DMT 125 Materials Science Chapter 7 Polymers Part








- Slides: 27

DMT 125 Materials Science Chapter 7: Polymers Part 1

Contents Overview Polymerization reactions Crystallinity & stereoisomerism Thermoplastics Thermosetting plastics (thermosets) Deformation & strengthening Creep & fracture

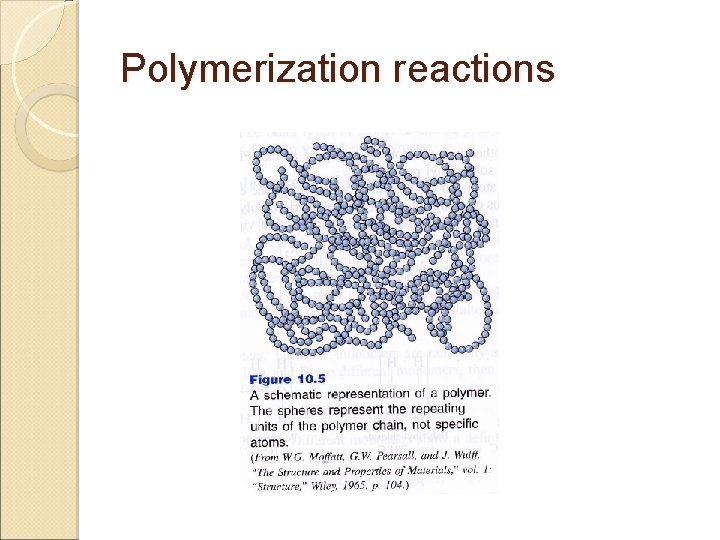
Overview Polymer means “many parts” Contains many chemically bonded parts or units that themselves are bonded together to form a solid 2 industrial important polymeric materials: ◦ Plastics: large & varied group of synthetic materials that are processed by forming or molding into shape Thermoplastics Thermosetting plastics (thermosets) ◦ Elastomers: can be elastically deformed a large amount when a force is applied to them & can return to their original shape when the force is released

Overview Thermoplastics ◦ Require heat to make them formable & after cooling, retain the shape they were formed into ◦ Can be reheated & reformed into new shapes a number of times without significant change in their properties ◦ Consist of very long main chains of carbon atoms covalently bonded together ◦ Long molecular chains are bonded to each other by secondary bonds

Overview Thermosets ◦ Cannot be remelted & reformed into another shape but degrade or decompose upon being heated too high a temperature ◦ Cannot be recyled ◦ Heat is required to permanently set the plastic ◦ Consist of a network of carbon atoms covalently bonded to form a rigid solid

Overview Advantages ◦ Relatively low in cost ◦ Elimination of parts through engineering design with plastics ◦ Elimination of many finishing operations ◦ Simplified assembly ◦ Weight savings ◦ Noise reduction ◦ Elimination of the need for lubrication of some parts

Polymerization reactions Most thermoplastics are synthesized by the process of chain-growth polymerization Small molecules are covalently bonded together to form very long molecular chains Monomers – simple molecules that are covalently bonded into long chains Polymer – long chain molecule formed from monomer units

Polymerization reactions Ethylene molecule, C 2 H 4 is chemically bonded by a double covalent bond between the carbon atoms & by 4 single covalent bonds between the carbon & hydrogen atoms Chain polymerization – covalently bonded together to form a polymer ◦ Polyethylene – polymer produced by the polymerization of ethylene

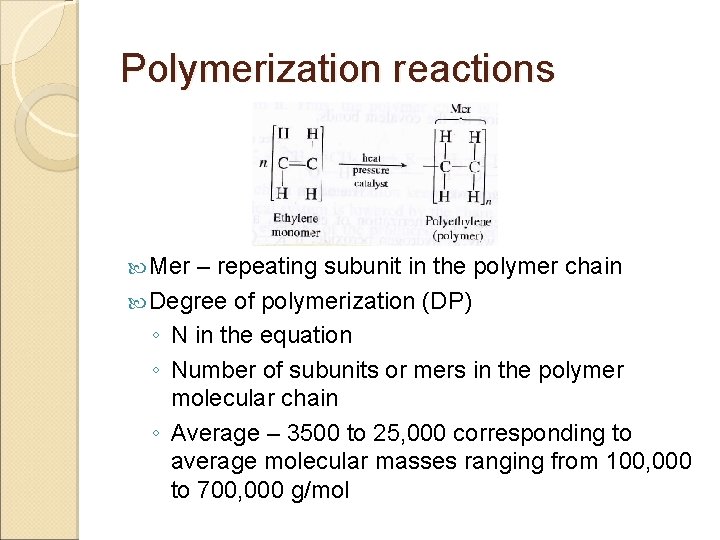
Polymerization reactions Mer – repeating subunit in the polymer chain Degree of polymerization (DP) ◦ N in the equation ◦ Number of subunits or mers in the polymer molecular chain ◦ Average – 3500 to 25, 000 corresponding to average molecular masses ranging from 100, 000 to 700, 000 g/mol

Polymerization reactions 3 steps of chain polymerization ◦ initiation ◦ Propagation ◦ Termination Initiation ◦ Free radical – atom that has an unpaired electron that can covalently bond to an unpaired electron of another atom or molecule

Polymerization reactions Example: Hydrogen peroxide, H 2 O 2 ◦ Upon heating, this peroxide can decompose into 2 free radicals ◦ Benzoyl peroxide – organic peroxide used to initiate some chain polymerization reactions Propagation ◦ Process of extending the polymer chain by the successive addition of monomer units Termination ◦ Can occur by the addition of a terminator free radical or when 2 chains combine ◦ Trace amounts if impurities may terminate the polymer chain

Polymerization reactions Thermoplastics consist of chains of polymers of many different lengths, each of which has its own molecular weight & degree of polymerization For a monomer to polymerize, it must have at least 2 active chemical bonds When it has 2 active bonds, it can react with 2 other monomers & by repetition of the bonding, others can form a long chain or linear polymer 3 D network molecules can be built up

Polymerization reactions Functionality – number of active bonds a monomer has Bifunctional – a monomer that uses 2 active bonds for the polymerization of long chains ◦ Example: ethylene Trifunctional – uses 3 active bonds to form a network polymeric material ◦ Example: phenol Bonding between the long molecular chains in polyethylene consists of weak, permanent dipole secondary bonds

Polymerization reactions

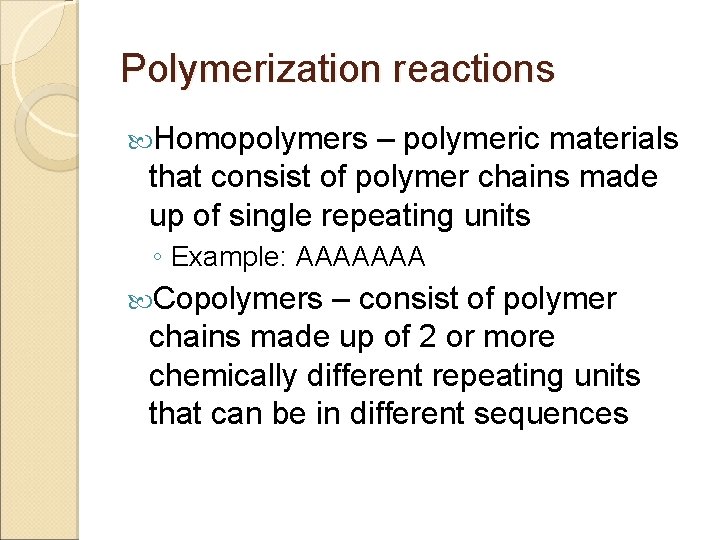
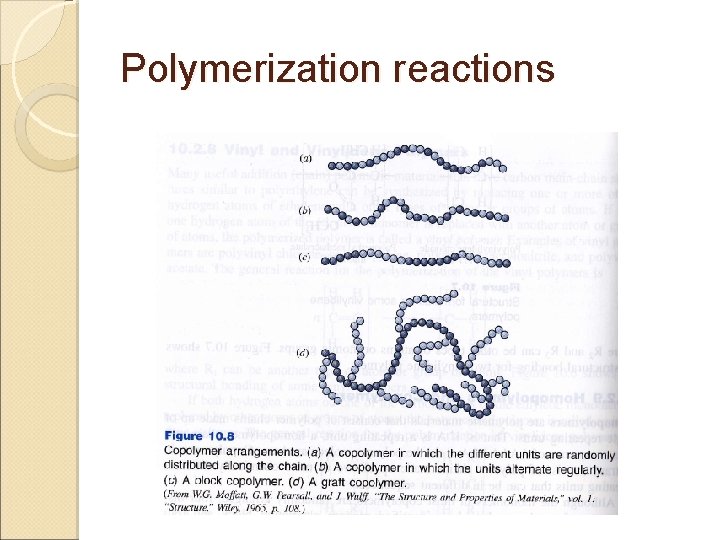
Polymerization reactions Homopolymers – polymeric materials that consist of polymer chains made up of single repeating units ◦ Example: AAAAAAA Copolymers – consist of polymer chains made up of 2 or more chemically different repeating units that can be in different sequences

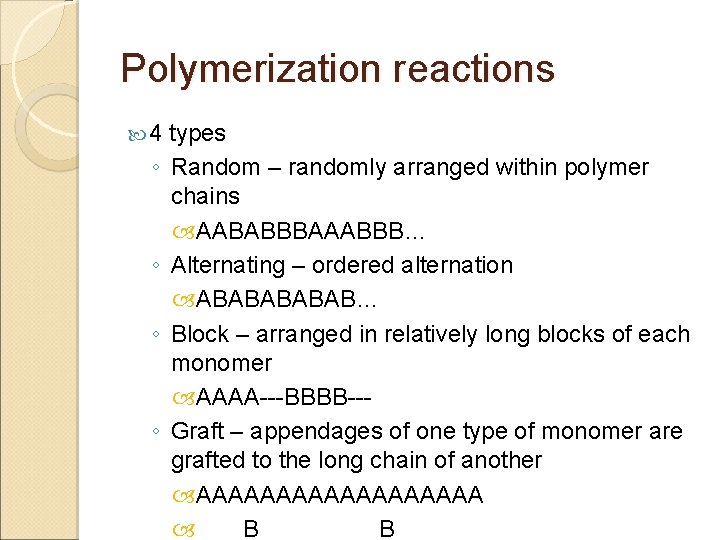
Polymerization reactions 4 ◦ ◦ types Random – randomly arranged within polymer chains AABABBBAAABBB… Alternating – ordered alternation ABABAB… Block – arranged in relatively long blocks of each monomer AAAA---BBBB--Graft – appendages of one type of monomer are grafted to the long chain of another AAAAAAAAA B B

Polymerization reactions

Polymerization reactions Other methods of polymerization ◦ Stepwise Monomers chemically react with each other to produce linear polymers ◦ Network Involving a chemical reactant with more than 2 reaction sites, a 3 D network material can be produced

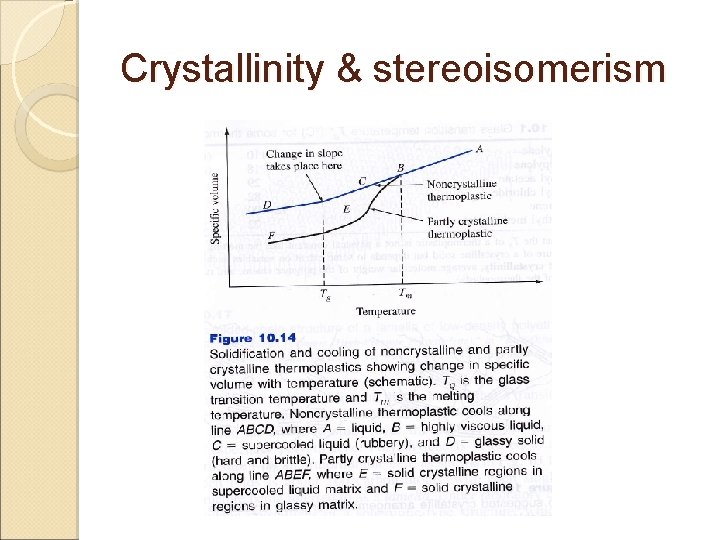
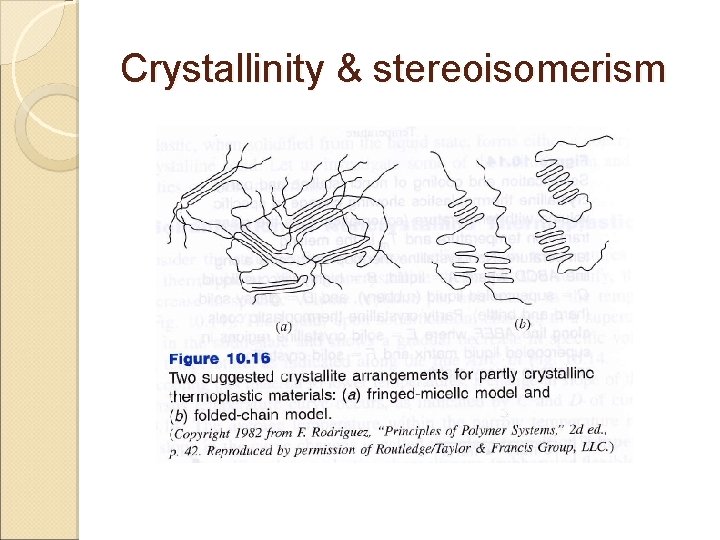
Crystallinity & stereoisomerism Thermoplastic when solidified from liquid state will form either ◦ Noncrystalline ◦ Partly crystalline solid Noncrystalline ◦ When noncrystalline thermoplastics solidify, there is on sudden decrease in specific volume as the temperature is lowered ◦ The liquid upon solidification, changes to a supercooled liquid that is in the solid state & shows a gradual decrease in specific volume with decreasing temperature

Crystallinity & stereoisomerism Upon cooling this material to lower temperatures, a change in slope of the specific volume versus temperature curve occurs Glass transition temperature, Tg ◦ Average temperature within the narrow temperature range over which slope in the curve changes ◦ Above Tg, viscous behavior is displayed (rubbery) ◦ Below Tg, glass-brittle behavior is displayed (due to molecular chain motion

Crystallinity & stereoisomerism ◦ Tg might be considered a DBT ◦ Example: polypropylene Partly crystalline ◦ When this material solidifies & cool, sudden decrease in specific volume occurs ◦ This is due to more efficient packing of the polymer chains into crystalline regions ◦ As cooling continued, glass transition is encountered ◦ In going through glass transition, supercooled liquid matrix transforms to glassy state ◦ Structure of thermoplastics consists of crystalline regions in a glassy noncrystalline matrix

Crystallinity & stereoisomerism

Crystallinity & stereoisomerism

Crystallinity & stereoisomerism Degree of crystallinity in partly crystalline ranges from 5 to 95 percent of their total volume Complete crystallization is not achievable even with polymeric materials that are highly crystallizable due to: ◦ Molecular entanglements ◦ Crossovers Degree of crystallinity increase,

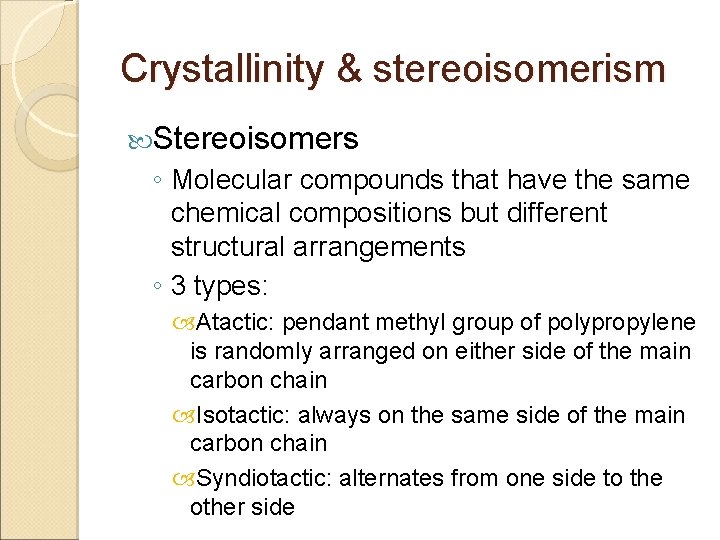
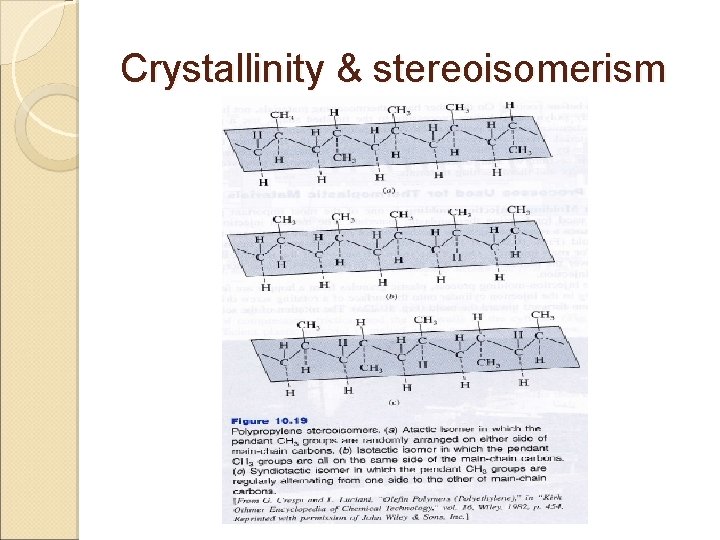
Crystallinity & stereoisomerism Stereoisomers ◦ Molecular compounds that have the same chemical compositions but different structural arrangements ◦ 3 types: Atactic: pendant methyl group of polypropylene is randomly arranged on either side of the main carbon chain Isotactic: always on the same side of the main carbon chain Syndiotactic: alternates from one side to the other side

Crystallinity & stereoisomerism

END End of Part 1 Copyright © Mr. Mohd. Azarulsani b. Md. Azidin March 2011