Option 2 A Materials polymers Polymers A polymer







- Slides: 19

Option 2 A Materials (polymers)

Polymers • A polymer is a large molecule made up of many identical repeating units called monomers. • Alkenes are the raw materials in the industrial manufacture of polymers. • In the formation of poly(ethene), the ethene molecule adds to itself to form a long chain consisting of repeating –CH 2 - units. • Poly(ethene), or polythene, is an example of an addition polymer.

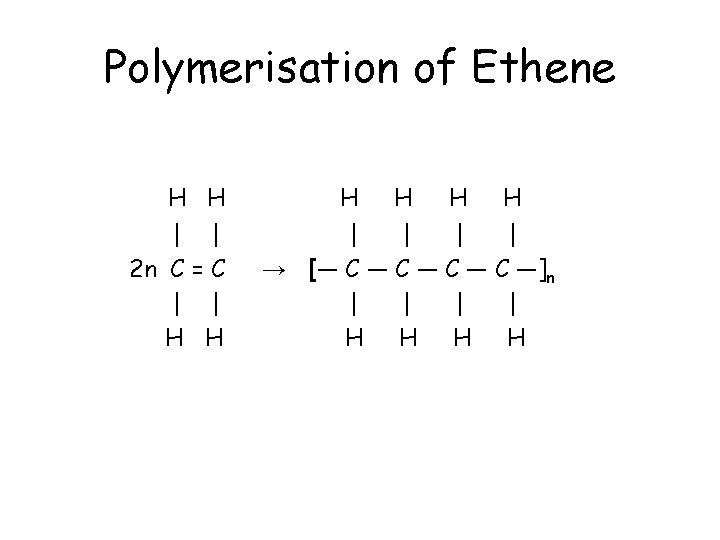
Polymerisation of Ethene H H | | 2 n C = C | | H H H | | → [― C ― C ―]n | | H H

Low-density Polythene • Significant degree of branching prevents molecules from packing closely together. • Density of the polymer is reduced. • Very flexible polymer, melting at 360 K. • Used to make plastic bags and squeezy bottles.

Discovery of Polythene • Discovered by accident by Fawcett and Gibson in 1933. • After an experiment at high pressure and temperature involving ethene, a white waxy solid was formed. • This was recognized to be polythene. • Later it was found that if a little oxygen was present with ethene, low-density polythene was formed.

High-density Polythene • Very little branching allows molecules to pack closely together. • Density of the polymer is increased. • More rigid polymer, melting at 400 K. • Used to make buckets and lunch boxes.

Discovery of High-density Polythene • Ziegler made polythene using organic catalysts that contains metal atoms in their molecules. • These catalysts allowed polythene to be made at lower pressure, thus more cheaply. • The polythene produced was relatively rigid with a higher MP, ie high-density polythene.

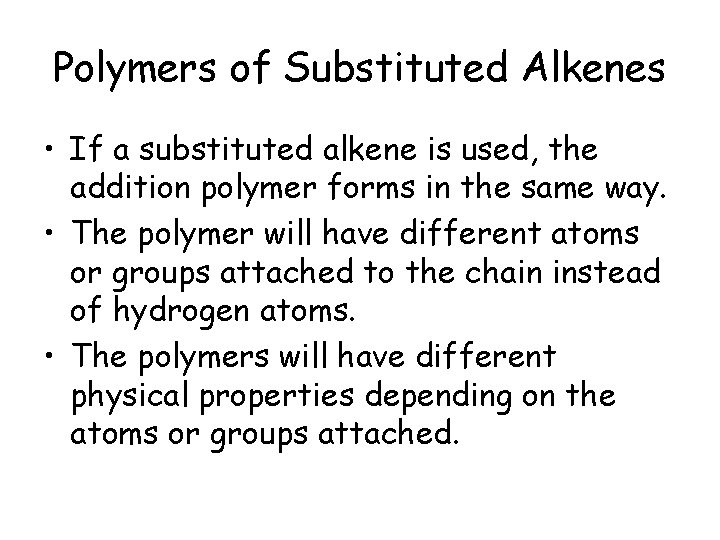
Polymers of Substituted Alkenes • If a substituted alkene is used, the addition polymer forms in the same way. • The polymer will have different atoms or groups attached to the chain instead of hydrogen atoms. • The polymers will have different physical properties depending on the atoms or groups attached.

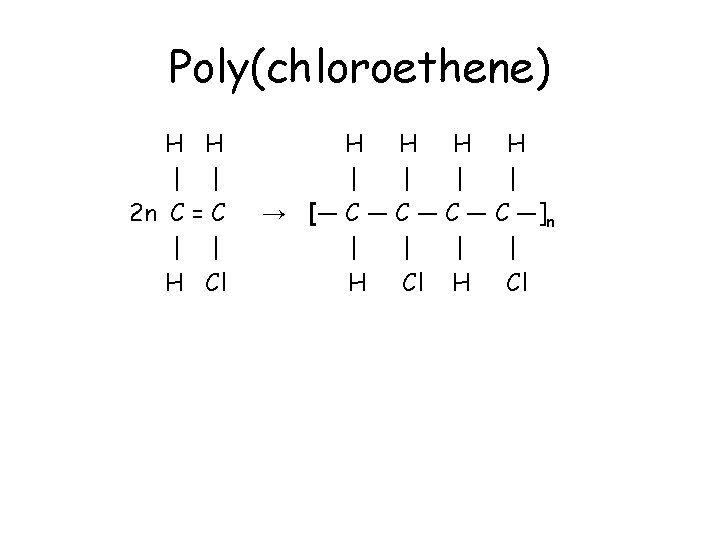
Poly(chloroethene) • Chloroethene has a chlorine atom replacing one hydrogen in the ethene molecule. • Chloroethene, CH 2=CHCl, polymerises to form poly(chloroethene). • Compared to poly(ethene), every second carbon in the chain has a chlorine atom attached. • The polymer is commonly known as PVC, and is used to make windows and gutters.

Poly(chloroethene) H H | | 2 n C = C | | H Cl H H | | → [― C ― C ―]n | | H Cl

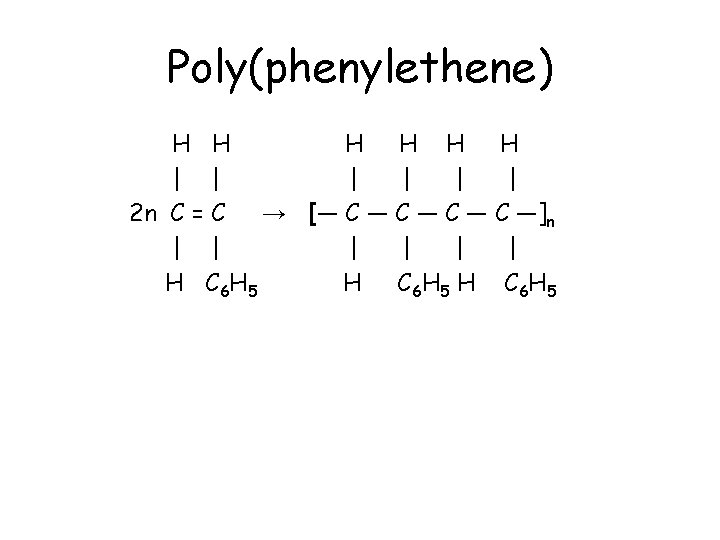
Poly(phenylethene) • Phenylethene has a phenyl group, C 6 H 5 (a benzene ring with one H removed), replacing one hydrogen in the ethene molecule. • CH 2=CHC 6 H 5 polymerises to form poly(phenylethene). • Compared to poly(ethene), every second carbon in the chain has a phenyl group attached. • The polymer is commonly known as polystyrene, and is used for insulation and packaging.

Poly(phenylethene) H H H | | | 2 n C = C → [― C ― C ―]n | | | H C 6 H 5

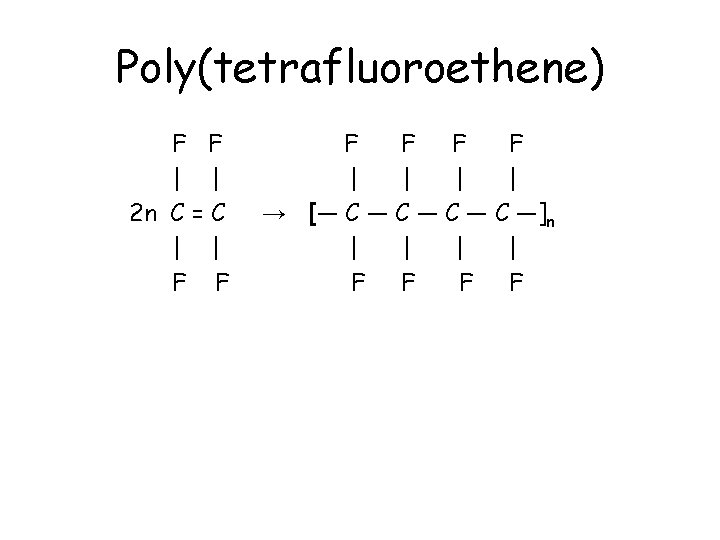
Poly(tetrafluoroethene) • Tetrafluoroethene, CF 2=CF 2, has fluorine atoms replacing each hydrogen atom in the ethene molecule. • Tetrafluoroethene, CF 2=CF 2, polymerises to form poly(tetrafluoroethene). • Compared to poly(ethene), every carbon in the chain has 2 fluorine atoms attached. • The polymer is commonly known as Teflon or PTFE, and is used for non-stick coatings.

Poly(tetrafluoroethene) F F | | 2 n C = C | | F F F | | → [― C ― C ―]n | | F F

Discovery of Poly(tetrafluoroethene) • US chemist Roy Plunkett opened a cylinder of C 2 F 4 but no gas came out. • Weight indicated a full cylinder. • Plunkett sawed open cylinder and found a waxy white powder inside. • He realised that a polymerisation reaction had occurred. • Poly(tetrafluoroethene), later called Teflon, was found to have remarkable properties.

Recycling of Plastics • Plastic products are labelled with numbers 1 to 6 to aid sorting. • The plastics are recycled after sorting. • Polystyrene is completely recyclable. • After sorting it is shredded, washed, dried, and then re-extruded.

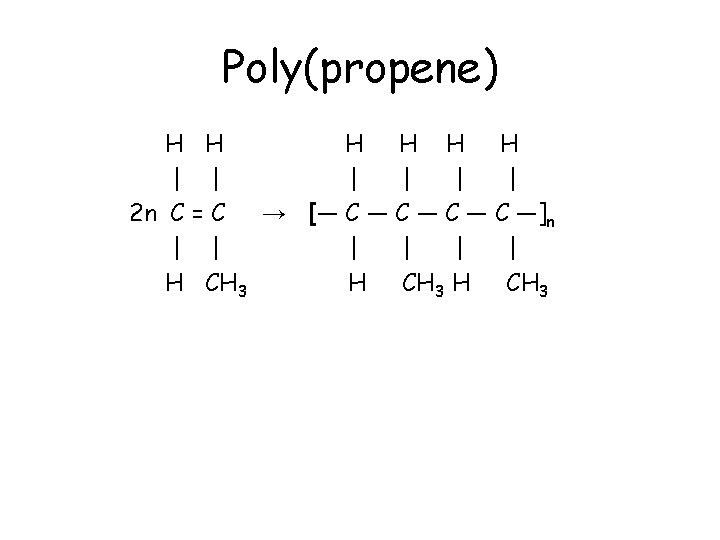
Poly(propene) • Propene, CH 2=CHCH 3, polymerises to form poly(propene). • Compared to poly(ethene), every second carbon in the chain has a methyl group attached. • The polymer is commonly known as polypropylene. • It is used to make ropes and stacking chairs.

Poly(propene) H H H | | | 2 n C = C → [― C ― C ―]n | | | H CH 3

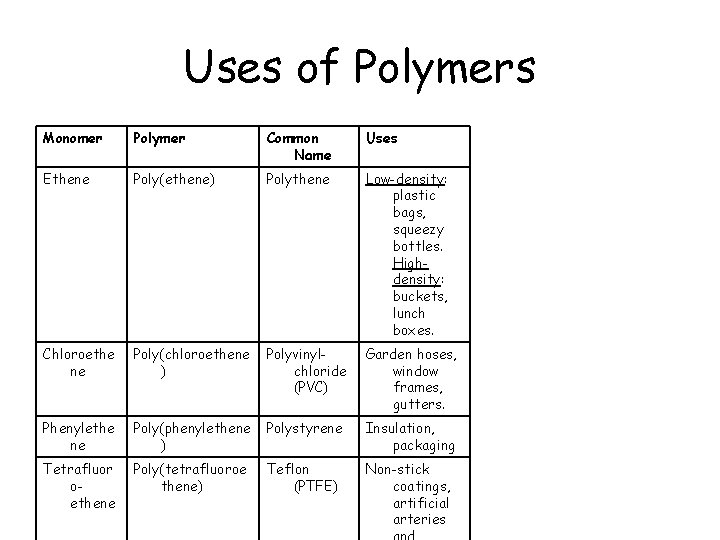
Uses of Polymers Monomer Polymer Common Name Uses Ethene Poly(ethene) Polythene Low-density: plastic bags, squeezy bottles. Highdensity: buckets, lunch boxes. Chloroethe ne Poly(chloroethene ) Polyvinylchloride (PVC) Garden hoses, window frames, gutters. Phenylethe ne Poly(phenylethene ) Polystyrene Insulation, packaging Tetrafluor oethene Poly(tetrafluoroe thene) Teflon (PTFE) Non-stick coatings, artificial arteries