Polymers A level Product Design Unit 2 Material





















- Slides: 36

Polymers A level Product Design Unit 2


Material Properties Uses

Plasticity material is a material property & not a ◦ “the ability to be shaped or formed” Plastic Materials ◦ Bone ◦ Horn ◦ Clay ◦ Concrete A polymer is a certain type of material There are natural & synthethic polymers ◦ We are only interested in synthetic polymers Whats the difference between a plastic & a polymer ?

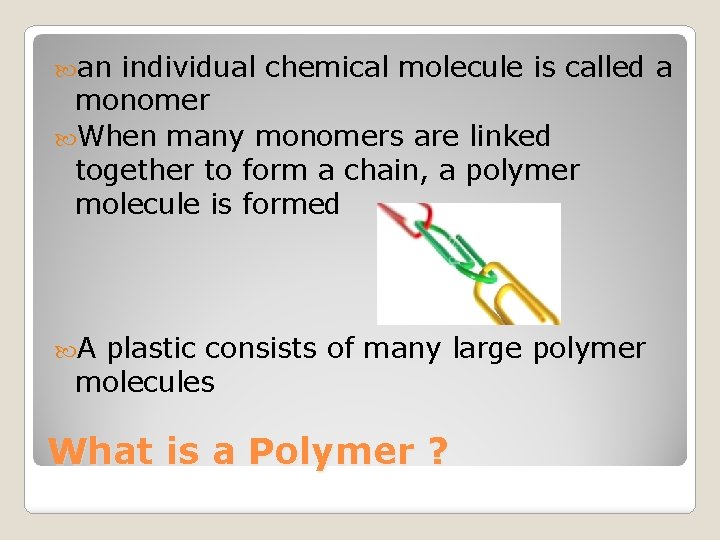
an individual chemical molecule is called a monomer When many monomers are linked together to form a chain, a polymer molecule is formed A plastic consists of many large polymer molecules What is a Polymer ?

A chemical reaction forms the polymer molecule (makes the chain) ◦ polymerisation Normally, chains are randomly arranged and form a 3 d pattern ◦ Imagine a piece of string scrunched up into a ball What is a polymer ?

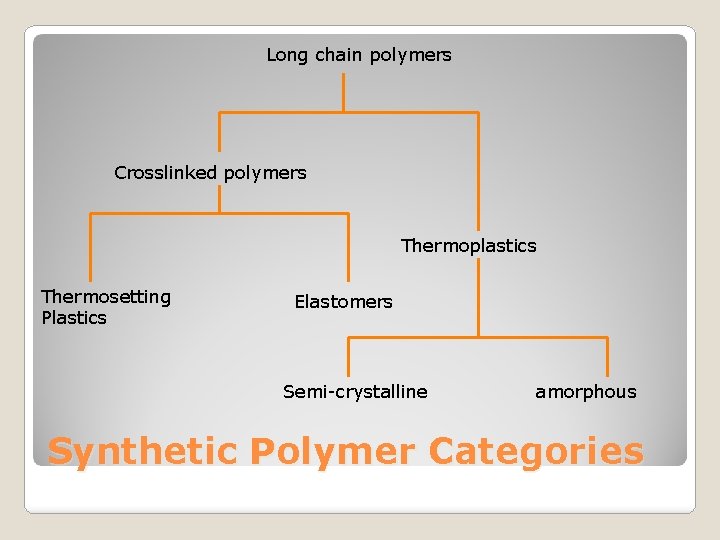
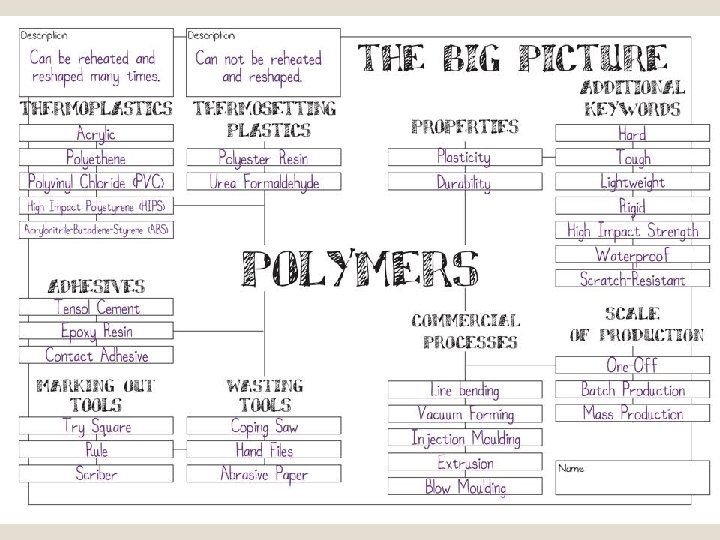
Long chain polymers Crosslinked polymers Thermoplastics Thermosetting Plastics Elastomers Semi-crystalline amorphous Synthetic Polymer Categories

Very strong ◦ Strong bonds between chains (crosslinks) and intra-chain Level of crosslinking determines Tg Thermosetting plastics (eg Araldite) are highly ◦ Chemical reaction forms the links ◦ One way process – cannot be reversed ◦ Will not soften with heat - very high Tg ◦ Araldite is a tradename for a resin based polymer ◦ The resin is mixed with an activator to start curing Materials like rubbers are lightly crosslinked ◦ Tg is below freezing ie. Is in a rubbery state at all temperatures above 0 ◦ Below Tg, material is hard & brittle (useless) Crosslinked Polymers linked

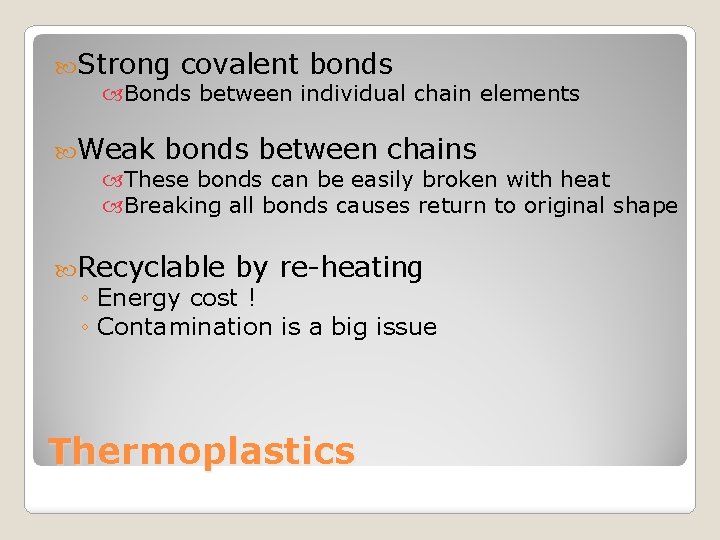
Strong covalent bonds Bonds between individual chain elements Weak bonds between chains These bonds can be easily broken with heat Breaking all bonds causes return to original shape Recyclable by re-heating ◦ Energy cost ! ◦ Contamination is a big issue Thermoplastics

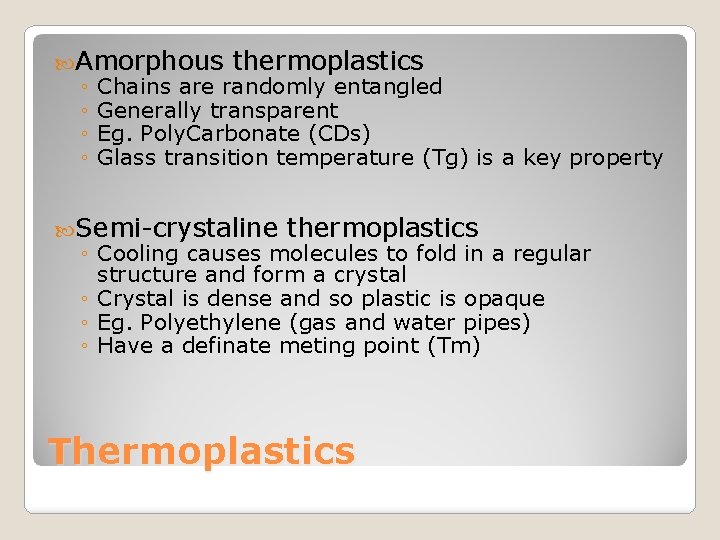
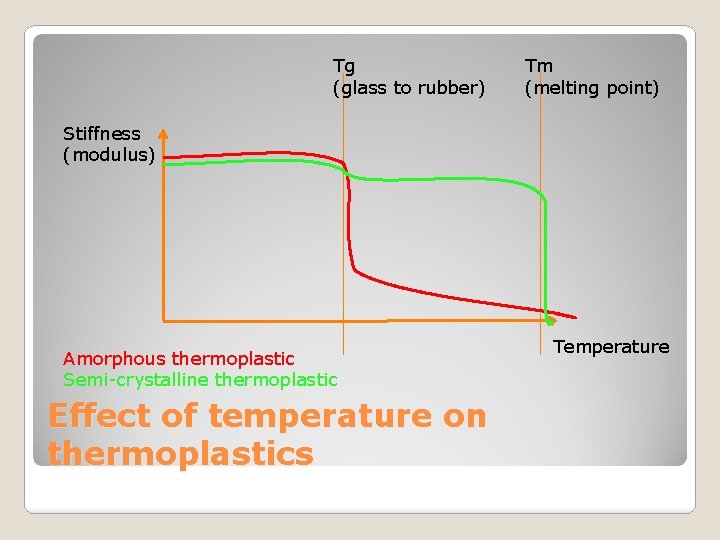
Amorphous thermoplastics ◦ Chains are randomly entangled ◦ Generally transparent ◦ Eg. Poly. Carbonate (CDs) ◦ Glass transition temperature (Tg) is a key property Semi-crystaline thermoplastics ◦ Cooling causes molecules to fold in a regular structure and form a crystal ◦ Crystal is dense and so plastic is opaque ◦ Eg. Polyethylene (gas and water pipes) ◦ Have a definate meting point (Tm) Thermoplastics

The more crystalline a material is: ◦ The stronger it gets ◦ It has more resistance to solvents Solvents need to dissolve into a material Amorphous plastics have greater free space ◦ Higher density ◦ Higher modulus (stiffness) ◦ Higher melting point (Tm) ◦ The lower its transparency ◦ Reduced impact resistance (brittle) ◦ Reduced ductility Ability to be deformed without cracking Semi-Crystalline Thermoplastics

Tg (glass to rubber) Tm (melting point) Stiffness (modulus) Amorphous thermoplastic Semi-crystalline thermoplastic Effect of temperature on thermoplastics Temperature

15% of plastic production Polyurethane Urea formaldehyde (UF) Phenol Formaldehyde (PF) Epoxy Resin Melamine formaldhyde ◦ Carpet underlay ◦ bed foam ◦ Mains plugs/sockets/light switches ◦ Tradename: Bakelite ◦ Tradename: Araldite ◦ Used as coatings & adhesives or to form composites eg. Carbon fibre ◦ Can cause health problems ◦ Work top laminates ◦ Office furniture Thermosetting Plastics

Oil (carbon) ◦ 4% of crude oil is used for plastics Sustainable sources ◦ Wheat & corn ◦ Carrot (biopolymers) Recycling ◦ Difficult: all recycled items must be of the same polymer ◦ Mixed plastics can be used for low level products such as road surfacing, wood replacement Sources of Polymers

Poly. Propylene (PP) ◦ Tupperware (lunch boxes) Poly Vinyl Chloride ◦ Window frames (PVC) Poly. Styrene (PS) ◦ Packaging ◦ Yoghurt pots / vending machine cups Carbon Based Polymers

Acrylic ◦ Paint ◦ Point of sale displays ◦ Baths ◦ Car lights HDPE (High Density PE) ◦ Bottles (biggest application) ◦ milk bottles (largest bottle sector) LDPE (low Density PE) ◦ Supermarket carrier bags ◦ Packaging film (eg. cling film) ◦ Washing up liquid bottles PET (PE Terephthalate) ◦ fizzy drinks bottles ◦ Carbonation makes HDPE unsuitable ◦ Space blankets ABS (Acrylonitrile Butadiene Styrene) ◦ Car batteries ◦ Calculators / mobile phones ◦ Safety helmets Carbon Based Polymers

Amorphous Good resistance against medium temperatures (< 1000 C) Hard tough antistatic. good resistance against chemicals. Poor resistance to UV-light Can be painted Min temp: Max Temp: Glass Temp: -250 C 800 C 1100 C Material Properties: ABS

Very light AKA: Polyester Can stand high tensile stress hard, stiff, strong dimensionally stable absorbs very little water good chemical resistance except to alkalis Medium resistance to UV most commonly recycled plastic Semi-crystaline Can degrade & become discoloured during heat treatment Adds an unwanted flavour to food (can be compensated for at addition cost) Min Temp: Max Temp: Glass transition temperature: Melting point: ◦ Often used for magnetic tape ◦ drinks bottles are made from PET ◦ Must be rapid cooled to make it amorphous & transparent -500 C 1700 C 82 o. C. 250 o. C. Material Properties: PET

Excellent for any food related products ◦ Not microwaveable Machines extremely well (cut, bond, drill etc. ) Good chemical resistance Good impact resistance light weight Poor UV tolerance very low moisture absorption high tensile strength Not a good candidate for gluing. Primarily used for blow moulding Colours fade over time Min Temp: Max temp: Melting point: Glass temp: Applications ◦ ◦ -1000 C 1100 C 1300 C -95 Milk bottles trays and tanks pipe fittings, wear plates, hinges cutting boards. Material Properties: HDPE

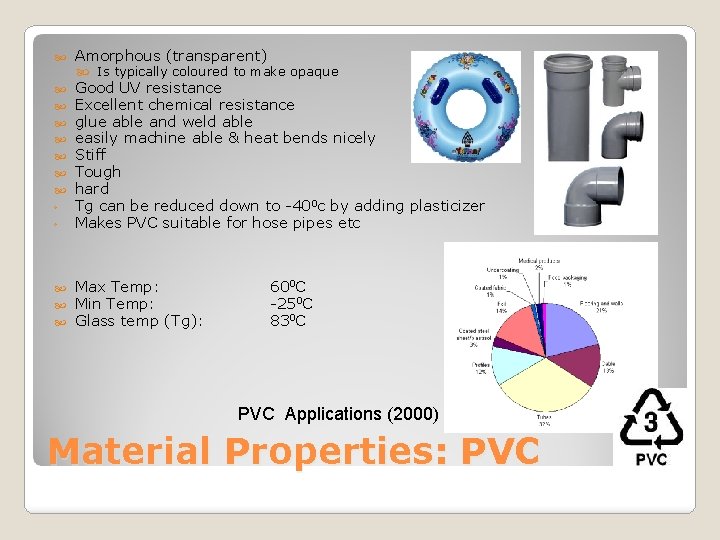
Amorphous (transparent) ◦ ◦ Good UV resistance Excellent chemical resistance glue able and weld able easily machine able & heat bends nicely Stiff Tough hard Tg can be reduced down to -400 c by adding plasticizer Makes PVC suitable for hose pipes etc Max Temp: Min Temp: Glass temp (Tg): Is typically coloured to make opaque 600 C -250 C 830 C PVC Applications (2000) Material Properties: PVC

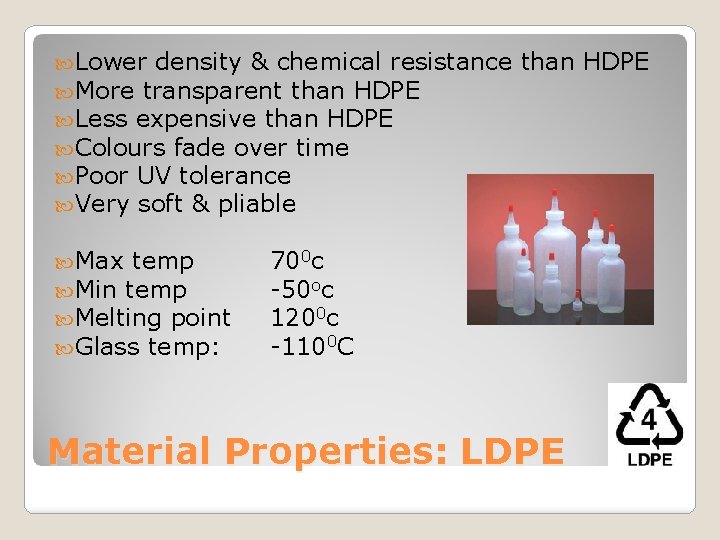
Lower density & chemical resistance More transparent than HDPE Less expensive than HDPE Colours fade over time Poor UV tolerance Very soft & pliable Max temp Min temp Melting point Glass temp: than HDPE 700 c -50 oc 1200 c -1100 C Material Properties: LDPE

Poor UV resistance Translucent (semi-crystaline) Rigid Very light Excellent chemical resistance Microwaveable Max temp Min temp Melting point: Glass temp: food storage applications Medical applications (syringes) Carpets 1350 C 00 C 1700 C -180 C Material Properties: PP

Amorphous Flammable Excellent thermal insulation ◦ Used in fridge linings Solid: ◦ Light, Hard, Stiff, Brittle Expanded: ◦ Light, Bouyant, Crumbles Min temp: Max temp: Glass transition point: -400 C 600 c 1000 C Material Properties: PS

amporphous Trade names: perspex & plexiglass Weather resistant (Can withstand sunlight for long durations) Difficult to recycle ◦ Can be done but is very expensive (not cost effective) Stiff (Flexible compared to glass) Less breakable than glass Scratches easily brittle Resistant to most chemicals and industrial fumes Can be cut by various methods Corrosion resistant Good electrical insulator Min Temp: Max temp: Glass temperature : 5 0 C 410 C 1100 C Material Properties: Acrylic

Some acrylic Products

Safety ◦ Many chemical plastisizers contain oestrogen Gender bending chemical ◦ Some plastics (eg. PET) degrade & emit cancerous material over time Life Cycle Sustainability Energy for manufacture Plastic is itself a fuel and can be incinerated Plastic can also be manufactured into a synthetic oil ◦ Carbon based plastics take thousands of years to degrade ◦ Biodegradeable plastics are being researched now ◦ What would land used to grow organic polymers normally be used for ? ◦ Are organic polymers at the expense of food grade crop ? ◦ Today: a 500 ML water bottle takes 3 fl/oz of crude oil to produce (+ energy to manufacture) ◦ All polymers (apart from elastomers) require heat to make them plastic ◦ All plastics must be sorted and washed before being recycled ◦ Where does this energy come from ? ◦ Toxic fumes are a consequence Issues

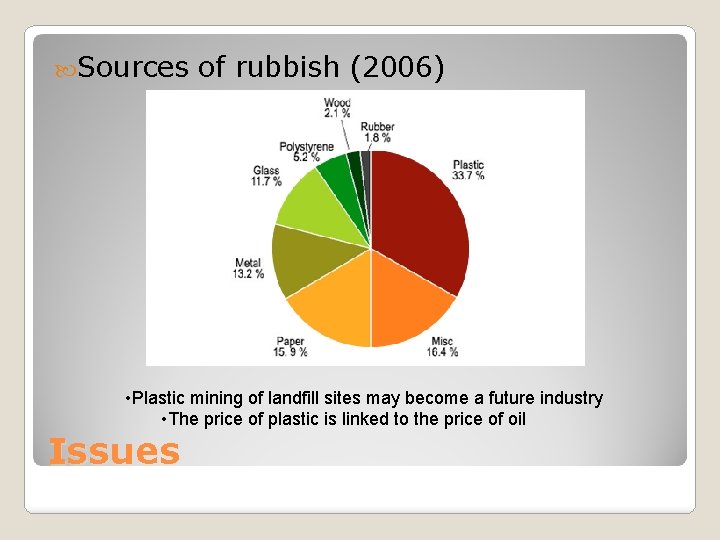
Sources of rubbish (2006) • Plastic mining of landfill sites may become a future industry • The price of plastic is linked to the price of oil Issues

http: //www. dynalabcorp. com/files/Use and Care of Plastics. pdf Useful Web Sites

Guess the plastic ….

Guess the plastic …. .

Guess the plastic ……

Guess the plastic ….

ABS HDPE PVC Acrylic Answers!

Exam Question. . .

Exam Question. . .
