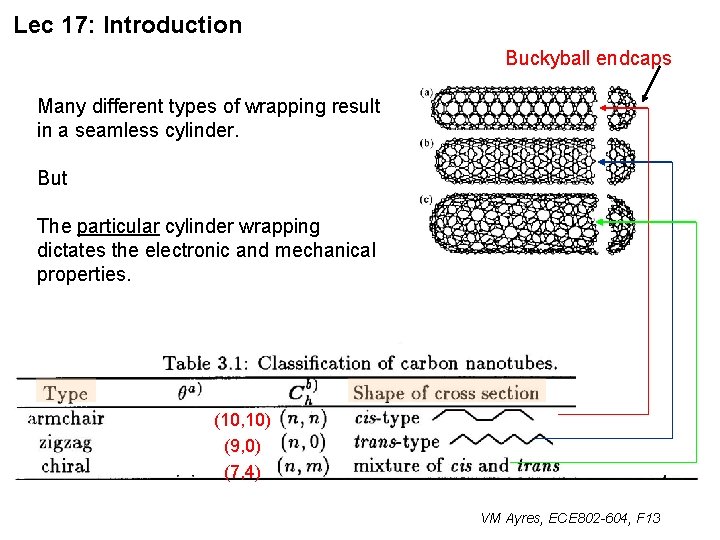
Lec 17: Introduction Buckyball endcaps Many different types of wrapping result in a seamless cylinder. But The particular cylinder wrapping dictates the electronic and mechanical properties. (10, 10) (9, 0) (7, 4) VM Ayres, ECE 802 -604, F 13

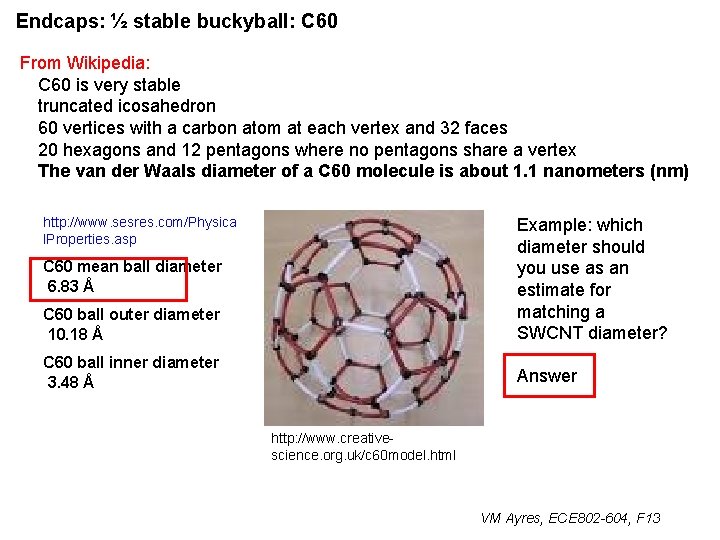
Endcaps: ½ stable buckyball: C 60 From Wikipedia: C 60 is very stable truncated icosahedron 60 vertices with a carbon atom at each vertex and 32 faces 20 hexagons and 12 pentagons where no pentagons share a vertex The van der Waals diameter of a C 60 molecule is about 1. 1 nanometers (nm) http: //www. sesres. com/Physica l. Properties. asp C 60 mean ball diameter 6. 83 Å C 60 ball outer diameter 10. 18 Å C 60 ball inner diameter 3. 48 Å http: //www. creativescience. org. uk/c 60 model. html VM Ayres, ECE 802 -604, F 13

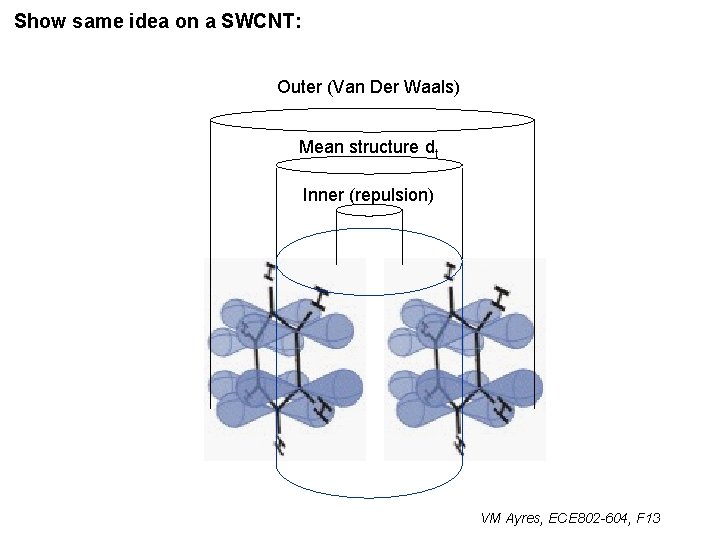
Show same idea on a SWCNT: Outer (Van Der Waals) Mean structure dt Inner (repulsion) VM Ayres, ECE 802 -604, F 13

Endcaps: ½ stable buckyball: C 60 From Wikipedia: C 60 is very stable truncated icosahedron 60 vertices with a carbon atom at each vertex and 32 faces 20 hexagons and 12 pentagons where no pentagons share a vertex The van der Waals diameter of a C 60 molecule is about 1. 1 nanometers (nm) http: //www. sesres. com/Physica l. Properties. asp Example: which diameter should you use as an estimate for matching a SWCNT diameter? C 60 mean ball diameter 6. 83 Å C 60 ball outer diameter 10. 18 Å C 60 ball inner diameter 3. 48 Å http: //www. creativescience. org. uk/c 60 model. html VM Ayres, ECE 802 -604, F 13

Endcaps: ½ stable buckyball: C 60 From Wikipedia: C 60 is very stable truncated icosahedron 60 vertices with a carbon atom at each vertex and 32 faces 20 hexagons and 12 pentagons where no pentagons share a vertex The van der Waals diameter of a C 60 molecule is about 1. 1 nanometers (nm) http: //www. sesres. com/Physica l. Properties. asp C 60 ball outer diameter 10. 18 Å Example: which diameter should you use as an estimate for matching a SWCNT diameter? C 60 ball inner diameter 3. 48 Å Answer C 60 mean ball diameter 6. 83 Å http: //www. creativescience. org. uk/c 60 model. html VM Ayres, ECE 802 -604, F 13

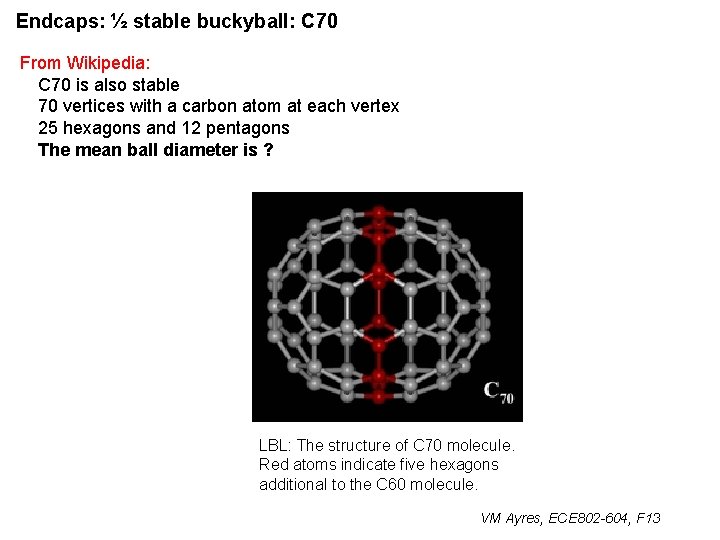
Endcaps: ½ stable buckyball: C 70 From Wikipedia: C 70 is also stable 70 vertices with a carbon atom at each vertex 25 hexagons and 12 pentagons The mean ball diameter is ? LBL: The structure of C 70 molecule. Red atoms indicate five hexagons additional to the C 60 molecule. VM Ayres, ECE 802 -604, F 13

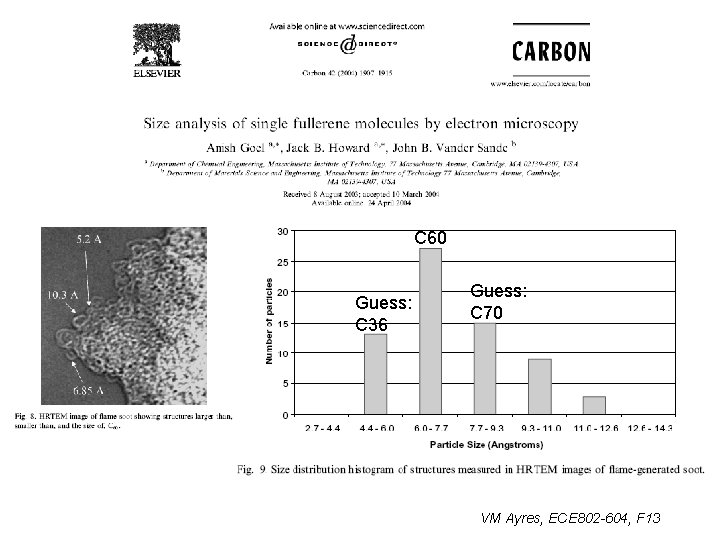
C 60 Guess: C 36 Guess: C 70 VM Ayres, ECE 802 -604, F 13

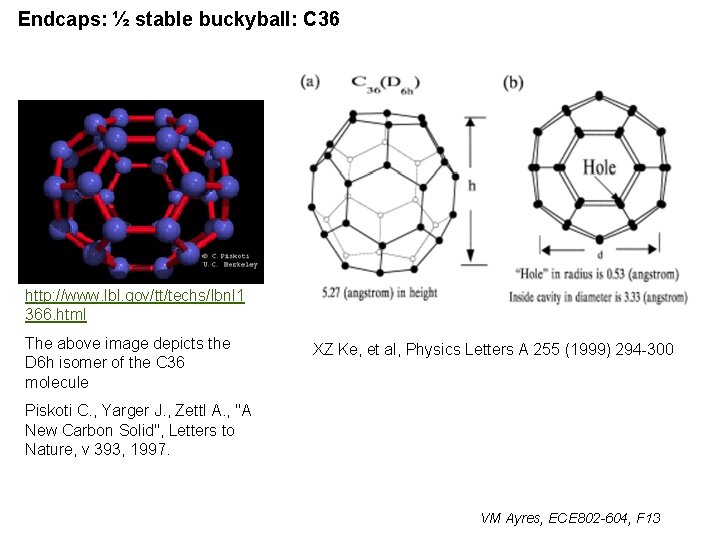
Endcaps: ½ stable buckyball: C 36 http: //www. lbl. gov/tt/techs/lbnl 1 366. html The above image depicts the D 6 h isomer of the C 36 molecule XZ Ke, et al, Physics Letters A 255 (1999) 294 -300 Piskoti C. , Yarger J. , Zettl A. , "A New Carbon Solid", Letters to Nature, v 393, 1997. VM Ayres, ECE 802 -604, F 13

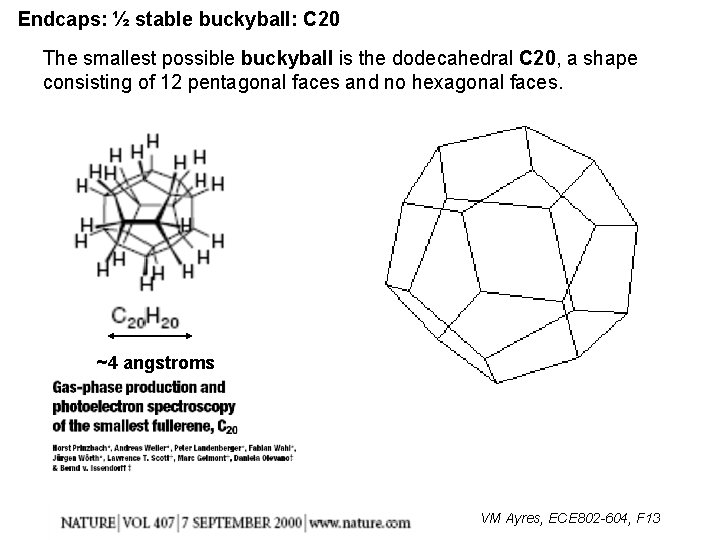
Endcaps: ½ stable buckyball: C 20 The smallest possible buckyball is the dodecahedral C 20, a shape consisting of 12 pentagonal faces and no hexagonal faces. ~4 angstroms VM Ayres, ECE 802 -604, F 13

Endcaps: ½ stable buckyball: C 20 smallest SWCNT inside MWCNT VM Ayres, ECE 802 -604, F 13

Example Problems: 8. Based on dt ~ 0. 4 nm, what ultrasmall SWCNT structures (n. m) are possible? 9. What types of CNT are they? 10. What physical issues would be involved in the self-assembly of these SWCNTs? HW 04: Find Ch, |Ch |, cosq, q, T, |T |, and N for the smallest SWCNT capped with a C 20. VM Ayres, ECE 802 -604, F 13

VM Ayres, ECE 802 -604, F 13

VM Ayres, ECE 802 -604, F 13
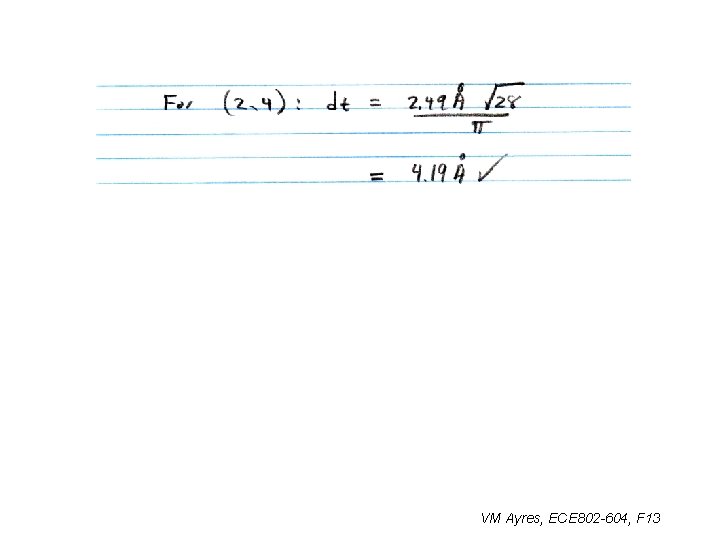
VM Ayres, ECE 802 -604, F 13

Lecture 19, 31 Oct 13 Carbon Nanotubes and Graphene Carbon nanotube/Graphene physical structure Carbon bond hybridization is versatile : sp 1, sp 2, and sp 3 sp 2: origin of CNT/Graphene mechanical and electronic structures CNT/Graphene electronic properties R. Saito, G. Dresselhaus and M. S. Dresselhaus Physical Properties of Carbon Nanotubes Imperial College Press, London, 1998. A. Beiser, Modern Physics, Chapter 13 E. Anderson, Quantum Mechanics, Chapter 7 VM Ayres, ECE 802 -604, F 13

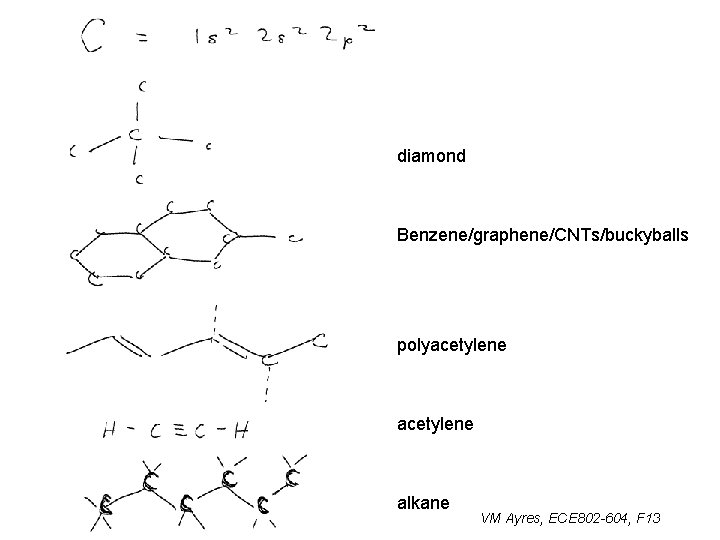
diamond Benzene/graphene/CNTs/buckyballs polyacetylene alkane VM Ayres, ECE 802 -604, F 13

~ VM Ayres, ECE 802 -604, F 13

~ VM Ayres, ECE 802 -604, F 13

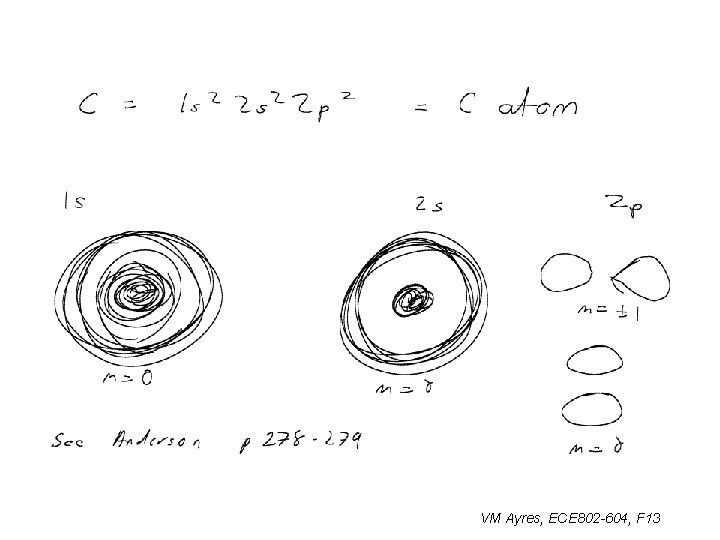
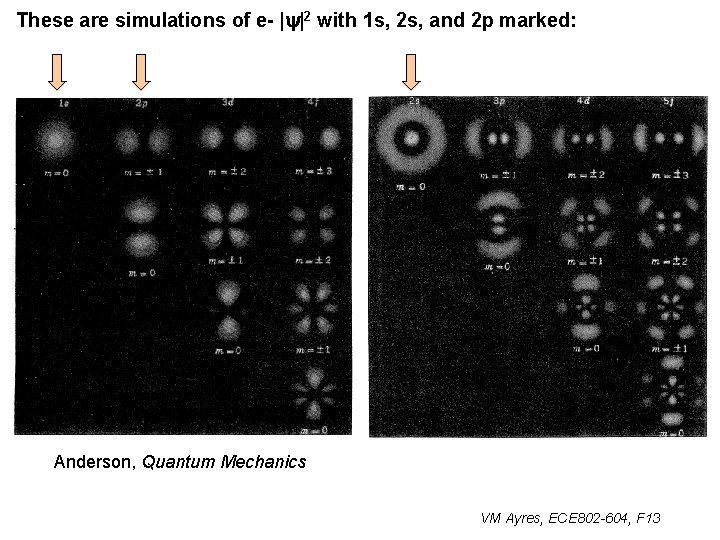
These are simulations of e- |y|2 with 1 s, 2 s, and 2 p marked: Anderson, Quantum Mechanics VM Ayres, ECE 802 -604, F 13

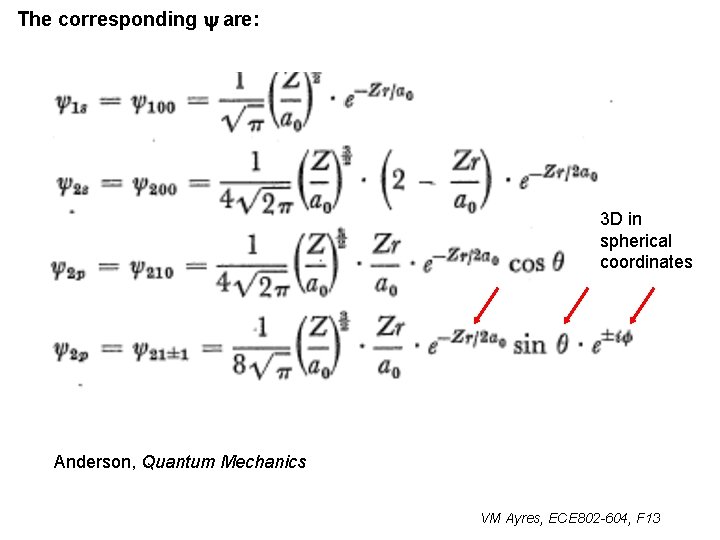
The corresponding y are: 3 D in spherical coordinates Anderson, Quantum Mechanics VM Ayres, ECE 802 -604, F 13

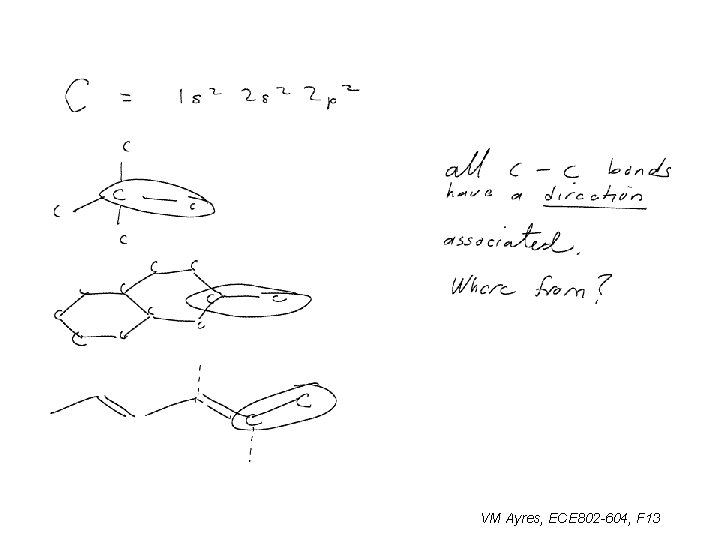
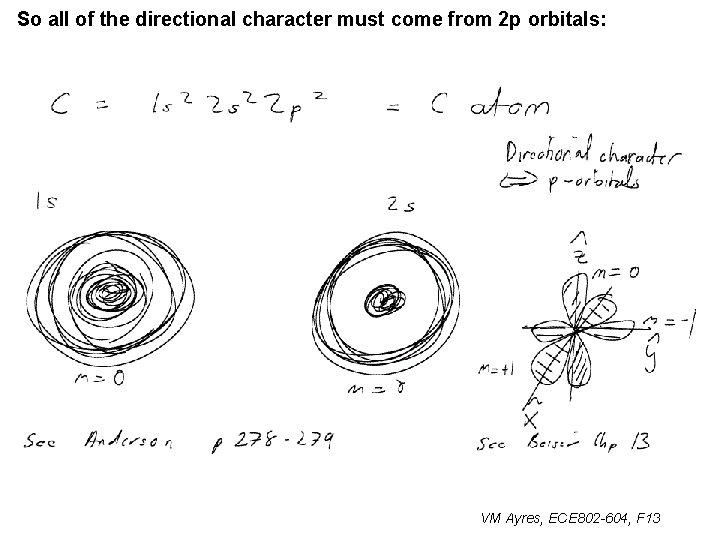
So all of the directional character must come from 2 p orbitals: ~ VM Ayres, ECE 802 -604, F 13

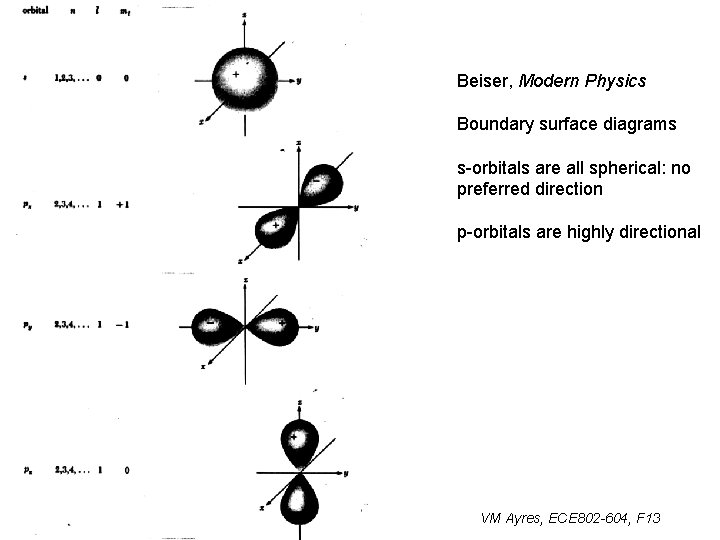
Beiser, Modern Physics Boundary surface diagrams s-orbitals are all spherical: no preferred direction p-orbitals are highly directional VM Ayres, ECE 802 -604, F 13

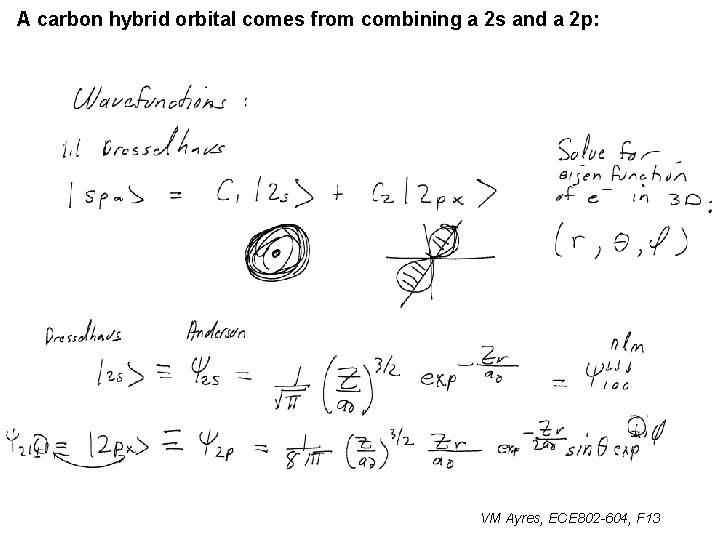
A carbon hybrid orbital comes from combining a 2 s and a 2 p: VM Ayres, ECE 802 -604, F 13

sp 1 (sp) hybridization: l sp 1 hybridization – Use orthonormality VM Ayres, ECE 802 -604, F 13

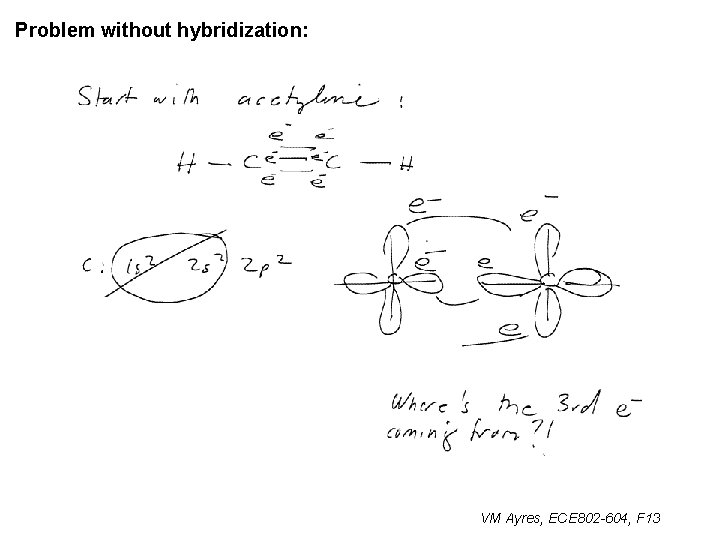
Problem without hybridization: ~ VM Ayres, ECE 802 -604, F 13

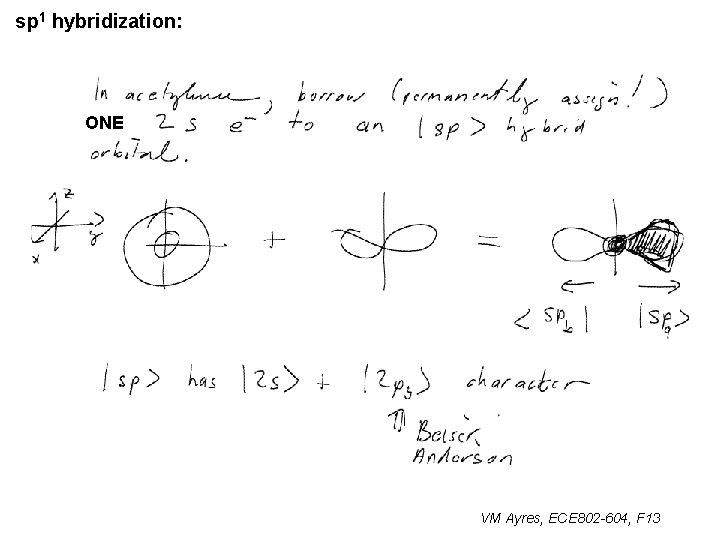
sp 1 hybridization: ONE ~ VM Ayres, ECE 802 -604, F 13

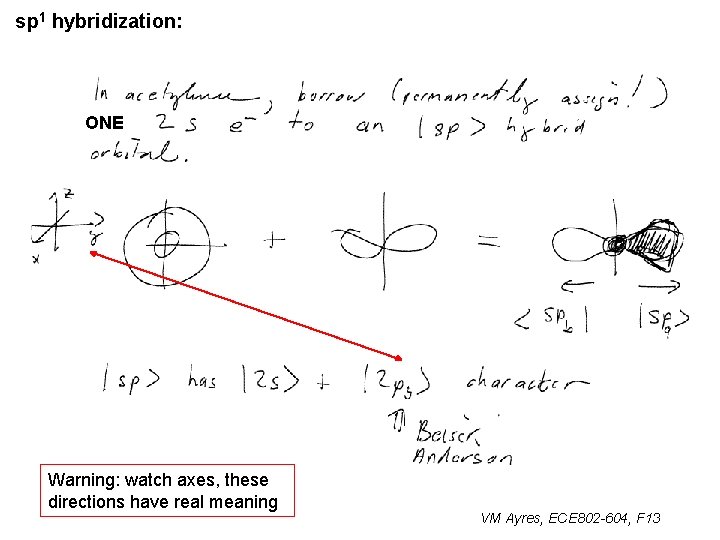
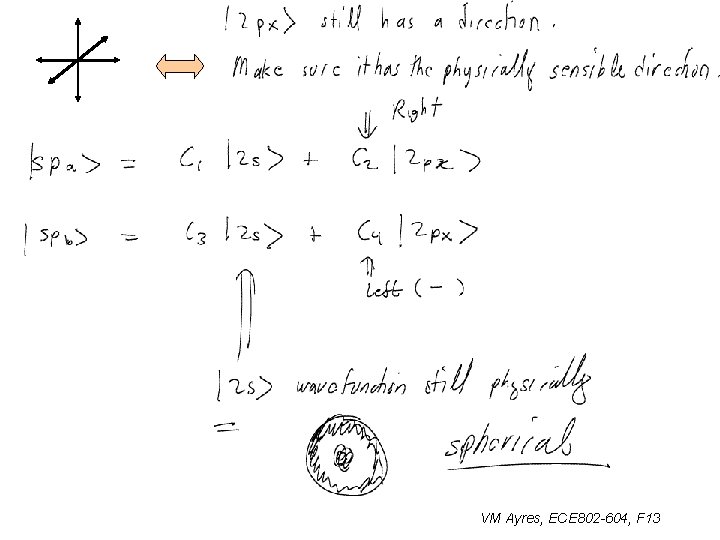
sp 1 hybridization: ONE ~ Warning: watch axes, these directions have real meaning VM Ayres, ECE 802 -604, F 13

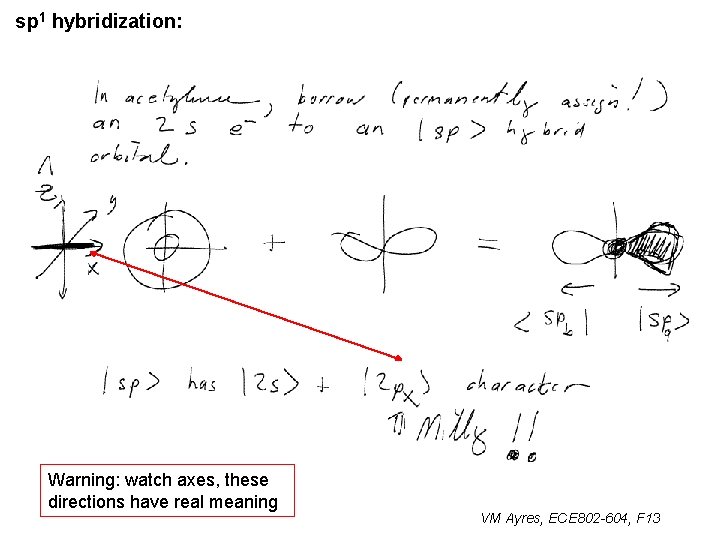
sp 1 hybridization: ~ Warning: watch axes, these directions have real meaning VM Ayres, ECE 802 -604, F 13

VM Ayres, ECE 802 -604, F 13

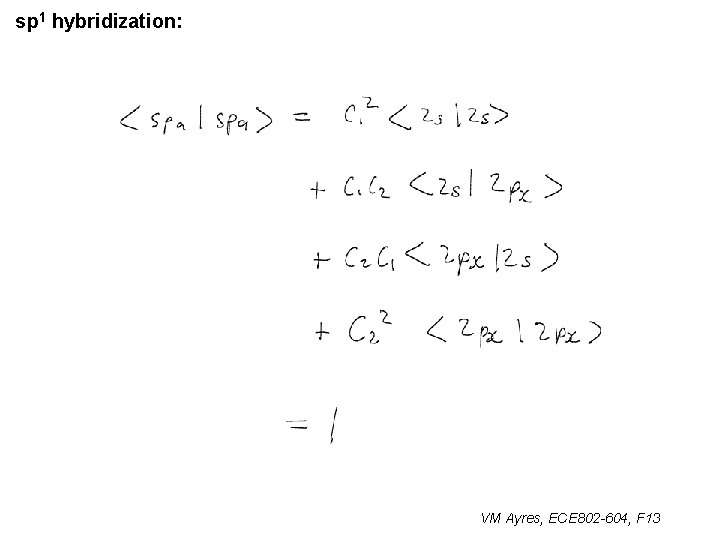
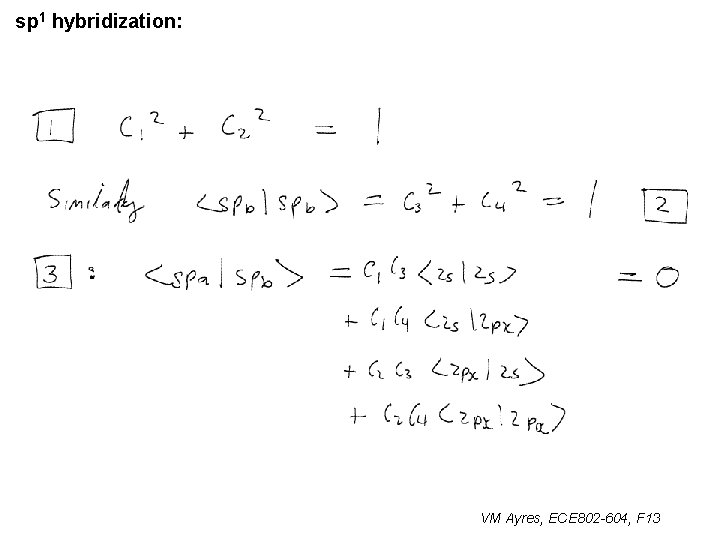
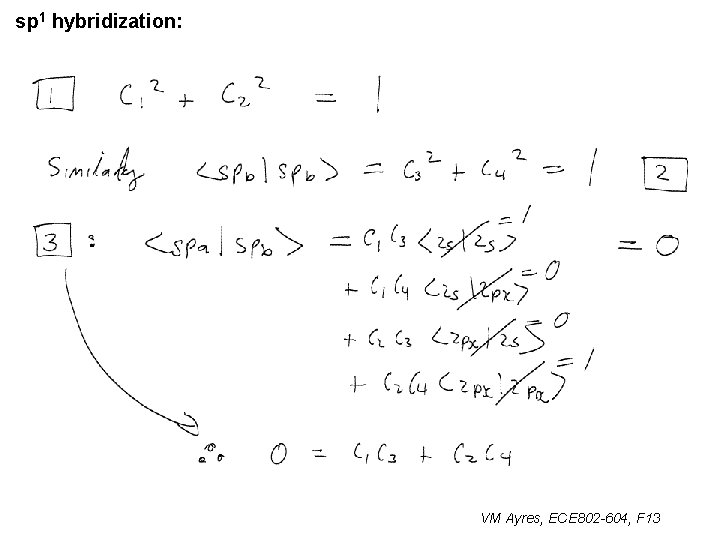
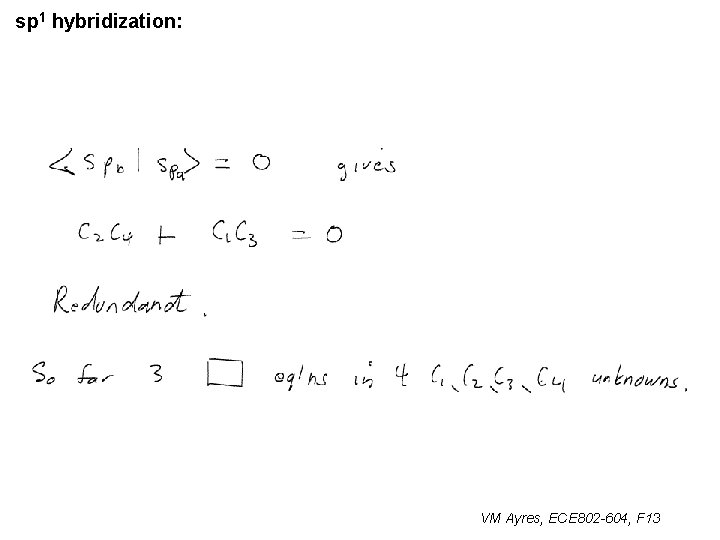
sp 1 hybridization: VM Ayres, ECE 802 -604, F 13

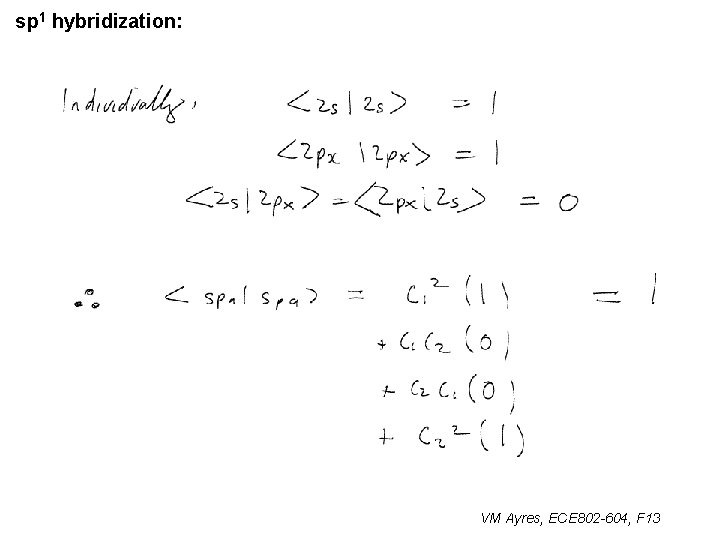
sp 1 hybridization: VM Ayres, ECE 802 -604, F 13

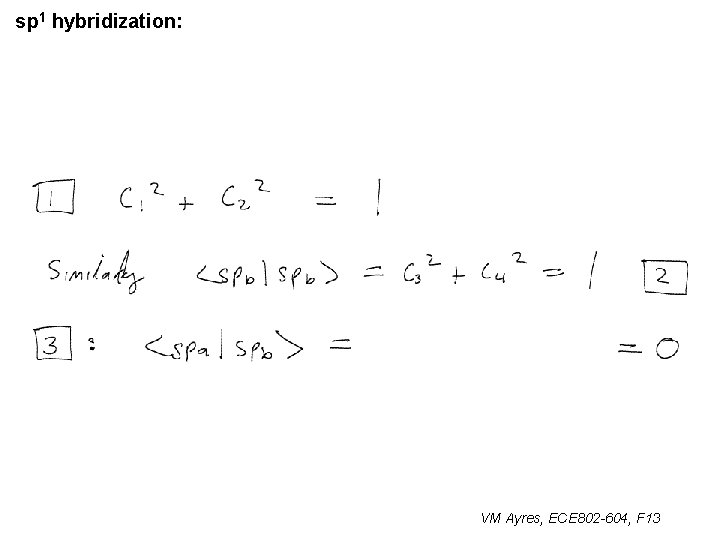
sp 1 hybridization: VM Ayres, ECE 802 -604, F 13

sp 1 hybridization: VM Ayres, ECE 802 -604, F 13

sp 1 hybridization: VM Ayres, ECE 802 -604, F 13

sp 1 hybridization: VM Ayres, ECE 802 -604, F 13

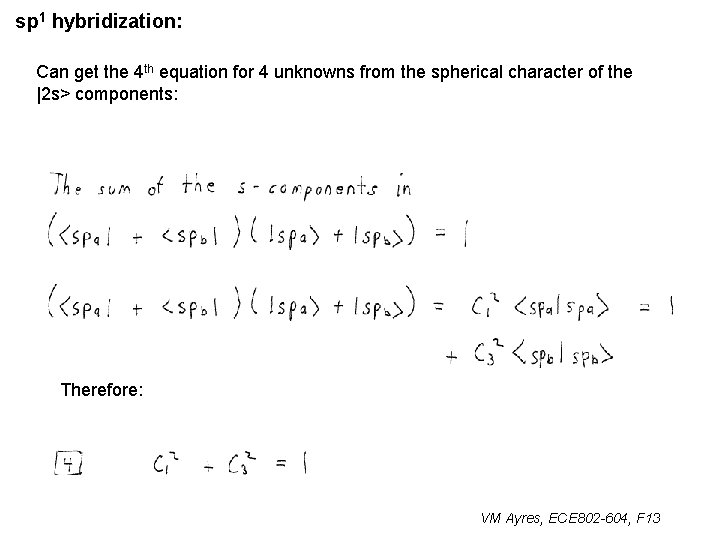
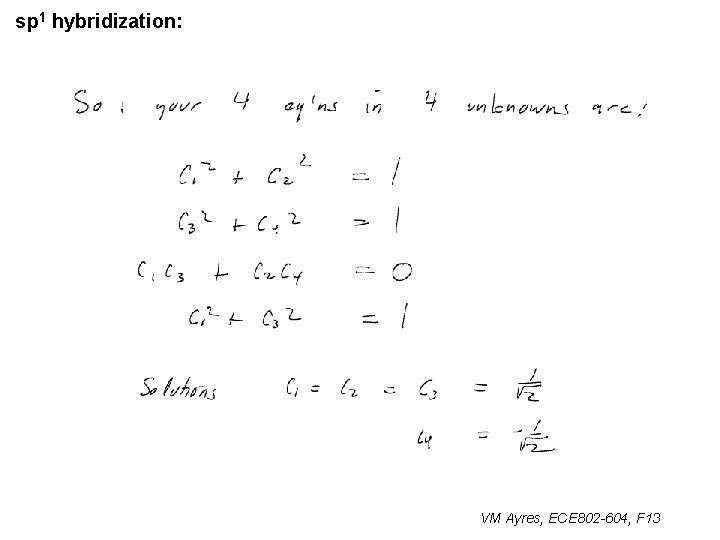
sp 1 hybridization: Can get the 4 th equation for 4 unknowns from the spherical character of the |2 s> components: Therefore: VM Ayres, ECE 802 -604, F 13

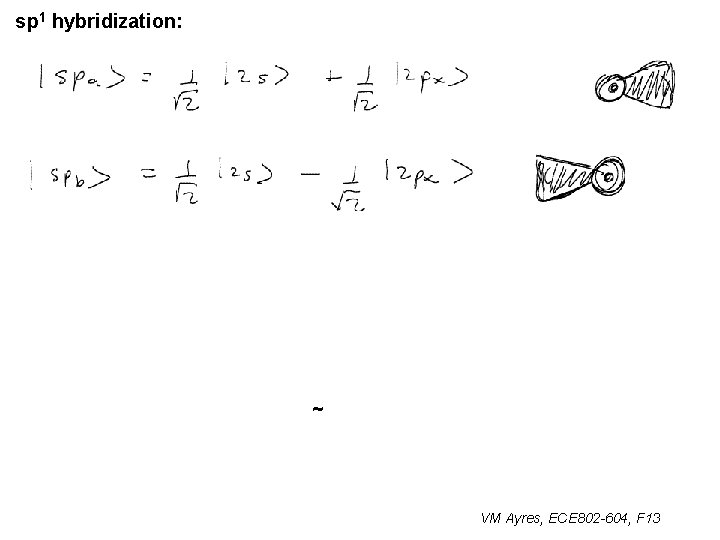
sp 1 hybridization: ~ VM Ayres, ECE 802 -604, F 13

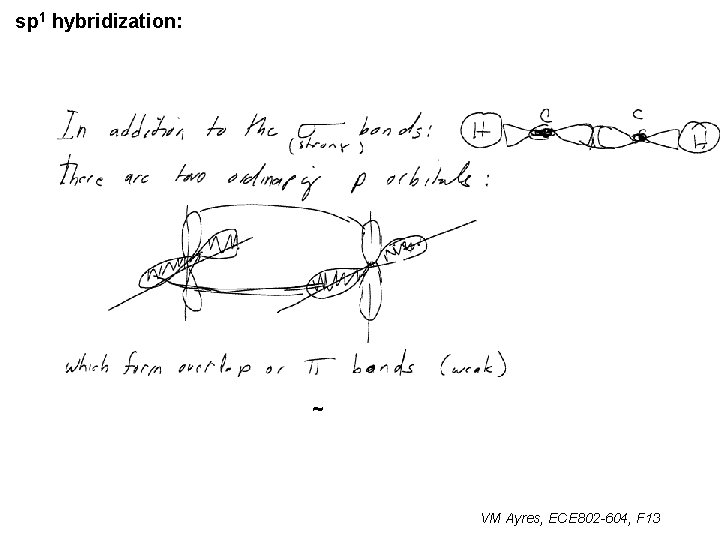
sp 1 hybridization: ~ VM Ayres, ECE 802 -604, F 13

sp 1 hybridization: ~ VM Ayres, ECE 802 -604, F 13

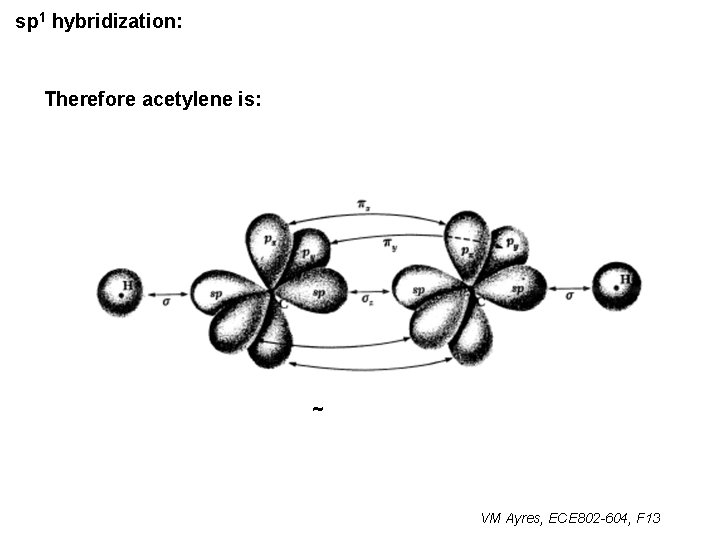
sp 1 hybridization: Therefore acetylene is: ~ VM Ayres, ECE 802 -604, F 13